Abstract
Synanthedon vespiformis L. (Lepidoptera: Sesiidae) is considered a rare insect in Sweden, discovered in 1860, with only a few observations recorded until a sex pheromone attractant became available recently. This study details a national survey conducted using pheromones as a sampling method for this species. Through pheromone trapping we captured 439 specimens in Southern Sweden at 77 sites, almost tripling the number of previously reported records for this species. The results suggest that S. vespiformis is truly a rare species with a genuinely scattered distribution, but can be locally abundant. Habitat analyses were conducted in order to test the relationship between habitat quality and the number of individuals caught. In Sweden, S. vespiformis is thought to be associated with oak hosts, but our attempts to predict its occurrence by the abundance of oaks yielded no significant relationships. We therefore suggest that sampling bias and limited knowledge on distribution may have led to the assumption that this species is primarily reliant on oaks in the northern part of its range, whereas it may in fact be polyphagous, similar to S. vespiformis found as an agricultural pest in Central and Southern Europe. We conclude that pheromones can massively enhance sampling potential for this and other rare lepidopteran species. Large-scale pheromone-based surveys provide a snapshot of true presences and absences across a considerable part of a species national distribution range, and thus for the first time provide a viable means of systematically assessing changes in distribution over time with high spatiotemporal resolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Woodland habitats have undergone significant anthropogenic change in recent centuries, giving way to land use focused on agriculture and housing development (Eliasson and Nilsson 2002). As well as habitat fragmentation, habitat alteration has also been an issue; whereby open, sunny woodland habitats have been transformed into shady, overgrown habitats less suitable for species dependent on sunlight (Kirby et al. 2005). One such light-dependent species associated with oak woodland habitat and the focus of this paper is the clearwing moth Synanthedon vespiformis (L.) (Lepidoptera: Sesiidae), first recorded in Sweden in 1860 (Eliasson 2007). This species is classified as Vulnerable on the Swedish Red List (Gärdenfors 2010) and considered ‘Nationally Scarce’ in the United Kingdom (Greatorex-Davies et al. 2003). The moth could potentially serve as a good indicator for the increasingly rare open woodland habitats. The location and distribution of clearwing moth populations are however difficult to assess, as the species of this family are relatively inconspicuous and frequently mistaken for members of the hymenoptera due to their mimicry (Duckworth and Eichlin 1974). As a result, knowledge of their true distribution and ecology remains relatively limited.
S. vespiformis is a particularly interesting example of a saproxylic clearwing, as its habitat preferences seems to vary significantly across its geographical range in Europe. In the southern part of its range it is thought to be fairly polyphagous and is in fact considered a pest, attacking numerous tree species including beech (Fagus silvatica L.), oak (Quercus spp.), poplar (Populus spp.), willow (Salix spp.) and fruit crops of blackberry (Rubus fruticosus L.), raspberry (Rubus idaeus L.) and peach (Prunus persica L.) (Szántóné-Veszelka et al. 2010). However, in the northern part of its range where the species is considered threatened and does not occur as an agricultural pest, the primary host of the larvae is assumed to be Quercus robur L. (pedunculate oak) with some suggestions of members of the Rosaceae and Betula spp. as secondary hosts (Eliasson and Nilsson 2002; Waring and Townsend 2003). However, the evidence is predominantly anecdotal or based on a small number of observations. Thus a better understanding of its ecology will aid in conservation of this species, particularly in places where it is considered to be in decline. These conservation efforts first require effective and accurate sampling and monitoring methods of the target species, something which has been identified as lacking in modern biodiversity management (Rademaekers et al. 2010).
Identifying, protecting and monitoring key areas or habitat types that support a high number of rare or threatened species is essential in conservation (Henle et al. 2013). However, broad systematic surveys are expensive, time-consuming and often reliant on a small number of taxonomic experts (e.g. Horák et al. 2013). Saproxylic habitats in particular are significantly more difficult and expensive to monitor using standard methods, with the number of site visits for establishing species assemblage being at least twice that of equivalent grassland habitats (pers. comm. David Heaver, Natural England). Thus for determining species distribution, systematic surveys of this nature risk generating expensive data of relatively low accuracy and precision.
As a result, bioindicator species are often used as a proxy in place of complete surveys in order to locate sites with high species richness and/or conservation value (Fleishman and Murphy 2009). The most commonly used indicators of terrestrial biodiversity are butterflies, grasshoppers and wild bees, mainly for open environments like grasslands (Rosenberg et al. 1986; Nilsson et al. 1995; Bazelet and Samways 2011, 2012; Bommarco et al. 2012; Gerlach et al. 2013). Bioindicator selection for assessing the quality of woodland or forest habitats is however heavily skewed towards saproxylic beetles due to their prevalence (Grove 2002), meanwhile Lepidoptera are under-represented in these habitats despite their potential for assessing human impacts on biodiversity (Fiedler and Schulze 2004). Some woodland moths are rather inconspicuous and are often under-reported in survey data despite being sampled regularly (Quinto et al. 2013; Jonason et al. 2013, 2014), possibly due to their predominantly nocturnal behavior, and sampling bias from recorders (Dennis and Thomas 2000). Thus an effective method of standardized recording for these potential bioindicators is highly desirable.
