Abstract
Although the recently reported results of the PADIT study [1] indisputably bring a positive message by showing that the infection rate within the first year after cardiac implantable electronic device (CIED) implantation in advanced care systems was “only” 1% in high-risk patients, infection associated with the use of CIED (CIEDI) remains a serious complication leading to significant morbidity and mortality. These infections can be the result of initial pocket infection, usually due to surgical site contamination (more frequently) or secondary to hematogenous seeding of the leads or pocket during an episode of bacteremia due to remote septic foci or associated with either intravascular catheters or invasive procedures. As previously discussed in Chaps. 3 and 4, the principal agents involved in the development of CIEDI are gram-positive Staphylococci, and the main factors promoting the infective process can be classified into (a) patient-related, (b) device-related, (c) procedure-related, and (d) related to operators’ experience. In this chapter, we will focus on the various aspects of periprocedural modifiable risk factors: anticoagulation, antisepsis, antibiotic prophylaxis, and wound care. We will discuss both available evidence supporting standard approaches and recently introduced devices to improve CIED procedures. On the contrary, prevention of CIEDI through adoption of new CIED technologies, patient-tailored choice of the device, implanting procedure, and long-term follow-up are discussed in Chaps. 10 and 12.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- CIED
- Infection
- Prevention
- Prophylaxis
- Pacemaker
- Defibrillator
- Surgical site infection
- Endocarditis
- Patient preparation
11.1 Introduction
Although the recently reported results of the PADIT study [1] indisputably bring a positive message by showing that the infection rate within the first year after cardiac implantable electronic device (CIED) implantation in advanced care systems was “only” 1% in high-risk patients, infection associated with the use of CIED (CIEDI) remains a serious complication leading to significant morbidity and mortality. These infections can be the result of initial pocket infection, usually due to surgical site contamination (more frequently) or secondary to hematogenous seeding of the leads or pocket during an episode of bacteremia due to remote septic foci or associated with either intravascular catheters or invasive procedures. As previously discussed in Chaps. 3 and 4, the principal agents involved in the development of CIEDI are gram-positive Staphylococci, and the main factors promoting the infective process can be classified into (a) patient-related, (b) device-related, (c) procedure-related, and (d) related to operators’ experience. In this chapter, we will focus on the various aspects of periprocedural modifiable risk factors: anticoagulation, antisepsis, antibiotic prophylaxis, and wound care. We will discuss both available evidence supporting standard approaches and recently introduced devices to improve CIED procedures. On the contrary, prevention of CIEDI through adoption of new CIED technologies, patient-tailored choice of the device, implanting procedure, and long-term follow-up are discussed in Chaps. 10 and 12.
11.1.1 Comorbidities
Greenspon et al. clearly showed an imbalance between increasing incidence of CIEDI and the trend in new CIED implants, underlying that the rising in the burden of comorbidities could serve as the more plausible explanation [2]. Notably, many of them not only predict the risk of CIEDI but also the long-term survival after successful lead extraction [3, 4] (Fig. 11.1). While we cannot avoid many of these factors (beyond excluding patients from the implant when risks clearly outweigh the benefits), we should carefully focus on those we can manage (Table 11.1). Several reports evidenced that presence of fever <24 h before CIED procedures is associated with an increased risk of CIEDI (OR 4.27; 95%CI 1.13–16.12) [5]. For this reason, the procedure should be postponed (whenever possible) in patients with fever (until >24 h apyrexia). In case of ongoing infections without fever, the best approach is less defined, and the role of systemic markers of infection, e.g., CRP or white cell count, has not been studied. However a similar conservative approach is rational, at least until resolution of systemic involvement [6]. Two additional risk factors deserving additional investigation are glycemic control and prevention of contrast-induced nephropathy. Diabetes mellitus has been identified as a predictor of CIEDI (OR 2.08, 95%CI 1.62–2.67; see Chap. 3), while it cannot be avoided, it has been reported that glucose levels >11.1 mmol/L in the immediate postoperative period are associated with increasing surgical site infection (SSI), and a strict glycemic control in the perioperative period significantly reduced major infectious morbidity and its associated socioeconomic costs [7]. These data suggest to extend the approach to a closer glycemic control also in the CIED setting, according to what is suggested in different surgical settings [6, 8, 9]. Renal failure is not only a leading risk factor for development of CIEDI but also for long-term survival after CIEDI treatment [3, 10]. Considering that up to 15% of the patients are undergoing complex CIED procedures [11], the adoption of measures should be carefully considered to avoid acute kidney injuries by properly managing periprocedural drugs and by adopting all measures for the prevention of contrast-induced nephropathy especially for candidates to cardiac resynchronization therapy. The last point to be discussed is the use of a temporary pacemaker which was reported to be associated with a more than doubled risk of CIEDI (see Chap. 3). Despite being recognized by several authors that it should be limited to very selected patients [12, 13] with severe symptomatic bradycardia (usually third-degree atrioventricular block with low escape rhythm or patients), it is not covered by many guidelines on about use of CIEDI or management/prevention of endocarditis [14,15,16,17]. Close monitoring of the patient coupled with timely implantation of permanent CIED and use of isoprenaline or adrenaline should always be considered [12, 13].
Long-term survival from death for any cause after complete system extraction according to the Shariff score at last CIED procedure. The Kaplan-Meier curves derive from a multicenter study on 169 patients after effective lead extraction for CIEDI (Reproduced with permission from Diemberger et al.) [3]. Patients were considered at high vs. low risk according to having <3 vs. ≥3 points at the 10-points Shariff score [4] as reported in the table on the right. CIED cardiac implantable electrical device, CIEDI CIED-related infection, PM pacemaker
11.1.2 Management of Anticoagulation and Antiplatelet Drugs
Pocket bleeding after CIED is a relevant complication since it causes patients discomfort and pain while prolonging/requiring hospital admission in many cases, and also it can lead to pocket revision (Fig. 11.2), thereby increasing the costs of CIED therapy [18]. More relevant, pocket hematoma has been associated with an increased risk of CIEDI of 8.46 (95%CI 4.01–17.86; see Chap. 3 for additional information). The principal risk factor for pocket hematoma is anticoagulation therapy (and dual antiplatelet, as more recently reported) [19], which has also been recognized as an independent risk factor for CIEDI. However, the association between CIEDI and pocket hematoma is not consistently reported among the studies [20], and various explanations could be advocated for this: study design, inhomogeneous definition/reporting of pocket hematoma [21], additional comorbidities, study settings (type of CIED and procedure involved), but more importantly the management of pocket hematoma [22].
