Abstract
Background
The avascular capsule around the generator of the cardiac implantable electronic device (CIED) could be susceptible to bacterial colonization and source of infection. Capsulectomy during CIED generator replacement may be beneficial in preventing device infection, but there is a lack of evidence.
Methods
This prospective randomized trial, conducted from December 2013 to December 2019, included 195 patients divided equally into two groups. In the intervention group (n = 97), capsule removal was performed on the floor of the pocket, while it was not performed in the control group (n = 98). In both groups, swab culture was performed in the pocket. The primary outcome was the occurrence of device infection requiring pocket revision.
Results
A total of 195 patients were included (mean age 70.2 ± 13.6 years, 55.4% women), with an average follow-up period of 54.3 ± 28.9 months. Among 182 patients undergoing microbiological cultures of pockets, 19 (10.4%) were confirmed positive, and Staphylococcus species were identified most frequently. The primary outcome occurred in 4 (2.1%), and there was no significant difference between the two groups (3.1% vs. 1.0%, p = 0.606). Hematoma has occurred in 10 patients (3.1% vs. 7.1%, p = 0.338), one of them required wound revision. In multivariable analysis, the occurrence of hematoma was the only independent risk factor associated with device infection (HR 13.6, 95% CI 1.02–181.15, p = 0.048).
Conclusions
In this long-term prospective study, capsulectomy during the replacement of the generator did not reduce the incidence of device infection. There was no association between bacterial colonization in the capsule around the generator and CIED infection.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The number of patients eligible for cardiac implantable electronic device (CIED) therapy has been increasing along with the aging society, leading to a gradual rise in procedural cases worldwide [1, 2]. Despite the benefits provided by CIEDs, there is a concern regarding the occurrence of CIED infections, which have been reported to develop in approximately 1% of CIED patients within the first year following implantation [3, 4]. These infections may cause potentially fatal complications that can lead to death [5, 6]. As the absolute number of patients undergoing CIED procedures continues to rise, the incidence of CIED infections is also increasing [7, 8]. While there are various risk factors for CIED infections, generator replacement is a crucial factor that increases the risk of infection more than de novo procedure [9, 10]. The fibrous capsule surrounding the CIED generator has been found to have poor vascularity and can undergo chronic inflammation [11]. According to several studies, bacterial colonization within the capsule has been observed in 37 to 56% of CIED patients [12,13,14], serving as a potential source of device infection [14, 15]. Additionally, there have been reports of malignancy arising from these chronic inflammation sites [16,17,18]. For these reasons, some clinicians believe it would be beneficial to remove the capsule during the generator replacement procedure. However, the capsule is adhered to the surrounding tissues, making its removal a time-consuming procedure and may develop the risk of hematoma formation, which itself can contribute to device infection. Despite the clinical interest in capsule removal, there is limited research on whether removal of the capsule prevents CIED infections. This study aimed to investigate the association between capsulectomy during CIED generator replacement and the occurrence of device infection. Additionally, the study sought to determine the prevalence of bacterial colonization within the capsule and assess its relationship with the occurrence of device infection.
2 Methods
2.1 Study population
This study was conducted as a prospective randomized trial, targeting patients who underwent CIED generator replacement at Seoul St. Mary’s Hospital, Catholic University of Korea, from December 2013 to December 2019. Patients who required generator replacement due to battery depletion or device upgrade were included, while those who underwent generator replacement due to infection were excluded from the study. One hundred sixty-eight out of 198 patients were enrolled in the initial four years. Later in the enrollment period, we were not actively enrolling patients. A total of 198 patients were included in the study and randomly assigned to the intervention group and control group in a 1:1 ratio. The study protocol conformed with the Declaration of Helsinki regarding investigations in humans and was approved by the Institutional Review Board of the participating center. All patients gave their written informed consents.
2.2 Procedure
Prior to the procedure, the discontinuation of antithrombotic agents and the duration of discontinuation were determined at the discretion of the physician. All patients received prophylactic antibiotic treatment with cefazolin intravenously within 1 h before the procedure. Skin preparation was performed using povidone-iodine and chlorhexidine. After removing the existing generator from the pocket, a swab culture was performed within the capsule in both groups. Subsequently, in the intervention group, the floor portion of the capsule was completely removed, while in the control group, only the minimum amount of capsule required for generator replacement or lead revision was removed. Then, the pocket was disinfected with hydrogen peroxide in both groups. No prophylactic antibiotics were administered routinely after the procedure. During the period when the study patients received the index procedure, antibacterial envelopes were not available in our country, so none of the patients in our study received antibacterial envelopes.