In order to provide greater levels of accuracy and sampling power at a lower cost, insect pheromones have been suggested as a supplement to existing sampling methods for insects (Larsson et al. 2003; Tolasch et al. 2007; Larsson et al. 2009; Harvey et al. 2010; Millar et al. 2010; Musa et al. 2013; Andersson et al. 2014). Given their widespread availability already in pest management for Lepidoptera and particularly clearwing moths (Braxton and Raupp 1995), these tools could be redirected to provide great benefits to biodiversity monitoring of saproxylics. Pheromone monitoring systems in insects are generally species-specific once optimized, although cross-attraction exists, e.g. in some other Lepidoptera (Löfstedt et al. 1991) and some groups of saproxylic beetle species (Hanks et al. 2012). Regardless, this selectivity would be advantageous for a focus on defined indicator species, whilst ultimately a guild of bioindicators would be desirable in order to reflect a wider range of microhabitats within a system. In the present study, we carried out pheromone monitoring of one potential indicator, the clearwing moth S. vespiformis, whose pheromone system has recently been characterized (Levi-Zada et al. 2011).
The first general aim of the present study was to establish whether pheromone monitoring could provide more accurate information about the species’ distribution than standard monitoring practices. Secondly, we wanted to establish whether the apparent rarity of the species in Sweden was a real phenomenon, or simply a result of poor detectability. Additionally, to conserve threatened species, knowledge of the species’ habitat requirements is essential. The type of habitat a species uses can be found by relating particular habitat elements to species abundances or occurrences, with quantity of habitat required being assessed at multiple scales (Bergman et al. 2012; Musa et al. 2013). Once the required type and quantity of that resource has been ascertained, an assessment of areas in the landscape suitable for a species is possible. The third aim of this study was therefore to establish the preferred habitat for S. vespiformis by correlating its abundance with habitat characteristics and, by extension, to establish whether it could be used as an indicator for the biodiversity potential of oak-dominated open woodlands with which it has traditionally been associated.
Materials and methods
Site selection
The study was conducted in the counties of Östergötland, Skåne, Blekinge and Kalmar where a number of sites with high density of old and/or hollow oaks were selected (Fig. 1). These sites generally have high species richness (Nilsson et al. 1995) but have suffered from severe decline and fragmentation over the last 200 years in Sweden, mainly due to the change in ownership of the oaks and shifts in farming and forestry practices (Eliasson and Nilsson 2002). Included in the selection were a number of sites with lower proportions of oaks, and a higher number of other hardwood species and hollow trees for comparison, covering a total of 251 sites (numbers of traps per county are shown in Table 1). Site selection was also based on a minimum separation distance of 500 m between traps, the closest two traps being separated by 512 m in this study. In this survey, a total of eight traps had another trap within a 1000 m radius, with the majority being separated by many kilometres. Our observations on the attractive range of similar moth pheromones in mark release recapture studies suggest that males may be able to detect lures at a maximum distance of 150–200 m (unpublished data). Therefore these distances were maintained in order to rule out the effect of inter-trap competition.
a Map showing pre-2010 records (www.artportalen.se) of S. vespiformis in Southern Sweden. b Recordings of S. vespiformis as of 2013 without pheromone survey. c Reported sites in 2013 after inclusion of pheromone survey data. d Map showing the distribution of pheromone traps in southern Sweden in the Skåne, Blekinge, Kalmar and Östergötland counties (Swedish “Län” = county). Filled and empty circles indicate sites where S. vespiformis. was recorded and was not recorded, respectively
Historical records of S. vespiformis
Pre-existing records were taken from Sweden’s nationwide repository for animal, fungi and plant distribution data, the “Swedish Species Gateway” (www.artportalen.se). In order to compare historical records with our own more recent survey efforts, we used data dating back to 1976 when the first database entry for this species occurred from a site in Stockholm. These sightings (often recorded using standard observational methods such as larval/pupal searches) (Fig. 1a, b) were then compared to recent inventories we carried out using pheromone lures in 2011–2012 (Fig. 1c, d). Artportalen also included a small number of historical pheromone-based sightings between 2005 and 2013 which were excluded from our analyses, but are shown in Fig. 1.
Biology of S. vespiformis
Very little is known about the biology of S. vespiformis, although it is considered to be saproxylic; feeding on the cortex of its various suggested host plants (Levi-Zada et al. 2011). The adult moth is considered to have a ‘moderate’ flight range (Van der Meulen and Groenendijk 2005) taking flight in the afternoon through into the early evening when male moths can be caught by pheromone lure (Levi-Zada et al. 2011). In Sweden the flight period begins in the last week of June and can last until the first week of August, with peak activity taking place in the second week of June in both northern and southern counties (Artportalen 2015). This species is also known to have an association with the gall-inducing bacterium Agrobacterium tumefaciens, which likely facilitates larval feeding in some host plants (Audemard and Vigouroux 1982).
Pheromone lures
We used a modified version of the pheromone blend for S. vespiformis, that has been found to be more attractive to Swedish populations than the blend from Levi-Zada et al., (2011) (Ryrholm unpublished). Pheromone lures were produced using a pre-prepared blend of four pheromone components dissolved in hexane. The blend was prepared so that 200 μl hexane solution contained 300 μg E3Z13-18:Ac, 90 μg E3Z13-18:OH, 30 μg Z3Z13-18:Ac and 3 μg E2Z13-18:OH. A blend volume of 200 μl was then pipetted onto the surface of a 20 mm diameter grey rubber septum (PheroNet, Sweden) and left in a fume cupboard to allow the solvent to fully evaporate. Septa were subsequently stored in the freezer until further use in order to preserve the attractiveness of the lure.