Different patterns of CIED pocket hematoma. The clinical relevance of the patterns progressively increases from a to f. In particular pattern a, b can be management with ambulatory surveillance. Pattern c, d deserves interruption of anticoagulation. Moreover, hospital admission should be seriously considered to avoid progressive dehiscence of the suture line (e) leading, if not urgently revised in the EP lab, to complete opening of the wound (f). At this point, it has to be considered complete CIED extraction
According to a comprehensive meta-analysis, the prevalence of pocket hematoma in current literature can be estimated around 4.6% ranging between 2.2% in untreated patients and 14.6% in patients undergoing heparin bridging [19] in accordance with the type of anticoagulant/antiplatelet therapy (Fig. 11.3). Notably, dual antiplatelet therapy provided a bleeding risk significantly higher than any oral anticoagulation approach without bridging. It is interesting to note that these figures are significantly higher than those reported by the meta-analysis of acute complications after ICD implant in randomized studies and registries (being, respectively, 1.2% and 0.86%) [23] and in a very large retrospective claim-based analysis [24] showing a range between 0.58 and 2.81%. These figures clearly underline the importance of providing a clear definition of pocket hematoma. According to De Sensi et al. [21] inside the literature, the definition of hematoma was recorded as an outcome ranged from any ecchymosis occurring in the surrounding area of the CIED pocket to any palpable mass requiring a dedicated intervention (reoperation, hospitalization, interruption of anticoagulation, blood transfusion) [25,26,27]. The authors recognized the importance of recording any phenomenon regarding the pocket, for the potential relationship with subsequent CIEDI, but stratifying it in a standardized manner, later modified by the Bristol Heart Institute scale [28] (Fig. 11.4).
Association between different anticoagulant/antiplatelet regimens and risk of bleeding complications both in terms of incidence (a) and odds ratio (b). AC anticoagulant, DAPT double antiplatelet therapy, HBS heparin bridging, SAPT single antiplatelet therapy (Figures adapted from Bernard et al. [19] with permission)
The Bristol Heart Institute scale to grade severity of pocket hematoma [28]
The evidence of the heavy impact of hematoma on patients’ outcome led to organization of many randomized studies aimed at verifying the impact of different strategies for managing oral anticoagulation (Table 11.2) [25, 26, 29,30,31,32]. In summary they confirmed data from previous observational studies [33] evidencing that interruption with oral anticoagulation and heparin bridging are associated with an increased risk of bleeding (mainly pocket hematoma), while perioperative continuation of warfarin reduces the occurrence of clinically significant CIED pocket hematoma and the duration of hospital stay, without any increase in thromboembolic events. Another finding that should be underlined is the very high ratio between bleeding and thromboembolic events explaining the results. The only limitation is the absence of a randomized study comparing warfarin interruption without bridging and uninterrupted warfarin. However, after the introduction of novel oral anticoagulants, the attention is shifting to these agents that are becoming the standard for anticoagulation for most of CIED patients. Beyond the reports on observational data [34, 35] and sub-analysis of authorization trials [36], the recently published BRUISE CONTROL-2 [30] trial evidenced that when considering direct oral anticoagulants, a strategy based on continuation (maximum interval between doses 12 h) and the choice of a brief interruption (median 72 h) are both associated with very low complications (Table 11.2).
The final consideration regards antiplatelet therapy. Single antiplatelet therapy is associated with an increased incidence of pocket hematoma [19], with inconsistent reports on higher effect provided by clopidogrel when interrupted for <5 days like to what occurs for other surgeries [37]. However, there is a considerable amount of data supporting a relevant increase in occurrence of pocket hematoma when CIED procedure is performed under dual antiplatelet therapy, estimated as a threefold increase [19, 37, 38].
According to the results of all these studies, it cannot be suggested a generalized approach to manage anticoagulation/antiplatelet therapy in patients candidates to CIED procedures. In general the use of heparin should be strongly discouraged, while the choice of interrupting or continuing oral anticoagulation should be balanced on patient thromboembolic risk, complexity of planned procedure, and risk of deferring the procedure. In particular, an uninterrupted approach should be considered in patients with a CHA2DS2-VASc score ≥ 4, previous stroke, recent ablation/cardioversion of atrial fibrillation, old mechanic valve prosthesis, and/or urgent procedure [39]. Conversely, other patients should be managed with appropriate interruption of oral anticoagulant. A recent report evidenced that a similar integrated approach has the potential to significantly decrease the incidence of pocket hematoma (from 6.5 to 1.6%) without paying in terms of an increase in ischemic events [40]. Notably, the authors adopted a lower INR value in the patients with uninterrupted anticoagulant therapy, a decision supported by the real values recorded in the BRUISE CONTROL trial [25]. Figure 11.5 provides a possible approach to manage anticoagulant/antiplatelet therapy considering the previously discussed literature.
Proposed management of antiplatelet for CIED procedures according to current literature [39, 46, 121]. ∗HIS high ischemic risk, see table in the left corner [121]; ∗∗ high thromboembolic risk, see table in the right corner [39]; ∗∗∗ preferably discussion between operator and interventional cardiologist. Note that submuscular CIED procedures and lead extraction are not covered by this scheme. DAPT dual antiplatelet therapy, DOAC direct oral anticoagulants, HIS high ischemic risk, MI myocardial infarction, SAPT single antiplatelet therapy, TE thromboembolic, VKA vit. K antagonists
The last consideration on the prevention of bleeding complications to reduce CIEDI is on operative technique. Each characteristic of CIED procedure can affect the bleeding risk, well beyond the underlying anticoagulant/antiplatelet treatment: (a) type of procedure; (b) vascular access (cephalic vs. subclavian); (c) creation of CIED pocket (site, tools, approach); and (d) preventive measures. While the points from (a) to (c) will be covered later (see the subsequent sections of this chapter and Chaps. 10 and 12), the last point needs a specific discussion. Several approaches have been suggested to reduce the incidence of pocket hematoma independently from the management of anticoagulation/antiplatelet therapy. Beton et al. [41] reported the results of a single-center case-control retrospective study on 135 patients under warfarin (>50%) or dual antiplatelet therapy (>25%) comparing use of topical tranexamic acid and showing an impressive reduction in hematomas (overall and clinically relevant) and reoperations. As recognized by the authors, this report can be only hypothesis generating, but in view of the low impact (in procedural time and costs), it should be considered for additional exploration. Another approach, routinely adopted in ophthalmologic and stomatologic procedures, is the topical infusion of epinephrine to promote vasoconstriction. Ilov et al. [42] reported the results of a randomized study on 133 patients to receive either epinephrine or saline solution, which were added to a local anesthetic administered during pacemaker implantation. Notably, only a half of the patients were under anticoagulant/antiplatelet therapy before CIED procedure. The study showed that use of local epinephrine was associated with an increased incidence of pocket hematoma (9% vs. 2%; OR = 5.95; CI: 2.1–7.3, p = 0.003). The provided explanation was that temporary vasoconstriction induced by epinephrine may lead to a false impression of adequate hemostasis with later bleeding at the end of the effect. A different approach adopted in three other reports is to put procoagulant agents inside CIED pocket. Ohlow et al. published a negative case-control study [43] on the use of D-Stat Flowable Hemostat™ (Vascular Solutions, Inc., USA). This device containins a mixture of thrombin and collagen approved by FDA indicated for use in the local management and control of bleeding in percutaneous and surgical procedures for patients at increased bleeding risk. Among the 163 enrolled patients, 50% were under anticoagulation, and 38% received dual antiplatelet therapy; the study arm presented more hematomas (14.6% vs. 3.7%; p = 0.03) but more importantly a trend for higher pocket infections (6.1% vs. 1.2%; p = 0.21) not associated with reoperations. In a second study, Tscholl et al. [44] evaluated the use of PerClot™ (CryoLife, Inc. Kennesaw, GA, USA) a CE-marked system to deliver a mixture of absorbable polysaccharide particles derived from purified plant starch with the properties to cause local dehydration accelerating clotting cascade through concentration of platelets, red blood cells, and procoagulant proteins. However, the study was stopped early, after enrollment of one third of the patients (n = 51) due to significant incidence of fever and raised inflammatory markers in the PerClot™ group even without clinical signs of infection or later device explantation. Finally, another option under evaluation [45] is the also the use of oxidized regenerated cellulose, a plant-based topical hemostatic agent, which couples procoagulant action with (in vitro) bactericidal properties (Surgicel® Fibrillar™ Hemostat; Ethicon Inc., USA). However, the only available report provides just feasibility data in a limited population.