2.3 Follow-up
The study subjects were scheduled to visit the outpatient center 10 days, 3 months, 12 months after the procedure, and every 24 months thereafter. During the follow-up period, if patients experienced symptoms or signs suspicious of device infection such as fever, chills, pain or tenderness at the wound site, or other related symptoms, they were instructed to visit the outpatient department for further evaluation. Wound examination and blood tests, including white blood cell count, C-reactive protein, and blood culture tests, were performed to confirm the presence of device infection.
2.4 Outcomes
The primary outcome of this study was the occurrence of CIED infection requiring wound revision after the index procedure. CIED infection was defined as a fever > 38.0 °C with one or more signs of wound inflammation, such as redness, swelling, tenderness, or purulent discharge. In cases where an additional pocket revision was conducted following the index procedure for any reason, subsequent device infections were excluded from the outcome analysis. The secondary outcomes included the occurrence of hematoma and pocket revision due to infection or hematoma. Hematoma is defined as the presence of a large effusion in the pocket leading to swelling and causing pain. Additionally, the prevalence of bacterial colonization was calculated by analyzing the results of swab cultures.
2.5 Statistical analysis
Continuous variables were presented as either the mean ± standard deviation (SD) or median (interquartile range (IQR)) and were analyzed using a one-way ANOVA test or Kruskal–Wallis test, depending on their distribution. Categorical variables were expressed as a number (percent or rate) and were analyzed using either the chi-square test or Fisher’s exact test, as appropriate. The calculation of the sample size was based on an endpoint event rate of 4 per 100 person-years for the control group and 1.4 per 100 person-years for the intervention group. This study planned to follow subjects for an average of 5 years. With a significance level set at 5% and a power of 70%, along with an anticipated dropout rate of 10%, it was determined that 94 subjects were required in each group. To assess the cumulative incidence of the primary outcome, the Kaplan–Meier method was employed, and the comparison between groups was conducted using log-rank tests. To further analyze the association between covariates and the primary outcome, a Cox proportional hazard model was applied, providing hazard ratios (HRs) and 95% confidence interval (CI). A p-value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) statistical software.
3 Result
3.1 Patient characteristics
Out of the initial cohort of 198 patients, three patients did not visit the outpatient center of the study institute after the procedure and were consequently excluded from the study. Thus, the final study population consisted of 195 patients. Among them, 108 (55.4%) were female, and the mean age was 70.2 ± 13.6 years. The intervention group consisted of 97 patients, while the control group consisted of 98 patients. The average follow-up duration was 54.3 ± 28.9 months. A total of 54 (27.7%) underwent lead revision concurrent with generator replacement and 8 (8.3%) had device upgrade from the pacemaker to an implantable cardioverter defibrillator (ICD) or a cardiac resynchronization therapy (CRT) device, or from an ICD to a CRT-D. There were no statistically significant differences between the two groups for both variables (25.8% vs. 29.6%, p = 0.663; 5.2% vs. 3.1%, p = 0.707 respectively). The intervention group had a higher proportion of patients with ICD or CRT-D devices than the control group (34.0% vs. 18.3%, p = 0.045). Patients taking anticoagulants (24.7% vs. 18.4%, p = 0.364) and antiplatelet agents (33.0% vs. 28.6%, p = 0.608) were more common in the intervention group, although these differences were not statistically significant. Swab culture inside the pocket during the generator replacement was performed in 182 (93.3%) patients. Detailed baseline characteristics are described in Table 1.