Trap methodology
The whole rationale of the present study was to use a viable and consistent sampling effort to obtain the first semi-quantitative data on presence and abundance across a large number of sites over a wide geographical range, in contrast to the scattered records previously available. For this purpose, we considered sticky traps as the best available option. Lures were hung 2 cm from the roof of a clear plastic delta trap sourced from CSalomon pheromone traps, Budapest, Hungary. Instead of the original sticky bottom inserts, we used cardboard sticky inserts from Oecos ltd, UK, which preserved the morphology of the wings more effectively for subsequent species identification. Studies on destructive sampling have shown that insect populations are robust against lethal sampling methods even when multiple killing traps are used per hectare (Haniotakis et al. 1999; Yamanaka et al. 2001; Gezon et al. 2015). Although we did not expect our sampling to affect the populations sampled significantly, we used a reduced sticky area in our traps (about 50 % compared to their original size, or 80 cm2) to reduce catches. This was a precaution considering that we were working with a rare species and a pheromone of unknown attractiveness. At each site, one trap was placed 1–2 m above ground from a nearby tree. Tree selection was based on availability of trees and not individual tree species. Traps were placed throughout the 10 day period which commenced on 1st July 2012, and were brought down in a 10 day period after 6th August 2012 ensuring that all traps had been placed in the field for a minimum of 4 weeks. Traps were also left for 4 weeks during July of 2011 (for a small pilot study in the Västervik region), with the majority of the survey work being carried out in 2012. Subsequent catches were identified and recorded after traps and sticky inserts were removed at the end of this sampling period. Only data from 2012 were used for subsequent habitat analyses.
Statistical analyses
In order to test the relative efficacy of pheromone lures compared to standard historical methods logged on Artportalen, a Kruskal–Wallis test was performed on the catch abundance data. The test was carried out in order to determine whether the median number of individuals caught by standard methods from historical survey was significantly different from the median number of catches made through the use of a pheromone lure.
The study of habitat characteristics was carried out only in Östergötland, which had the largest proportion of occupied sites. This county also had the largest and most consistent set of tree data in Sweden whilst tree data were partially incomplete in other counties. Thus the 102 sites in this region were deemed most suitable for study of habitat characteristics preferred by S. vespiformis. Tree data used for this study were derived from the most recent survey, which was a 10 year old survey of the region commissioned by the County Administration Board of Östergötland. From these data, tree groups were categorized as oaks or non-oaks in order to test the hypothesis that S. vespiformis is associated more strongly with Quercus spp. in this northern part of its range. European aspen (Populus tremula L.) and silver birch (Betula pendula Roth.) were excluded from the study due to incomplete survey data as well as the following coniferous trees: Norway spruce [Picea abies (L.) H.Karst], larch (Larix sp.), common juniper (Juniperus communis L.), and scots pine (Pinus sylvestris L.).
Additionally, trees were classed by a further six groupings as follows; (1) all trees, (2) trees >450 cm circumference, (3) hollow stage >3 (where stages 1–3 have no significant hollows and 4–7 have hollows of increasing size categories larger than 10 cm in diameter) (Claesson and Ek 2009), (4) trees located in open areas, (5) trees >450 cm circumference located in grasslands, (6) hollow stage >3 located in grasslands. These groupings would allow us to further determine preferences for different types of oak woodland/semi pasture. A national database on semi-natural grasslands (TUVA) was used to locate targeted trees situated in semi-natural grasslands. The “open areas” category included targeted trees with open canopy cover and open surrounding vegetation. Further explanations of the canopy cover and surrounding vegetation categories can be found in Claesson and Ek (2009).
Since saproxylic insect populations can respond to a wide range of geographical scales (Bergman et al. 2012), 28 radii, ranging from 30 to 6000 m (Fig. 2), were used to calculate tree densities around each site (Quantum GIS 1.8.0-Lisboa 2013). The reasoning behind selecting 30 m as the minimum scale was that it needed to be small while still maintaining variation in tree densities among sites. The maximum scale of 6000 m was selected based on the maximum dispersal range of other moths as well as to retain enough sites without the largest radii overlapping.
The scale(s) at which the species responds most strongly to the habitat variables (characteristic scale of response) was estimated for each tree group separately. At each scale, a negative binomial general linear model was run with abundance of S. vespiformis as response and tree counts as predictor variable. The computer program Focus (Geomatics and Landscape Ecology Research Lab, Canada) was used to extract 500 combinations of non-overlapping buffers at each radius: no radii overlapped below 500 m. The median of the regression results were used to condense the results for one scale and tree group. The characteristic scale of response for a tree group was defined as the scale with the largest absolute Z-value. It is possible to use other variables, and rings instead of circle as buffers, to determine the characteristic scale of response. Tree content in ring buffers are less correlated between scales, and regression coefficients on standardized explanatory variables make comparisons of effect size easier. However, the connection between response to circle buffers and ecological processes is easier to understand. Furthermore, the interpretation of standardized coefficients need a measure of variance which the Z-value already provides. Results from ring buffers were also similar to circle buffers and standardized coefficients scaled with Z-value, thus the alternatives did not change the interpretation. There was generally a weak correlation between oak and non-oak trees which was negative (r = −0.1) for small radii and positive for larger radii (r = 0.1) while correlations within tree-groups of oak or non-oak were higher and positive. Running multiple correlations reduces the pseudoreplication of data points because each iteration uses spatially independent sites (Holland et al. 2004). However, the repeated analysis uses the same data so pseudoreplication may not be entirely avoided. The Focus program therefore allows for optimization of the data available and an increase in the power of the analysis. All analysis and data management was done in the statistical software R (R Core Team 2014).