11.1.3 Skin Preparation
Several measures are routinely undertaken by many operators in current practice with the aim to reduce bacterial skin colonization (Table 11.3). However, we have limited and contrasting data supporting them. Removal of adhesive left by monitoring electrodes is rational, but it should be carried out gently, with the use of alcoholic solutions avoiding excessive rubbing to prevent skin erythema [6]. Preoperative shaving derives from the common belief that hair removal could reduce the incidence of wound infection, and in many institutions, it is performed the night before. However, microscopic injuries secondary to this procedure can theoretically increase the risk of infection. There is evidence that the use of razors is associated with an increase in SSI, leading to the practical suggestion to use clippers (with a single-use head) on the day of the procedure [8, 9, 46]. Another possibility could be use of depilatory cream the day before the operation, but it has no supportive evidence [6]. Chlorhexidine shower proved to diminish skin bacterial count (particularly, Staphylococcus spp.), but no robust data confirm a reduction in postoperative infections [8, 9]. More recently, the guidelines for the prevention of SSI issued by the US Centers for Disease Control and Prevention in 2017 [47] provide a strong recommendation to advise patients to shower/bathe (full body) with soap at least the night before the operative day. However, this recommendation is supported as an “accepted practice,” since there is uncertainty regarding the optimal timing, the total number of soap/antiseptic agent applications, and the type of agent (as clearly reported by the supplementary material). Notably, the same guidelines did not mention the practice of removing hair before surgery. More data support the disinfection of the surgical site to reduce bacterial colonization (without irritating the skin). The results of a recent study on 1326 patients showed no difference between aqueous and alcoholic povidone-iodine solutions regarding CIEDI prevention [48]; in general it is suggested to adopt alcohol-based (for higher skin penetration) antiseptic agent for intraoperative skin preparation (unless contraindicated) [46, 47]. Notably, single-use units should be preferred to avoid contamination during repeated opening of large bottles, while the skin preparation should be left on for a minimum contact time of 30 s and should not be allowed to pool (to avoid the fire risk from diathermy) [46, 49]. Iodine and chlorhexidine both in 70% alcohol are the two most effective skin antiseptics [6, 50]. The comparison between these agents presents conflicting results in available literature. In the EHRA survey, the centers are split with 57.8% using povidone-iodine solution [51]. Povidone-iodine was also the preferred antiseptic agent in the participating centers at higher infection rates in the large REPLACE registry suggesting a higher protective action provided by chlorhexidine [52]. This was supported by a randomized multicenter trial in candidates to different types of surgery (CIED procedures were not included) showing a significant reduction in SSI with chlorhexidine (9.5% vs. 16.1%, p = 0.004) [53]. However, in a very recent retrospective cohort including 2792 patients undergoing 2840 CIED procedures, no difference in infection rates was found between povidone-iodine and chlorhexidine groups [54]. This inhomogeneity is also present in current guidelines [46, 47] showing a preference for chlorhexidine only for the Joint British guidelines on CIEDI management and prevention [46] mainly based on the extension of the EPIC3 guidelines recommendation for central line [55]. On the contrary, the US guidelines on SSI prevention prefer a more conservative approach, not reporting a preference for any of the two agents [47] but acknowledging the divergent data. Remarkably, a recent report showed an interesting strategy based on a staged bundled antiseptic skin preparation including (a) application of 75% alcohol over the anterior chest and covering with sterile gauzes after taking a shower on the night before the procedure, (b) povidone-iodine at the incision site 10 min before operation, and (c) standard antiseptic preparation [56]. 270 patients prepared according to this protocol were compared with 395 patients who received the standard skin antiseptic preparation in the same institution in the 2 years before. The authors reported a drastic decrease in CIEDI (0.7 vs. 4.3%, P = 0.007) confirmed by multivariate analysis. Albeit interesting the limitations in study design do not permit to solve the concerns on the best approach for antisepsis before CIED procedures. A final remark involves the adoption of warmed antiseptic solutions to improve patient comfort. This approach was found to be at least non-inferior to use of a standard antiseptic regimen in a recent randomized comparison [57]. Finally, also the use of adhesive sheets to preserve sterility of the surgical site has no definite proof of reducing SSI. Anyway if adopted, it is recommended to choose an iodophor-impregnated adhesive incision drape [6, 46, 47]. When used, it should be carefully considered that partial or complete removal before suturing can contaminate the wound [58], and different antiseptics can influence the adherence of the incisional drape [59].
11.2 Procedural Aspects
Table 11.4 reports suggested behavior according to best clinical practice in surgery interventions, to minimize the risk of infections [6, 46]. Beyond these recommendations, mainly based on consensus and established clinical practice, there are some data supported by scientific evidence. Kozon et al. recently reported an interesting study [60] on 60 candidates to CIED procedure. Both first operator and assistant imprinted their outer gloves on agar plates before manipulating the device while a wound swab was performed. Samples were cultured, and the presence of bacteria. Contamination occurred in 80% of replacements and 67% of primary implantations. Coagulase-negative Staphylococcus occurred in 52%, and Propionibacterium spp. occurred in 84% of positive cases. According to these data, the authors suggest changing outer gloves before handling the device. However, we deserve additional confirmatory data to suggest a similar, albeit rational, approach since contamination of the other area involved in the procedure could dramatically reduce the impact of a similar preventive measure. Notably, the additional evidence that contamination of the operators’ glove significantly increased every 15 min of procedure time stress the need to simplify procedures as much as possible.