3.2 Outcomes
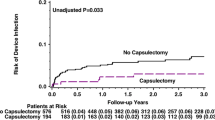
During the follow-up period, a total of 3 cases (3.1%) of the primary outcome occurred in the intervention group, while the control group had 1 case (1.0%) of the primary outcome. However, the difference between the two groups was not statistically significant (p = 0.61). In the control group, one patient underwent lead revision 6 months after generator replacement due to lead injury. Subsequently, this patient experienced a pocket infection 4 months after the lead revision. However, based on the assessment that the previously performed generator replacement procedure was not the cause of the pocket infection, this case was not included in the primary outcome. No other patients were excluded due to additional pocket revision in the two groups. Among the 4 patients who experienced device infection, 3 had pacemaker as their device type, while the remaining patient in the intervention group had a CRT-D device. Additionally, there were 3 cases (3.1%) of hematoma occurrence in the intervention group and 7 cases (7.1%) in the control group. Among the intervention group, one case with hematoma required pocket revision. The incidence of secondary outcomes, including hematoma and wound revision due to infection or hematoma, did not show a significant difference between the two groups (p for hematoma = 0.338, p for wound revision = 0.996) (Table 2). All patients who underwent wound revision remained free from any additional complications during the follow-up period. The Kaplan–Meier curve for the occurrence of primary outcomes is presented in Fig. 1. The Cox multivariable regression analysis revealed that only the occurrence of hematoma was independently associated with the primary outcome (HR 13.6, 95% CI 1.02–181.15, p = 0.048). Age, sex, capsulectomy, lead revision status, and device type did not show statistically significant associations with the primary outcome as indicated by the results presented in Table 3 and Fig. 2. The results of the multivariable analysis indicated that none of the variables were associated with the risk of hematoma occurrence.
3.3 Prevalence of bacterial colonization
Among the 182 patients (93.3%) who underwent swab culture inside the pocket, a total of 23 species of bacteria were isolated in 19 patients, representing an overall prevalence of 10.4%. Within this group, Gram-positive cocci (GPC) accounted for the majority of the identified species, comprising 16 species in total (69.6%). Staphylococcus species were the most frequently identified, present in 10 cases (43.5%). Furthermore, Enterococcus species and Gram-negative bacilli (GNB) were identified in 4 (17.4%) cases each. Interestingly, despite the presence of bacterial colonization in these 19 patients, no occurrences of device infection were documented during the follow-up period.
4 Discussion
The main findings of this study are as follows:
Firstly, capsulectomy during the generator replacement procedure did not reduce the occurrence of device infection. Secondly, a swab culture conducted inside the pocket revealed bacterial colonization in 10.4% of patients, but none of these cases progressed to device infection. Thirdly, the occurrence of hematoma was the only independent factor associated with device infection. The capsule surrounding the CIED is a fibrotic tissue with poor vascularity. According to a study describing the histology of CIED pockets, the average thickness of the pocket is 0.8 to 1.1 mm, and in over 80–90% of patients; chronic inflammation is observed in the capsule or subcapsular area. Additionally, exudative material and calcification can be observed, and neovascularization within the capsule is observed in only about one-third of cases [11]. Furthermore, bacterial colonization has also been reported within the capsule, which can contribute to device infection [14, 15]. Therefore, it might be thought that removing the capsule could be helpful in preventing device infection. However, there is some controversy based on several reported studies. The MAKE IT CLEAN study, a single-center randomized trial, included a total of 258 patients [19]. The intervention group underwent complete removal of the whole capsule surrounding the CIED, while the control group did not have the capsule removed. The results showed no significant difference in the rate of pocket infection between the two groups. However, a retrospective study by Goldenberg et al. involving 773 patients reported a significantly lower risk of CIED infection during CIED replacement when capsule debridement was performed [20]. A substudy of the WRAP-IT trial, a large study investigating the efficacy and safety of antibacterial envelopes, reported that complete capsulectomy was associated with an increased risk of device infection compared to partial or no capsulectomy [21]. Our study showed similar results to previous randomized trials and substudy of the WRAP-IT trial, with no significant difference in the occurrence of device infection between the two groups. The average follow-up duration of this study is 4.5 years, and to our knowledge, this study has the longest follow-up period among studies that investigated the impact of capsulectomy on device infection. According to a Danish device-cohort study with 97,750 patients, 47.9% of all device infections occurred 1 year after the index procedure, with a median time to infection of 1053 (interquartile range 643–1762) days [22]. Therefore, to adequately assess the presence