Results
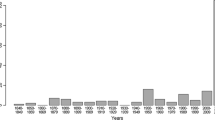
In total 439 individual specimens of S. vespiformis were caught across the four counties, accounting for 77 newly identified localities (Fig. 1c, d). We also recorded a total of 174 sites where the species was not found. The breakdown of records per county is shown in Table 1. The sampling effort from this study almost tripled the number of reported localities for this species during only two field seasons (47 previously reported sites found in Artportalen were increased to 124 occupied sites in total as a result of sites located by pheromone lure), after decades of reports by classical methods of surveying (Fig. 3). The result is a slight apparent expansion of S. vespiformis range due to increased sampling effort/accuracy, and a much higher density of occupied sites noted within the existing range (Fig. 1).
Frequency of site reports of S. vespiformis since 1976 (www.artportalen.se). The implementation of pheromone lures contributes significantly towards the greatly increased numbers of reported sites in 2011 and 2012
The new inventory of the species revealed a scattered pattern of distribution amongst woodland habitats sampled in southern Sweden. In some cases moths were locally abundant, but their absence is notable across significant areas of most counties despite the extensive and systematic sampling effort and high efficacy of the traps. Comparison of the median number of individuals caught by standard versus pheromone methods however showed significantly higher numbers to be observed via pheromone trapping overall (H = 18.58, d.f. = 1, p < 0.001). The median catch using the standard method was one individual, compared to a median of two individuals for the pheromone method.
Habitat analyses in Östergötland (Fig. 4), showed no significant relationship between the numbers of individuals caught in traps, with any of the habitat variables included at any scale. No absolute median Z-values were larger than 1.96 which is the 5 % significance level. Focusing on effect size there was a relatively larger positive response to hollow oaks close to the trap (30–200 m), while other tree species in general, and hollow trees in particular, seemed to have a negative effect on species presence. The reversed relationship between oak and non-oak was not a result from explanatory variables being highly correlated at lower scales (r was approximately −0.1 between 30 and 200 m).
The relationship (median Z-value from 500 negative binomial regressions at each scale and tree group) between abundance of S. vespiformis and amount of a oak trees with various characteristics at multiple scales and b non-oak deciduous trees at multiple scales. Light and dark gray circles denote negative and positive relationships respectively, and ring size above 1.96 is significant at 5 % level
In addition to catches of S. vespiformis, some traps in Skåne and Östergötland also caught another clearwing species, Pennisetia hylaeiformis Laspeyres. This species was present at fifteen sites in Skåne and nine in Östergötland, with the number of individuals totaling 128 and 23 across all traps in these regions respectively.
Discussion
S. vespiformis is considered a rare insect in Sweden, first recorded in 1860, and with just a few observations logged until the sex pheromone was identified and its synthetic constituents became available more recently. To our knowledge, our approach to addressing this situation represents the first systematic application of pheromones for any regional survey of a lepidopteran conservation target species, although large-scale pheromone surveys have previously targeted pests among the Lepidoptera (e.g. Tobin et al. 2007) and other orders, and also the threatened click beetle Elater ferrugineus (Kadej et al. 2015). This study shows that pheromone-based monitoring of woodland moths can provide a significant increase in accuracy and sampling effort of surveys targeting saproxylic insects. By use of sex pheromones an additional 77 sites with S. vespiformis were found; thereby increasing the known localities from 47 to 124. This represents a near tripling of the number of reported sites in just two field seasons. In addition, the number of individuals caught represents a significant increase in sampling efficiency. Previous site visits often report single insects, whereas we uncovered a total of 439 individuals across our survey sites, with the highest number of individuals at one site being 41 (a site in Västervik municipality, Kalmar county). This increased efficiency is further supported by a significant difference noted in the median number of insects caught when comparing recording based on observation to recording using a pheromone lure.
A minor limitation which was noted during this study was the tendency for this pheromone blend to cause cross-attraction to Pennisetia hylaeiformis; a similar looking sesiid which could potentially lead to misidentification of S. vespiformis sites. It was also noted that some potential predation had occurred in traps left for a period of time longer than a few weeks, which may have even led to an under-sampling at certain sites. Thus is suggested that for the inventory of this species, voucher specimens should be retained for taxonomic determination, and traps checked at regular intervals where possible.
We have not yet studied the specific accuracy of our pheromone lures in detecting populations of S. vespiformis, e.g. through recapture experiments or repeated sampling of known populations, but we would argue that empty pheromone traps in our study usually represent truly unoccupied sites, lacking reproducing populations. The generally high efficiency of these types of classical female-produced sex pheromones in detecting their target species has been well established in lepidopteran pest species (Zhang and Schlyter 1996) and in many other insect groups (Östrand and Anderbrant 2003), including the threatened click beetle E. ferrugineus (Svensson et al. 2012; Zauli et al. 2014). In most cases it appears highly unlikely that a monitoring trap would fail to detect any males over a full flight season at a site with a local reproducing population. Absence data generated from sex pheromone lures have been shown to be much more reliable than stochastic methods such as unbaited pitfall or window traps in establishing presence or absence (Andersson et al. 2014; Zauli et al. 2014). The data generated by this methodology can therefore allow for better resolution in ecological studies, which rely strongly on accurate presence and absence data to characterize not only the species’ habitat, but also its ‘non-habitat’. Future studies of the use of pheromones would benefit from a full assessment of their monitoring accuracy in order to assess exactly how precise moth pheromone systems are for determining absence, similar to studies on emerging eDNA technology for species assessment of great crested newt (Biggs et al. 2015).