Another relevant issue relates the surgical technique and tools adopted, especially during upgrade and replacement procedures. Nichols et al. provided an interesting analysis on a US claim database on >40,000 patients undergoing a CIED replacement procedure. Incidence of lead damage was 0.46% for PM, 1.27% for ICD, and 1.94% for CRT (p < 0.001). After adjustment patients with ICD and CRT-D presented a risk of lead damage that was, respectively, double and > 2.5 times that of patients with PM [61]. Lead damage occurred at a median of 107 days following the CIED replacement procedure, while age had a protective effect with a halved incidence for patients >65 years old. These issues were associated with an average cost of $25,797. Previous studies showed an incidence of lead failure ranging between 0.6 to 1.2% for PM replacement and 2.2 to 5.1% for ICD replacements [62,63,64]. These figures are not negligible since repairmen procedures are at increased risk of CIED infections [65]. At this regard, the technique and the tools adopted for the procedure can be as important as the operators’ experience. Lim et al. analyzed effects of standard cautery blade transvenous lead insulation materials considering different outputs, pulse duration, orientation of the blade, and composition of the outermost insulations of the lead [66]. They evidenced a significant insulation damage, especially in polyurethane leads or when the blade was used with a perpendicular direction with outputs >20 W. To overcome these issues, two devices have been recently studied: PlasmaBlade™ (Medtronic, Minneapolis, MN, USA) and PhotonBlade™ (Invuity, San Francisco, CA, USA); until now no report regarding other devices (e.g., laser or ultrasonic scalpels) has been reported in CIED procedures. In a retrospective study comparing 508 patients undergoing CIED replacement with standard approach (including use of scalpel, scissors, and electrocautery) with 254 patients in which the operators used the PlasmaBlade™ device, Kypta et al. [67] showed a dramatic reduction of lead damages occurred (5.3% vs. 0.4%; p < 0.001), and the procedure time was significantly longer with standard approach (47.9 ± 24.9 and 34.1 ± 18.1 min; p < 0.001). These results turned into an average return of €81 for each patient. However, the retrospective design coupled with the very high incidence of lead failure in the control group limits transferability in other settings. On the contrary, Wasserlauf et al. proposed a direct comparison of PlasmaBlade™ and PhotonBlade™ on an animal model (each lead positioned into grooves 1–2 cm deep made in a chicken breast. Later it was positioned on a grounding pad for monopolar cautery) [68]. Applied force and duration of contact were also controlled. The authors tested different operative settings (COAG vs. CUT; 20 W, 35 W, 40 W; blade orientation) and lead external insulation. Lead damage was scored on an ordinal scale of 0–4. They found a lower incidence of lead damages with PhotonBlade™ (75% vs. 40% at higher power; 39% vs. 13% at CUT 20 W settings). Moreover, they underlined the compatibility of the PhotonBlade™ with any standard electrosurgical generator. Despite the limitations of the design of these findings, requiring verification in clinical studies, they underline the importance of tailoring the different settings of these new cutting devices when used for CIED procedures. Another interesting suggestion is provided by an observational retrospective report on the single-center adoption of the PlasmaBlade™ for standard CIED procedures [69]. Their aim was to evidence possible benefits of this device in reducing pocket complications in view of good data in other settings (e.g., ear, nose, and throat procedures and) where it showed good precision with lower local damages (thermal injury, inflammatory response, and scar formation) in comparison to conventional electrocautery. Despite these premises, they found an overall perioperative complication rate of 3.9%, mainly driven by pocket hematoma (3.2%) without any lead failure (among 282 patients) within 6 months. The authors suggest as the most plausible explanation a sub-optimal management of anticoagulation that was not in line with the results of the BRUISE CONTROL study [25]. However, the retrospective design of the study does not permit to rule out the real mechanism.
In different settings, Servello et al. [70] proposed the use of PlasmaBlade™ to perform complete “capsulectomy” during elective replacement of generators used for deep brain stimulation. The reason to perform elective “capsulectomy” rises from the evidence of a higher prevalence of device infections after replacement procedures (vs. first implant), similar to what occurs in CIED settings [65, 71]. Among the possible explanations, it has been suggested the theory of a lower penetration of antibiotics used for prophylaxis due to a “barrier-effect” provided by fibrotic tissue surrounding the generator. After a CIED procedure, the pocket tissue undergoes all the process of wound repair: (a) inflammation, (b) reepithelialization, (c) keratinocyte proliferation, (d) matrix metalloproteinase deposition, (e) angiogenesis, and (f) contraction and closure [72]. Coupled with this process, there is the physiologic response to a foreign body leading to formation of this fibrotic avascular capsule that Kleemann et al. [73] showed to be associated with a high prevalence of bacterial colonization (about one third in this report). Moreover the authors evidenced that after a median follow-up time of 203 days after CIED revision, CIEDI occurred in 7.5% of patients with culture-positive vs. 2.4% in culture-negative patients not reaching significance in view of the small size of the involved population (122 patients). However, they underline that culture-positive patients later developing overt device infection presented the same type of agent. Albeit highly intriguing this study presents several limitations: samples were taken after CIED removal (increasing the risk of contamination both of samples and pocket), and two among three CIEDI in culture-positive patients were lead endocarditis, while all underwent only CIED replacement (without lead revision). This concept was challenged in the MAKE IT CLEAN trial [74] where 258 patients were randomized to pocket revision (i.e., complete capsule excision including floor, roof, and surrounding the leads) and a more conservative/standard approach. Patients in the first group experienced significantly more hematoma (6.1% vs. 0.8%, P = 0.03) (despite not being bridged with heparin) but without any difference in terms of CIEDI (1.5% vs. 4.7%; p = 0.13). Notably, despite being a “negative” trial, the presence of conflicting results between hematoma (which is a recognized risk factor for CIEDI) and CIEDI can be interpreted as a partial confirmation of the role of CIED capsule in promoting later development of infection. However, a similar approach cannot be suggested in current practice. Interestingly there are data suggesting both a relationship between disposition of the leads inside CIED pocket and amount of fibrotic tissue on one side [75] and presence of fibrous tissue in CIED pocket and adhesions during lead extraction [76]. These elements will require additional studies in the near future to identify modifiable factors or predictors of later development of fibrosis.