of device infection, a sufficient follow-up period is essential. The strength of this study lies as the only long-term prospective study that observed outcomes following capsulectomy. In the MAKE IT CLEAN study, the intervention group exhibited a higher incidence of hematoma [19]. However, in our investigation, there was no significant difference in the incidence of hematoma between the two groups. Moreover, among the patients with hematoma, only one patient required pocket revision. We believe that the difference in results could be attributed to the use of different surgical approaches. While the MAKE IT CLEAN study involved the complete resection of the entire capsule, our study resected only the posterior wall, relatively easier to control bleeding. In this study, bacterial colonization was observed in 10.4% of swab cultures inside of the CIED pocket. Previous studies have reported varying diagnostic rates of bacterial colonization depending on the culture method. Swab culture has shown a diagnostic rate of colonization ranging from 10 to 45% [15, 23,24,25], while tissue culture has shown a colonization rate ranging from 37 to 56% [12,13,14]. In the aforementioned studies, the incidence of device infection caused by the same bacterial species in patients diagnosed with colonization ranged from 0 to 7.5%. In this study, none of the 19 patients with bacterial colonization experienced an infection during the follow-up period, suggesting no association between bacterial colonization and device infection. Our study has several limitations. Firstly, it is a single-center study with a small sample size, which may limit the generalizability of the findings. But, as previously mentioned, this study is a long-term prospective study that observed the occurrence of device infection over a sufficient duration. Secondly, there may be variations in procedure details among different physicians. Thirdly, the discontinuation of antithrombotic agents and the duration of discontinuation prior to the procedure were not controlled, which could have a potential influence. Fourthly, there was a significant difference in device type between the two groups, which may have a potential impact on the study’s results. However, since there was only one case of device infection following ICD/CRT-D device implantation, it is unlikely that this difference significantly influenced the study’s primary results. Lastly, due to the low outcome rate, there were limited variables that could be included in the Cox regression model of this study, potentially leading to overfitting of the regression model.
5 Conclusion
Capsulectomy during CIED generator replacement did not reduce the risk of device infection, and hematoma occurrence was the only independent factor associated with device infection. There was no association between bacterial colonization in the capsule around the generator and CIED infection.
Data Availability
The datasets generated and analyzed during this study are not publicly available. Data are available from the corresponding author upon reasonable request.
References
Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60(16):1540–5. https://doi.org/10.1016/j.jacc.2012.07.017.
Bradshaw PJ, Stobie P, Knuiman MW, Briffa TG, Hobbs MS. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart. 2014;1(1):e000177. https://doi.org/10.1136/openhrt-2014-000177.
Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran P, Rinne C, Coutu B, Low RA, Essebag V, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol. 2018;72(24):3098–109. https://doi.org/10.1016/j.jacc.2018.09.068.
Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, Gallastegui J, Pickett RA, Evonich R, Philippon F, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019;380(20):1895–905. https://doi.org/10.1056/NEJMoa1901111.
Ngiam JN, Liong TS, Sim MY, Chew NWS, Sia CH, Chan SP, Lim TW, Yeo TC, Tambyah PA, Loh PH, et al. Risk factors for mortality in cardiac implantable electronic device (CIED) infections: a systematic review and meta-analysis. J Clin Med. 2022;11(11):3063. https://doi.org/10.3390/jcm11113063.
Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR, Love JC, Augostini R, Faerestrand S, Wiggins SS, et al. Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT trial. Circ Arrhythm Electrophysiol. 2020;13(5):e008280. https://doi.org/10.1161/CIRCEP.119.008280.
Dai M, Cai C, Vaibhav V, Sohail MR, Hayes DL, Hodge DO, Tian Y, Asirvatham R, Cochuyt JJ, Huang C, et al. Trends of cardiovascular implantable electronic device infection in 3 decades: a population-based study. JACC Clin Electrophysiol. 2019;5(9):1071–80. https://doi.org/10.1016/j.jacep.2019.06.016.
Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001–6. https://doi.org/10.1016/j.jacc.2011.04.033.
Blomstrom-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, Poole J, Boriani G, Costa R, Deharo JC, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;41(21):2012–32. https://doi.org/10.1093/eurheartj/ehaa010.
Palmeri NO, Kramer DB, Karchmer AW, Zimetbaum PJ. A review of cardiac implantable electronic device infections for the practicing electrophysiologist. JACC Clin Electrophysiol. 2021;7(6):811–24. https://doi.org/10.1016/j.jacep.2021.03.021.
Massaro G, Leone O, Valzania C, Angeletti A, Corti B, Martignani C, Diemberger I, Baldovini C, Ziacchi M, Biffi M. Pocket histology at cardiac implantable electronic device replacement: what’s new? Heart Rhythm. 2023;20(2):198–206. https://doi.org/10.1016/j.hrthm.2022.10.017.