Under-reporting of occupancy is a significant issue in biodiversity monitoring where sampling methods show low detection probability (Pellet and Schmidt 2005), but this could be easily resolved with the high levels of detectability that pheromones provide (Andersson et al. 2014). The previous lack of occupancy records are likely contributors to our view of S. vespiformis as a species with a very sparse distribution in Sweden. Our findings suggest this species is perhaps a little less rare than previously thought, and occasionally abundant locally despite notable long-term declines in the type of Swedish oak forest considered to be its primary habitat. The slight expansion of the species range, and higher density of known sites within its range seen in Fig. 1 is likely to be reflective of increased sampling efficacy rather than actual recent expansion of the species itself. However, the distribution of S. vespiformis is still relatively sparse, with a large proportion of sites where the species was absent despite an apparent abundance of saproxylic resources, suggesting the species may still be under threat or that very little is known about its ecological requirements.
During the course of the study, S. vespiformis was found at a number of sites outside of its ‘expected’ habitat type. Singleton catches outside of the expected habitat may be a result of chance migrants caught nearby a ‘true’ locality. However, sampling bias has also been highlighted as a limiting factor in insect surveying particularly with Lepidoptera (Dennis et al. 1999), and this may contribute towards the oak-biased recordings seen in previous survey data. Resource-limited county administrative boards and field entomologists can tend to focus their efforts on known sites, or sites similar to those where the species has been found already. This circular approach may obscure hidden biodiversity and niches that might otherwise be uncovered by a less biased search effort in the field. Our analyses of S. vespiformis’ habitat further highlight this sampling bias when considering S. vespiformis’ preferences in northern Europe. In Sweden, the species is considered to be reliant on oaks (Waring and Townsend 2003; Eliasson 2007), but our results showed only weak relationships with old oaks and oaks with holes for number of moths caught and oak and non-oak tree abundance (Fig. 4). This is in stark contrast to results from the click beetle E. ferrugineus using similar methodology in relation to similar veteran tree habitats, which demonstrated a very strong association between the beetle and its nominal habitat (Musa et al. 2013). This casts some doubt on the previous assumptions made regarding host specificity and preference in S. vespiformis. Our findings were also echoed in a recent Swedish inventory, where it was noted that “Pheromone lures placed on several premises in Skåne and Öland and in eastern Småland have shown that the species is not as tied to large veteran trees as previously assumed, but is reliant on this habitat when others are not available.” (Palmqvist 2014).
In the south of its range S. vespiformis is found to be rather polyphagous, yet its visibility in these regions is undoubtedly much higher due to its high population numbers in agricultural crops (Levi-Zada et al. 2011), and thus the likelihood of observing the species on alternative hosts is higher. Based on these findings we suggest that Swedish S. vespiformis could be more polyphagous than previously expected, possibly in line with its southern siblings. This hypothesis could be further tested by using pheromones to survey in regions significantly outside of its known range (for example Småland), in habitats which are not typical oak woodland and contain higher numbers of “secondary” host species. Incidentally, a recent study on saproxylic beetles also showed that most were polyphagous (Milberg et al. 2014), and the presumed oak-specificity was most likely due to sampling bias. It is therefore suggested that sesiids would benefit from a significant re-inventory in order to assess their true host relationships and conservation status.
In principle, the methods applied here could encompass all previous observations and attempts at surveying S. vespiformis in Sweden and surpass them several times over, in a single season, with comparably little effort. The high quality and potentially low bias of data generated by pheromone monitoring is also important, as knowledge on species distribution needs to inform decision making at both a local, national and international level (Pereira and Cooper 2006). This increased sampling efficacy has the potential to solve a number of issues highlighted by the European Commission in their efforts to mitigate biodiversity loss by 2020. Firstly, it has been suggested that one of the major challenges in meeting this target is development of effective and standardized methods of monitoring for national or international biodiversity (Pereira and Cooper 2006; Henry et al. 2008). Additionally, such international monitoring efforts must be viable within a restricted pool of resources (Bates et al. 2007), since cost effectiveness is a significant consideration in any environmental monitoring system (Hauser et al. 2006; Lovett et al. 2007). In light of the reduced or unstable funding for conservation/biodiversity monitoring in many member states (Lindenmayer et al. 2012), pheromone attractants could provide an essential tool for insect inventories across Europe. Not only are the pheromones relatively cost effective (costs are relatively low for these simple compounds where the synthesis method is already established), but the relative sampling effort in terms of working hours for survey is also greatly reduced (Burman and Thackery, unpublished data). This is particularly promising, as time is also a limiting resource in biodiversity monitoring (Yoccoz et al. 2001). Pheromones could allow those with limited resources the opportunity to spread their efforts further afield, and thereby improve the quality of data obtained. We demonstrate in this study that national level insect species surveys are feasible using sex pheromones, covering large spatial and potentially temporal scales to provide data relevant to international monitoring (Pereira and Cooper 2006). Large-scale pheromone-based surveys provide a snapshot of presences and absences across a considerable part of a species national distribution range, and thus for the first time provide a viable means of systematically assessing changes in distribution over time with high spatiotemporal resolution.