Another field of research is the approach of wound closure. Standard approach to closure of CIED pocket is performed by multiple layers of sutures, with a tendency to favor intradermal suturing for the superior layer. However, several devices have been developed to improve wound closure: (a) tissue adhesive (2-octylcyanoacrylate) [77]; (b) barbed sutures [78]; and a (c) new adhesive device, the Zip™ Surgical Skin Closure (ZipLine Medical, Inc., Campbell, CA, USA). This device, approved for low-tension noninfected surgical wounds, is a sterile single-use system with two self-adhesive hydrocolloid pressure-sensitive strips linked with individually adjustable self-locking fasteners [79, 80]. All of them have been previously studied in different surgical settings, usually without involvement of implantable devices, with good results in terms of reduction of closure time, esthetic results, and wound healing. However, in the specific CIED settings, the only available benefits reported with respect to standard suture are a reduction of closure time for the Zip™ Surgical Skin Closure [80] (Fig. 11.6) showing a reduction of 5 min in closure time (14.9 ± 6.8 vs. 20.1 ± 11.09 min, p = 0.0003). The same authors claimed for a reduction in overall procedure time coupled with a tendency for less CIEDI. However, the non-randomized design coupled with a greater prevalence of ICD in the control arm which was also followed for a longer time represents a significant limitation. It has to be recognized that standard sutures/staples hold incisions together at single points (in which the material passes through the incision) creating an area of increased wound tension where the lesion created by the suture material can promote spreading of bacteria. For this reason, a monofilament continuous absorbable intradermal suture probably provided the best healing process among the “standard approaches” which could be exceeded, albeit theoretically, by the “noninvasive” Zip™ Surgical Skin Closure device. However, future studies are needed to confirm this hypothesis.
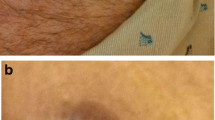
Example of the ZIP™ device for noninvasive wound closure. The two self-adhesive hydrocolloid pressure-sensitive strips are linked with individually adjustable self-locking fasteners (a) and should be positioned along the suture after completion of subcutaneous or other deep, tension-reducing sutures (b). The final result is good also in difficult sites like after implant of subcutaneous defibrillator (c) (Panel b, c are courtesy of Elia De Maria Cardiology Unit, Ramazzini Hospital, 41012 Carpi (Modena), Italy)
The use of elasticated pressure dressings has been studied with positive results in patients undergoing breast surgery/lymph node clearance [81]. In the CIED setting, it has been proposed a postsurgical elastic vest to prevent pocket hematoma, in view of the associated risk of CIEDI. The device, manufactured by L & M Innovations (Leawood, KS, USA), is a disposable synthetic expandable vest available in different sizes with two adjustable straps (shoulder and chest) to appropriately fit the body habitus of the patient and with a specially designed pocket containing a support wedge for additional pressure. This device was evaluated in a feasibility case-control study involving 40 anticoagulated patients, assuming also antiplatelet treatment in >85%, candidates to CIED procedures. Turagam et al. [82] evidenced a significant reduction of pocket hematoma, showing a significant reduction at 7 days of pocket hematoma in the vest group (0 vs. 30%, p = 0.02), despite a significantly higher INR in the vest group (2.7 ± 0.4 vs. 2.2 ± 0.3 = <0.001). These highly promising results deserve additional confirmations in larger randomized studies including various types of CIED procedures and anticoagulation/antiplatelet regimens.
11.3 Antibiotic Prophylaxis
11.3.1 Evidence Supporting Antibiotic Prophylaxis
Intravenous antibiotics targeted against Staphylococci, which are involved in more than 70% of CIED infections, should be used in all the candidates to CIED procedures [6]. This approach is supported by a large amount of data, but the first randomized experience supporting the use of antibiotic prophylaxis for CIED procedures dates to 1994 when Mounsey et al. [83] showed a significant reduction of CIEDI with the administration of flucloxacillin (clindamycin if patient was allergic): the rate was 0% vs. 4% in the control arm (p = 0.003). Notably, several interesting features characterize this study: the antibiotics were continued for 48 h, and reoperation was described as a risk factor for CIEDI as far as prolonged procedures or reduced operators volume. However, the most robust evidence derives from the landmark double trial by de Oliveira et al. [84] randomizing patients to receive 1 g i.v. cefazolin or placebo immediately before CIED procedure. The study was stopped early before enrolling the planned 1000 patients in view of the dramatic reduction in CIEDI (0.63% vs. 3.28%, p = 0.016). These findings have been later confirmed by other randomized studies and two meta-analyses showing that antibiotic prophylaxis grants a reduction of CIEDI in a range between one third and one eighth [5, 85,86,87,88]. In 2010, the American Heart Association published a scientific statement “Update on Cardiovascular Implantable Electronic Device Infections and Their Management” and recommended that a parenteral-administered antibiotic be given 1 h before the procedure [17]. However, several aspects were less investigated: (a) type of antibiotic; (b) timing of administration; (c) the need for postoperative antibiotics; and (d) the use of local pocket antibiotics (Table 11.5) [6, 13, 46, 72, 89].
11.3.2 Antibiotic Agents and Routes of Administration
Among the different agents, cefazolin is the first choice in view of the data in CIED procedures, cardiac surgery, and for the wide spectrum of activity on gram-positive agents. Notably, it has been shown to be not inferior to glycopeptides even in the case of high prevalence of methicillin resistance with a high tolerability and low costs [84, 90,91,92,93,94,95]. Obviously, the final choice critically depends on site-specific prevalence of bacteria species and antibiotic resistance. For example, in a study of over 50,000 isolates from 495 hospitals in 26 European countries, methicillin-resistant Staphylococcus aureus prevalence varied from 1% to over 40% [13]. In case of an institution with known high prevalence of antibiotic resistance or in case of coexistence of other risk factors (i.e., prolonged hospitalization, recurrent admissions, chronic bed rest, living in a retirement home, or recent antibiotic treatment), glycopeptides, in particular vancomycin and teicoplanin, are probably the best choice [6, 46, 96]. Teicoplanin use is simpler since it can be administered in a single bolus which lasts also for long procedures. However, teicoplanin resistance is more frequent, and dose-response is more affected by patient-specific characteristics [46, 97, 98]. Adding gentamicin to glycopeptides can be useful to increase the antibacterial spectrum, but the benefits are unproven, and it may be advisable to avoid gentamicin in patients with impaired renal function, particularly those where a deterioration in renal function may precipitate the need for long-term renal replacement therapy [46]. However, an increase in nephrotoxicity was not seen with a 2 mg/kg single-dose prophylaxis regimen in cardiac surgical patients [99].