Dy Chua J, Abdul-Karim A, Mawhorter S, Procop GW, Tchou P, Niebauer M, Saliba W, Schweikert R, Wilkoff BL. The role of swab and tissue culture in the diagnosis of implantable cardiac device infection. Pacing Clin Electrophysiol. 2005;28(12):1276–81. https://doi.org/10.1111/j.1540-8159.2005.00268.x.
Pichlmaier M, Marwitz V, Kuhn C, Niehaus M, Klein G, Bara C, Haverich A, Abraham WR. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace. 2008;10(9):1067–72. https://doi.org/10.1093/europace/eun191.
Chu XM, Yu H, Sun XX, An Y, Li B, Li XB. Identification of bacteriology and risk factor analysis of asymptomatic bacterial colonization in pacemaker replacement patients. PLoS ONE. 2015;10(3):e0119232. https://doi.org/10.1371/journal.pone.0119232.
Kleemann T, Becker T, Strauss M, Dyck N, Weisse U, Saggau W, Burkhardt U, Seidl K. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace. 2010;12(1):58–63. https://doi.org/10.1093/europace/eup334.
Smith SC, Bernacki KD, Haft JW, McHugh JB. Internal cardiac defibrillator implant-associated angiosarcoma presenting as suspected implant pouch infection. Cardiovasc Pathol. 2013;22(1):105–8. https://doi.org/10.1016/j.carpath.2012.03.007.
Kang D, Do K, Nattiv J, Zareh M, Doshi R. Malignant transformation of a chronically infected implantable cardioverter-defibrillator pocket. HeartRhythm Case Rep. 2018;4(7):307–9. https://doi.org/10.1016/j.hrcr.2018.03.012.
Keyser A, Schopka S, Stadlbauer A, Zerdzitzki M, Jungbauer C, Schmid C. B-cell lymphoma at the site of pacemaker generator. HeartRhythm Case Rep. 2020;6(8):528–30. https://doi.org/10.1016/j.hrcr.2020.05.013.
Lakkireddy D, Pillarisetti J, Atkins D, Biria M, Reddy M, Murray C, Bommana S, Shanberg D, Adabala N, Pimentel R, et al. Impact of pocket revision on the rate of infection and other complications in patients requiring pocket manipulation for generator replacement and/or lead replacement or revision (MAKE IT CLEAN): a prospective randomized study. Heart Rhythm. 2015;12(5):950–6. https://doi.org/10.1016/j.hrthm.2015.01.035.
Goldenberg GR, Barsheshet A, Bishara J, Kadmon E, Omelchencko A, Strasberg B, Golovchiner G. Effect of fibrotic capsule debridement during generator replacement on cardiac implantable electronic device infection risk. J Interv Card Electrophysiol. 2020;58(1):113–8. https://doi.org/10.1007/s10840-019-00581-4.
Tarakji KG, Krahn AD, Poole JE, Mittal S, Kennergren C, Biffi M, Korantzopoulos P, Dallaglio PD, Lexcen DR, Lande JD, et al. Risk factors for CIED infection after secondary procedures: insights from the WRAP-IT trial. JACC Clin Electrophysiol. 2022;8(1):101–11. https://doi.org/10.1016/j.jacep.2021.08.009.
Olsen T, Jorgensen OD, Nielsen JC, Thogersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J. 2019;40(23):1862–9. https://doi.org/10.1093/eurheartj/ehz316.
Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, Erne P, Trampuz A. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation. 2010;121(15):1691–7. https://doi.org/10.1161/CIRCULATIONAHA.109.906461.
Okada M, Kashiwase K, Hirata A, Nemoto T, Matsuo K, Murakami A, Ueda Y. Bacterial contamination during pacemaker implantation is common and does not always result in infection. Circ J. 2015;79(8):1712–8. https://doi.org/10.1253/circj.CJ-15-0133.
El-Ashry AH, Hussein MSA, Saad K, El Elhoufey A. Clinical utility of sonication for diagnosing infection and colonization of cardiovascular implantable electronic devices. Med Microbiol Immunol. 2021;210(5–6):245–50. https://doi.org/10.1007/s00430-021-00717-2.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, Catholic University of Korea.
Informed consent
All patients provided their written informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Kim, S., Park, S. et al. Clinical impact of capsulectomy during cardiac implantable electronic device generator replacement: a prospective randomized trial. J Interv Card Electrophysiol 67, 1211–1217 (2024). https://doi.org/10.1007/s10840-024-01765-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-024-01765-3