In the present study destructive monitoring of S. vespiformis was a prerequisite for obtaining comparable semi-quantitative occupancy data with high resolution over an extensive geographical range, and was carried out in agreement with conservation authorities although neither the species nor many of the sites are protected. The species is believed to generally have a 2-year life cycle in Sweden (Eliasson 2007), which would mitigate any risk to individual populations. Whilst destructive monitoring of the kind carried out in this study should be avoided year on year, it does provide a new snapshot of species distribution previously unavailable to conservationists. This includes a significant number of previously unreported sites for this species, many of which receive no statutory protection or management at the time of writing, but which could now be considered for their conservation value. Repeated future monitoring of these sites over extended time intervals perhaps combined with more regular use of pheromone live-trapping at a limited number of sites, could provide regular insights into how these habitats are faring in the longer term in response to different management regimes.
Conclusions
In this study we have shown that hidden biodiversity and species preferences can be substantial due to sampling bias and less effective sampling methodology. The vast majority of species sampled by standard observational methods are likely to be underreported, and as a result the drop in data quality can lead to problems in conducting ecological studies to uncover true habitat characteristics (Pellet and Schmidt 2005). By comparison, pheromone-based methods can significantly increase the sampling accuracy and give a much more reliable idea of a species’ distribution. S. vespiformis appears to be genuinely quite rare still, despite a significant number of new sites being located. In addition, the increased resolution of data, showed no particular association for large oaks, despite previous assumptions.
We believe as a result, that pheromone-baited traps bring much promise for conservation, both for surveying and monitoring targeted species, and could be used as a powerful tool to achieve the EU’s optimistic goals of halting insect biodiversity loss by 2020, as well as an invaluable resource for carrying out landscape level ecological studies.
References
Andersson K, Bergman KO, Andersson F, Hedenström E, Jansson N, Burman J, Winde I, Larsson MC, Milberg P (2014) High-accuracy sampling of saproxylic diversity indicators at regional scales with pheromones: the case of Elater ferrugineus (Coleoptera, Elateridae). Biol Conserv 171:156–166
Artportalen (2015) Swedish Species Gateway. http://www.artportalen.se/. 3 Nov 2015
Audemard H, Vigouroux A (1982) Une curieuse association parasitaire sur Pecher: Sesie (Synanthedon vespiformis) et tumeur bacterienne du collet (Agrobacterium tumefaciens). Phytoma 336:28–29
Bates CR, Scott G, Tobin M (2007) Weighing the costs and benefits of reduced sampling resolution in biomonitoring studies: perspectives from the temperate rocky intertidal. Biol Conserv 137:617–625
Bazelet CS, Samways MJ (2011) Identifying grasshopper bioindicators for habitat quality assessment of ecological networks. Ecol Indic 11:1259–1269
Bazelet CS, Samways MJ (2012) Grasshopper and butterfly local congruency in grassland remnants. J Insect Conserv 16:71–85
Bergman K-O, Jansson N, Claesson K, Palmer MW, Milberg P (2012) How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles. For Ecol Manag 265:133–141
Biggs J, Ewald N, Valentini A, Gaboriaud C, Dejean T, Griffiths RA, Foster J, Wilkinson JW, Arnell A, Brotherton P, Williams P, Dunn F (2015) Using eDNA to develop a national citizen science-based monitoring programme for the great crested newt (Triturus cristatus). Biol Conserv 183:19–28
Bommarco R, Lundin O, Smith HG, Rundlöf M (2012) Drastic historic shifts in bumble-bee community composition in Sweden. Proc Roy Soc B Biol Sci 279:309–315
Braxton SM, Raupp MJ (1995) An annotated checklist of clearwing border pests of ornamental plants trapped using commercially available pheromone lures. J Arboricult 21:177–180
Claesson K, Ek T (2009). Skyddsvärda träd i Östergötland 1997–2008. Länsstryrelsen Östergötland, 1–12
Dennis RLH, Thomas CD (2000) Bias in butterfly distribution maps: the influence of hot spots and recorder’s home range. J Insect Conserv 4:73–77
Dennis RLH, Sparks TH, Hardy PB (1999) Bias in butterfly distribution maps: the effects of sampling effort. J Insect Conserv 3:33–42
Duckworth WD, Eichlin TD (1974) Clearwing moths of Australia and New Zealand (Lepidoptera: Sesiidae). Smithsonian Institution Press, USA
Eliasson CU (2007) Synanthedon vespiformis. Fact sheet. ArtDatabanken, Swedish University of Agricultural Sciences, Uppsala
Eliasson P, Nilsson SG (2002) ‘You should hate young oaks and young noblemen’: the environmental history of oaks in eighteenth-and nineteenth-century Sweden. Environ Hist 7:659–677