In a meta-analysis, preoperative prophylaxis was found to be superior to postoperative antibiotics (RR = 0.14 (0.03–0.60); p = 0.008); however no trial formally addressed this question [86]. To achieve the appropriate concentration of antibiotic in the tissues during CIED procedures, timing of administration is crucial [17, 100, 101]. When cefazolin is adopted, it should be administered 30 min before the procedure, considering the half-life of 1.6 h and a peak concentration in 30–60 min, with a repeated dose in case of procedures taking >3 h. When considering the use of vancomycin, having a half-life of 6–12 h with a peak tissue concentration around 60 min, it should administered 1–2 h prior to surgery and requires a slower rate of infusion (1 g/h) to prevent systemic vasodilatation and erythema. As previously stated, teicoplanin can be administered in a single 5 min i.v. bolus eliminating the longer infusion of vancomycin.
The necessary duration of the treatment is also poorly established. Although prolonged courses of antibiotics may be theoretically useful in selected circumstances, available data does not support this behavior [86, 102]. In particular, Dwivedi et al. found no difference in the rate of CIEDI following 1 week of postoperative antibiotics compared to 2 days [87]. In the prospective REPLACE study which included a pre-specified infection analysis, a higher infection rate was seen in patients treated with postoperative antibiotics. However, in this registry, the use of any or no postoperative antibiotics was left to the individual investigator, thus limiting any specific conclusions [52]. More recently, Krahn et al. [103] published the results of the PADIT Trial. The study prospectively evaluated the practice of postoperative antibiotic administration to reduce CIED infection. This investigative strategy involved an investigative center-based cluster-crossover design to evaluate the role of incremental antibiotics before, during, and after the CIED procedure. Each implanting center was randomized to pre-incision cefazolin (or vancomycin in penicillin-allergic patients) alone or with intraoperative bacitracin 50,000 U in normal saline wound irrigation and a 2-day postoperative course of oral cephalexin or clindamycin in penicillin-allergic patients. Patients eligible for inclusion are those who present for generator replacement, revision or upgrade procedures, or cardiac resynchronization procedures [72]. 19,603 patients were enrolled among 28 centers, 12,842 were defined at high risk. Infection occurred in 99 patients (1.03%) under conventional treatment and in 78 (0.78%) receiving incremental treatment (OR 0.77; 95%CI: 0.56–1.05; p = 0.10). In high-risk patients, hospitalization for infection occurred in 77 patients (1.23%) receiving conventional antibiotics and in 66 (1.01%) receiving incremental antibiotics (OR: 0.82; 95%CI: 0.59–1.15; p = 0.26). Subgroup analysis did not identify any characteristics with significant benefit from incremental therapy.
The last point is the optimal route of antibiotic administration. Darouiche et al. examined two studies comparing systemic vs. intraoperative local antibiotics and found no significant difference (OR 0.45; 95%CI 0.10–2.03) [86]. However, it should be noted that they were both clearly underpowered also for being metanalyzed since they reported 7 CIEDI events among 177 patients overall. The meta-analysis also found no evidence that concomitant local antimicrobials offered any benefit and concluded that local instillation of antimicrobials did not reduce infection rates. Moreover, also pocket irrigation with povidone-iodine showed no additional benefit in reducing CIEDI in a small study [104]. Irrigation of the pocket with saline probably reduces the bacterial concentration, though there is no evidence to indicate that it reduces CIEDI.
11.3.3 The Antibacterial Envelope
The concept of using antibiotic coating to decrease SSI was tested more than 20 years ago, showing good results both for central venous catheters [105, 106] and ventricular drain catheters [107]. However, in the same years, it was launched a different approach to fight bacterial colonization, through introduction of a different coating. St. Jude Medical (SJM, St. Paul, MN, USA) addressed this problem by introducing a modified prosthesis, the “Silzone” valve, with the sewing ring made of a dense layer of a silver-based alloy bound to the surface of the polyester fibrils by ion beam-assisted vapor deposition [108, 109]. Notably, this was based on the known capacity of silver to behave as a broad-spectrum antimicrobial agent. However, after launching a randomized controlled trial to test the superiority in reducing early endocarditis, the product was withdrawn voluntarily on the basis of a higher incidence of paravalvular dehiscence (4.4% vs. 1.0%) probably driven by inhibition of fibroblastic reparative action [110]. At the end of the 1990s, also the antibacterial envelope, currently known as TYRX™ Absorbable Antibacterial Envelope, was born using the same antibiotics: minocycline and rifampin. The first name was AIGIS (from the Greek word meaning shield), and the polymer technology was invented in the laboratories of Prof. Joachim Kohn at Rutgers University. Later it was tested on four different bacterial strains in a rabbit model with good results [111]. In 2010 the Food and Drug Administration (FDA) approved the use of the AGISRX to reduce infection after CIED implant. Before closure of the pocket, the generator is inserted into an antibacterial polypropylene mesh sleeve that releases within approximately 7 days minocycline and rifampicin in the generator pocket (Fig. 11.7). Both minocycline and rifampin have broad-spectrum antibacterial coverage, and biofilm penetration and local concentrations of the drugs are very high (with negligible systemic concentrations). The first-generation envelope was nonabsorbable; the last one uses a fully bioabsorbable polymer that dissolves within 9 weeks, now called TYRX™ (Medtronic, Minneapolis, MN). Observational studies showed favorable outcomes in reducing the rate of CIED infections in high-risk patients (Table 11.6) except the one of Hassoun et al. [112] which was conducted in a rather small population and showed higher infection rates in patients who received the envelope. Reasons for this result could include the presence of severe comorbidities and a higher incidence of revision surgery in the TYRX™ group. The authors also suggest that the envelope may have acted as a nidus for infection. A meta-analysis was performed on controlled studies of the antibiotic envelope. Five studies were included, corresponding to 1798 patients implanted with an antibiotic envelope and 2692 without [113]. The envelope was associated with a 69% relative risk reduction in CIED infection (0.31 [0.17, 0.58] 95% CI, p = 0.0002). Propensity-matched data from three studies were analyzed to ensure accurate comparison. In the risk-matched cohort, infections were significantly lower in the envelope group (3 vs. 26, p < 0.0003).