Fiedler K, Schulze CH (2004) Forest modification affects diversity (but not dynamics) of speciose tropical pyraloid moth communities. Biotropica 36:615–627
Fleishman E, Murphy DD (2009) A realistic assessment of the indicator potential of butterflies and other charismatic taxonomic groups. Conserv Biol 23:1109–1116
Gärdenfors U (2010) The 2010 Red List of Swedish Species. Artdatabanken, SLU, Uppsala
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850
Gezon ZJ, Wyman ES, Ascher JS, Inouye DW, Irwin RE (2015) The effect of repeated, lethal sampling on wild bee abundance and diversity. Met Ecol Evol 6:1044–1054
Greatorex-Davies N, Sparks T, Woiwod I (2003) Changes in the Lepidoptera of Monks Wood NNR. Ten years of change: Woodland research at Monks Wood NNR, 90
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annu Rev Ecol Syst 33:1–23
Haniotakis GE, Koutroubas A, Sachinoglou A, Lahlou A (1999) Studies on the response of the leopard moth, Zeuzera pyrina I (Lepidoptera: Cossidae) to pheromones in apple orchards. IOBC wprs Bull 22:105–114
Hanks LM, Millar JG, Mongold-Diers JA, Wong JC, Meier LR, Reagel PF, Mitchell RF (2012) Using blends of cerambycid beetle pheromones and host plant volatiles to simultaneously attract a diversity of cerambycid species. Can J Forest Res 42:1050–1059
Harvey DJ, Hawes CJ, Gange AC, Finch P, Chesmore D, Farr I (2010) Development of non-invasive monitoring methods for larvae and adults of the stag beetle, Lucanus cervus. Insect Con Diver 4:4–14
Hauser CE, Pople AR, Possingham HP (2006) Should managed populations be monitored every year? Ecol Appl 16:807–819
Henle K, Bauch B, Auliya M, Külvik M, Pe‘er G, Schmeller DS, Framstad E (2013) Priorities for biodiversity monitoring in Europe: a review of supranational policies and a novel scheme for integrative prioritization. Ecol Indic 33:5–18
Henry PY, Lengyel S, Nowicki P, Julliard R, Clobert J, Čelik T, Gruber B, Schmeller DS, Babij V, Henle K (2008) Integrating ongoing biodiversity monitoring: potential benefits and methods. Biodivers Conserv 17:3357–3382
Holland JD, Bert DG, Fahrig L (2004) Determining the spatial scale of a species’ response to habitat. Bioscience 54:227–233
Horák J, Vodka Š, Pavlíček J, Boža P (2013) Unexpected visitors: flightless beetles in window traps. J Insect Conserv 17:441–449
Jonason D, Franzén M, Pettersson LB (2013) Transient peak in moth diversity as a response to organic farming. Basic Appl Ecol 14:515–522
Jonason D, Franzén M, Ranius T (2014) surveying moths using light traps: effects of weather and time of year. PLoS ONE 9:e92453
Kadej M, Zajac K, Ruta R, Gutowski JM, Tarnawski D, Smolis A, Olbrycht T, Malkiewicz A, Myskow E, Larsson MC, Andersson F, Hedenström E (2015) Sex pheromones as a tool to overcome the Wallacean shortfall in conservation biology: a case of Elater ferrugineus Linnaeus, 1758 (Coleoptera: Elateridae). J Insect Conserv 19:25–32
Kirby KJ, Smart SM, Black HIJ, Bunce RGH, Corney PM, Smithers RJ (2005) Long-term ecological change in British Woodlands (1971–2001): a re-survey and analysis of change based on the 103 sites in the Nature Conservancy ‘Bunce 1971’ Woodland Survey. English Nature
Larsson MC, Hedin J, Svensson GP, Tolasch T, Francke W (2003) Characteristic odor of Osmoderma eremita identified as a male-released pheromone. J Chem Ecol 29:575–587
Larsson MC, Svensson GP, Ryrholm N (2009) Monitoring rare and threatened insects with pheromone attractants. In: Samways MJ, New T, McGeoch M (eds) Insect conservation: a handbook of approaches and methods. Oxford University Press, Oxford, pp 114–116
Levi-Zada A, Ben-Yehuda S, Dunkelblum E, Gindin G, Fefer D, Protasov A, Kuznetsowa T, Manulis-Sasson S, Mendel Z (2011) Identification and field bioassays of the sex pheromone of the yellow-legged clearwing Synanthedon vespiformis (Lepidoptera: Sesiidae). Chemoecology 21:227–233
Lindenmayer DB, Gibbons P, Bourke MAX, Burgman M, Dickman CR, Ferrier S, Fitzsimons J, Freudenberger D, Garnett ST, Groves C, Hobbs RJ, Kingsford RT, Krebs C, Legge S, Lowe AJ, McLean R, Montambault J, Possingham H, Radford J, Robinson D, Smallbone L, Thomas D, Varcoe T, Vardon M, Wardle G, Woinarski J, Zerger A (2012) Improving biodiversity monitoring. Austral Ecol 37:285–294
Löfstedt C, Herrebout WM, Menken SB (1991) Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae). Chemoecology 2:20–28
Lovett GM, Burns DA, Driscoll CT, Jenkins JC, Mitchell MJ, Rustad L, Shanley JB, Likens GE, Haeuber R (2007) Who needs environmental monitoring? Front Ecol Environ 5:253–260
Milberg P, Bergman K-O, Johansson H, Jansson N (2014) Low host-tree preferences among saproxylic beetles: a comparison of four deciduous species. Insect Conserv Diver 7:508–522
Millar JG, McElfresh JS, Romero C, Vila M, Mari-Mena N, Lopez-Vaamonde C (2010) Identification of the sex pheromone of a protected species, the Spanish moon moth Graellsia isabellae. J Chem Ecol 36:923–932
Musa N, Andersson K, Burman J, Andersson F, Hedenström E, Jansson N, Paltto H, Westerberg L, Winde I, Larsson MC, Bergman K-O, Milberg P (2013) Using sex pheromone and a multi-scale approach to predict the distribution of a rare saproxylic beetle. PLoS ONE 8:e66149
Nilsson SG, Arup U, Baranowski R, Ekman S (1995) Tree-dependent lichens and beetles as indicators in conservation forests. Conserv Biol 9:1208–1215
Östrand F, Anderbrant O (2003) From where are insects recruited? A new model to interpret catches of attractive traps. Agric For Entomol 5:163–171
Palmqvist G (2014) Intressanta fynd av storfjärilar (Macrolepidoptera) i Sverige 2013 [Remarkable records of Macrolepidoptera in Sweden 2013]. Entomol Tidskr 135:63–76
Pellet J, Schmidt BR (2005) Monitoring distributions using call surveys: estimating site occupancy, detection probabilities and inferring absence. Biol Conserv 123:27–35
Pereira HM, Cooper DH (2006) Towards the global monitoring of biodiversity change. Trends Ecol Evol 21:123–129
Quinto J, Marcos-García MA, Brustel H, Galante E, Micó E (2013) Effectiveness of three sampling methods to survey saproxylic beetle assemblages in Mediterranean woodland. J Insect Conserv 17:765–776
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. 25 Nov 2015
Rademaekers K, Braat LC, Eichler L, Widerberg O, Jones-Walters L (2010) Costs and benefits assessment of monitoring approaches for measuring progress towards the EU 2020 biodiversity target. http://edepot.wur.nl/173781
Rosenberg DM, Danks HV, Lehmkuhl DM (1986) Importance of insects in environmental impact assessment. Environ Manag 10:773–783
Svensson GP, Liedtke C, Hedenström E, Breistein P, Bång J, Larsson MC (2012) Chemical ecology and insect conservation: optimizing pheromone-based monitoring of the threatened saproxylic click beetle Elater ferrugineus. J Insect Conserv 16:549–555
Szántóné-Veszelka M, Poós B, Szőcs G (2010) Blackberry and raspberry, new hosts of the yellow legged clearwing moth, Synanthedon vespiformis: what can the recently developed sex attractant offer in monitoring and beyond. In: IOBC working group, integrated plant protection in fruit crops subgroup “Soft Fruits”, 7th workshop on integrated soft fruit production, pp 20–23
Tobin PC, Liebhold AM, Roberts EA (2007) Comparison of methods for estimating the spread of a non-indigenous species. J Biogeogr 34:305–312
Tolasch T, Von Fragstein M, Steidle JLM (2007) Sex pheromone of Elater ferrugineus L. (Coleoptera: Elateridae). J Chem Ecol 33:2156–2166
Van der Meulen J, Groenendijk D (2005) Assessment of the mobility of day-flying moths: an ecological approach. Proc Exp Appl Entomol 16:37–50
Waring P, Townsend M (2003) Field guide to the moths of Great Britain and Ireland. British Wildlife Publishing Ltd, UK
Yamanaka T, Satoda S, Senda S, Tatsuki S (2001) Mass-trapping trials of the fall webworm, Hyphantria cunea (Drury)(Lepidoptera: Arctiidae), with synthetic sex pheromone in urban street trees. Jpn J Appl Entomol Z 12:175–183
Yoccoz NG, Nichols JD, Boulinier T (2001) Monitoring of biological diversity in space and time. Trends Ecol Evol 16:446–453
Zauli A, Chiari S, Hedenström E, Svensson GP, Carpaneto GM (2014) Using odour traps for population monitoring and dispersal analysis of the threatened saproxylic beetles Osmoderma eremita and Elater ferrugineus in central Italy. J Insect Conserv 18:801–813
Zhang Q-H, Schlyter F (1996) High recaptures and long sampling range of pheromone traps for fall web worm moth Hyphantria cunea (Lepidoptera: Arctiidae) males. J Chem Ecol 22:1783–1796
Acknowledgments
Stiftelsen Eklandskapet i Linköpings kommun, Marie-Claire Cronstedts Stiftelse, Swedish WWF, the Tranemåla Foundation, Skogssällskapet, Region Skånes miljövårdsfond, SLU Partnerskap Alnarp, and the IC-E3 Linnaeus grant (Formas, SLU) to the Division of Chemical Ecology at SLU provided grants for this project. Assistance was provided by Stefan Ekroth (expert advice, field work and species determination), Henrik Nguyen (pheromone bait and administrative support); Klas Andersson (field work); Tomas Burén and co-workers at Kalmar Municipality, Anders Jörneskog at Linköping Municipality and Kjell Antonsson at the County Administrative Board of Östergotland (various support). Thanks also go to David Heaver at Natural England for comments on the research from a national monitoring perspective. Finally, a special thanks to all landowners who gave their permission to set up traps, and their commitment to the historical legacy of their land as well as its future management.
Author’s contribution
M.C.L., L.W., P.M., N.R. & K.O.B. conceived and designed the study. J.B., M.C.L., I.W., S.O. and F.N. collected most of the data. L.W., J.B., P.M., S.O. & M.C.L. analyzed the data. J.B., M.C.L., N.R., F.N. & I.W. contributed reagents/materials/analysis tools. J.B., P.M. & M.C.L. wrote the paper with assistance from all other coauthors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burman, J., Westerberg, L., Ostrow, S. et al. Revealing hidden species distribution with pheromones: the case of Synanthedon vespiformis (Lepidoptera: Sesiidae) in Sweden. J Insect Conserv 20, 11–21 (2016). https://doi.org/10.1007/s10841-015-9835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-015-9835-9