In their study, Shariff et al. [4] have considered economic implications related to the use of TYRX™. Out of 1476 patients undergoing CIED procedures, 365 received the TYRX™ envelope. Nineteen patients in the no-TYRX™ group experienced CIED infection versus 0 in the TYRX™ group (p = 0.006). The mean duration of hospitalization stay related to infection was 13 ± 11 days. The average cost of treating CIED infections was calculated $54,926 ± $11,374 per patient, mostly attributable to inpatient care. Applying the infection rate observed in the no-TYRX™ group, it was estimated that 6.2 additional patients would have experienced infection if the device had not been used in the TYRX™ group. The estimated cost of treating those infections was similar to the cost of using TYRX™ in every patient. Patient subsets in which greater cost-efficiency was observed included those with high preoperative infection risk score and those who had undergone early reintervention. It was calculated that, even at an infection rate of 1.59% (instead of the observed 1.71%), the cost of infection care would be approximately balanced by the cost of using TYRX™ in every patient. In the study of Kay et al. [114], the cost-effectiveness of TYRX™ vs. standard of care was assessed from the UK National Health Service perspective. Probabilities of infection were derived from the literature, and resource use included mainly drugs, hospitalization, device extraction, and replacement. Over a 12-month time horizon, TYRX™ was less costly and more effective than standard of care when utilized in patients with an ICD or CRT-D. The results of the randomized WRAP-IT clinical trial (Table 11.1) confirmed the efficacy of the antibacterial envelope. 6983 patients were randomly assigned in a 1:1 ratio to receive or not the envelope at the end of the CIED procedure. The use of TYRX was associated with a 40% reduction of major CIEDI at 1 year (0.7% vs. 1.2%; HR 0.60; 95%CI 0.36–0.98; p = 0.04). The same result was confirmed at the end of the entire follow-up of 20.7 ± 8.5 months (HR 0.63; 95%CI 0.40–0.98). [126] The published subgroup analysis did not show any specific group with significantly higher benefit from use of TYRX, albeit this analysis is clearly limited by the small number of events that was far below what it was expected in the development of the study design. In fact, the sample size was calculated assuming a 2% 12-month infection rate, about the double of what the actually authors found. Notably, there was an evident discrepancy between the expected and found incidence of CIEDI in high-power vs. low-power devices (respectively, 2.4%/1.3% vs. 0.6%/0.8%) leaving much room for future analysis [89]. At this regard, we are still waiting for the results of the ENVELOPE study to better clarify these aspects. The study will evaluate whether the TYRX™ envelope alone offers protection against CIED infections without the use of intraoperative antibacterial solution and postoperative oral antibiotics in patients at high risk for infection. This randomized non-inferiority study will enroll nearly 1500 patients at one site in the USA. In summary, the use of a minocycline/rifampin envelope in patients requiring a CIED proved to be effective in reducing CIEDI, but further research is still needed to define the most appropriate patient groups that would benefit more from this approach.
11.4 Integrated CIEDI Infection Protocols
Several interventions showed a dramatic reduction in the incidence of CIEDI; as discussed in the previous sections and chapters (see Chaps. 1, 3, 10), some have been already introduced in current guidelines (e.g., antibiotic prophylaxis and avoidance of temporary pacemaker); some others not (e.g., use of antibiotic envelope). Regarding CIEDI, like to many other diseases, several recommendations present in current guidelines/consensus documents are based on common practice and opinion from the experts [14, 46, 47, 115] since it would be almost impossible to demonstrate any single intervention both from a feasible and ethical point of view (e.g., preparation of the operatory field). Moreover, it is extremely difficult to analyze any single intervention alone with complete control of all other variables in such a complex setting with relatively few events. For these reasons, it is extremely interesting looking at the effects of wide protocols including multiple interventions. Ahsan et al. reported the results of a retrospective analysis of the impact of a specifically designed infection-control protocol (including antibiotic prophylaxis determined by risk stratification, improved glycemic control, specific skin preparation, and closure techniques, as well as different diathermy settings) on the incidence of CIEDI in a tertiary referral center [116]. They found a 54% reduction in the incidence of CDI (from 1.3 to 0.6%; p = <0.03) associated with a relevant cost saving (about 70,000 GBP per annum) driven by the reduction in the costs associated with management of new cases of CIEDI, while the cost per patient varied between 85 GBP and 115 GBP according to infection risk and drug intolerance. The same protocol was adopted in completely different settings, on five low-volume centers in China [117], showing a dramatic reduction in CIEDI from about 4 to 1% in these centers justifying the authors suggestion for the adoption of an integrated protocol in all low-volume centers. More recently Manolis reported the results of his personal preventive strategy to CIEDI evidencing the occurrence of only two infections among 762 patients [118]. Despite the limitations of the design (single operator, teaching hospital, long-time window), this report provides really interesting results with a rather different protocol from the previous one. The preventive strategy evaluated in the PADIT trial was narrower compared to the two previously reported, being more focused on antibiotic treatment [119]. However, looking deeply in the design, at least four different aspects were covered: (a) risk stratification (only high-risk patients are considered); (b) pre-procedure antibiotic; (c) use of pocket wash; and (d) post-procedure continuation of antibiotic. This was a well-conducted randomized controlled trial which however failed to demonstrate a significant benefit of the incremental antibiotics strategy [103], but the principal reason seems to be the very low incidence of CIEDI (1.03% in the control group vs. 0.78%; p = 0.10) which made the study underpowered. Notably, these results are different from the before-and-after design of previous reports in this topic (e.g., CITADEL/CENTURION studies or the report from Ahsan et al.) [116, 120] but similar to the results of the WRAP-IT trial [89]. The more plausible reason is the presence of a bias introduced by the investigational setting, maybe due to operator/site selection or modification of operator’s behavior in response to participation to the study. Additional sub-analysis of these two landmark trials integrated with future studies will probably help us identify the factors we have to focus on in order to drastically reduce CIEDI (Fig. 11.8).
Comparison of infection rates among the three main trials on the TYRX antibacterial envelope (WRAP-IT, CENTURION, and CITADEL trials) (data from [120, 126]). From left to right, the bars represent incidence of 12 months CIEDI with matched control in the whole WRAP-IT cohort, in the same cohort after exclusion of pacemaker patients (CRT + ICD), and in the combined CENTURION/CITADEL cohorts (CRT + ICD). ∗ Site-matched controls (see [120]), ∗∗ published controls (see [120])
11.5 Conclusions
Newly developed technologies and strategies represent attractive options to reduce the incidence of the highly concerning issue of CIEDI, in particular, avoidance of promoting factors (e.g., temporary pacemaker, pre-operatory fever), tailored management of anticoagulation, appropriate antibiotic prophylaxis, accurate intra-procedure aseptic approach, and careful post-procedural follow-up. All these factors probably concur to the final result, and we should not focus on a single ingredient but in the whole receipt that should be tailored to each costumer. However, rooms of improvement are clearly present to optimize our preventive strategies and obtain the best results for our patients.
References
Abrahamyan L, et al. Insights into the contemporary management of heart failure in specialized multidisciplinary ambulatory clinics. Can J Cardiol. 2013;29(9):1062–8.
Greenspon AJ, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001–6.
Diemberger I, et al. The “subtle” connection between development of cardiac implantable electrical device infection and survival after complete system removal: an observational prospective multicenter study. Int J Cardiol. 2018;250:146–9.
Shariff N, et al. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol. 2015;26(7):783–9.
Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17(5):767–77.
De Maria E, et al. Prevention of infections in cardiovascular implantable electronic devices beyond the antibiotic agent. J Cardiovasc Med (Hagerstown). 2014;15(7):554–64.
Furnary AP, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–60; discussion 360–2
Woodhead K, et al. Behaviours and rituals in the operating theatre. A report from the Hospital Infection Society Working Party on Infection Control in Operating Theatres. J Hosp Infect. 2002;51(4):241–55.
Leaper D, et al. Prevention and treatment of surgical site infection: summary of NICE guidance. BMJ. 2008;337:a1924.
Diemberger I, et al. Predictors of long-term survival free from relapses after extraction of infected CIED. Europace. 2018;20(6):1018–27.
Kowalczyk J, et al. Contrast-induced acute kidney injury in patients undergoing cardiac resynchronization therapy-incidence and prognostic importance. Sub-analysis of data from randomized TRUST CRT trial. J Interv Card Electrophysiol. 2014;40(1):1–8.
Boriani G, Diemberger I. A closer look into the complexity of our practice: outcome research for transvenous temporary cardiac pacing. Int J Cardiol. 2018;271:117–8.
Padfield GJ, et al. Preventing cardiac implantable electronic device infections. Heart Rhythm. 2015;12(11):2344–56.
Habib G, et al. ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J, 2015. 2015;36(44):3075–128.
Nishimura RA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252–89.
Al-Khatib SM, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91–e220.
Baddour LM, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458–77.
Tobin K, et al. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol. 2000;85(6):–6): p. 774, A9
Bernard ML, et al. Meta-analysis of bleeding complications associated with cardiac rhythm device implantation. Circ Arrhythm Electrophysiol. 2012;5(3):468–74.
Essebag V, et al. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol. 2016;67(11):1300–8.
De Sensi F, et al. Pocket hematoma: a call for definition. Pacing Clin Electrophysiol. 2015;38(8):909–13.
Klug D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116(12):1349–55.
Ezzat VA, et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real-world’ data an underestimation? Open Heart. 2015;2(1):e000198.
Nichols CI, Vose JG. Incidence of bleeding-related complications during primary implantation and replacement of cardiac implantable electronic devices. J Am Heart Assoc. 2017:6(1).
Birnie DH, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368(22):2084–93.
Airaksinen KE, et al. Safety of pacemaker and implantable cardioverter-defibrillator implantation during uninterrupted warfarin treatment—the FinPAC study. Int J Cardiol. 2013;168(4):3679–82.
Poole JE, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553–61.
Yeoh A, et al. Wound haematoma following de fibrillator implantation: incidence and predictors in the Shockless Implant Evaluation (SIMPLE) trial: comment. Europace. 2018;20(8):1388.
Michaud GF, et al. A randomized trial comparing heparin initiation 6 h or 24 h after pacemaker or defibrillator implantation. J Am Coll Cardiol. 2000;35(7):1915–8.
Birnie DH, et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur Heart J. 2018;39(44):3973–9.
Tolosana JM, et al. Preparation for pacemaker or implantable cardiac defibrillator implants in patients with high risk of thrombo-embolic events: oral anticoagulation or bridging with intravenous heparin? A prospective randomized trial. Eur Heart J. 2009;30(15):1880–4.
Douketis JD, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–33.
Proietti R, et al. Risk of pocket hematoma in patients on chronic anticoagulation with warfarin undergoing electrophysiological device implantation: a comparison of different peri-operative management strategies. Eur Rev Med Pharmacol Sci. 2015;19(8):1461–79.
Rowley CP, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol. 2013;111(8):1165–8.
Jennings JM, et al. Cardiovascular implantable electronic device implantation with uninterrupted dabigatran: comparison to uninterrupted warfarin. J Cardiovasc Electrophysiol. 2013;24(10):1125–9.
Essebag V, et al. Short-term dabigatran interruption before cardiac rhythm device implantation: multi-centre experience from the RE-LY trial. Europace. 2017;19(10):1630–6.
Kutinsky IB, et al. Risk of hematoma complications after device implant in the clopidogrel era. Circ Arrhythm Electrophysiol. 2010;3(4):312–8.
Koh Y, et al. Cardiac implantable electronic device hematomas: risk factors and effect of prophylactic pressure bandaging. Pacing Clin Electrophysiol. 2017;40(7):857–67.
Sticherling C, et al. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS). Europace. 2015;17(8):1197–214.
Malagu M, et al. Frequency of “pocket” hematoma in patients receiving vitamin K antagonist and antiplatelet therapy at the time of pacemaker or cardioverter defibrillator implantation (from the POCKET study). Am J Cardiol. 2017;119(7):1036–40.
Beton O, et al. Bleeding complications during cardiac electronic device implantation in patients receiving antithrombotic therapy: is there any value of local tranexamic acid? BMC Cardiovasc Disord. 2016;16:73.
Ilov N, et al. Arguments to apply epinephrine for pocket hematoma reduction. The MAITRE Study. J Atr Fibrillation. 2016;9(1):1391.
Ohlow MA, et al. Pocket related complications in 163 patients receiving anticoagulation or dual antiplatelet therapy: D-Stat Hemostat versus standard of care. Int J Cardiol. 2012;159(3):177–80.
Tscholl V, et al. Prospective randomized study evaluating the effects of PerClot(R) (polysaccharide hemostatic system) application in patients with high bleeding risk undergoing cardiac rhythm device implantation. Int J Cardiol. 2017;248:84–91.
Chia PL, Foo D. Use of oxidized regenerated cellulose to prevent pocket hematomas after cardiac electronic device implantation in patients on anticoagulants or dual antiplatelet therapy. Int J Cardiol. 2013;168(4):4406–7.
Sandoe JA, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70(2):325–59.
Berrios-Torres SI, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA. 2017;152(8):784–91.
Da Costa A, et al. Preoperative skin antiseptics for prevention of cardiac implantable electronic device infections: a historical-controlled interventional trial comparing aqueous against alcoholic povidone-iodine solutions. Europace. 2015;17(7):1092–8.
MHRA, Dangerous liaisons: diathermy burns with alcohol based skin preparations. http://www.mhra.gov.uk/home/groups/clin/documents/publication/con140853.pdf.
McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–79.
Bongiorni MG, et al. How European centres diagnose, treat, and prevent CIED infections: results of an European Heart Rhythm Association survey. Europace. 2012;14(11):1666–9.
Uslan DZ, et al. Cardiovascular implantable electronic device replacement infections and prevention: results from the REPLACE Registry. Pacing Clin Electrophysiol. 2012;35(1):81–7.
Darouiche RO, et al. Chlorhexidine-alcohol versus Povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
Qintar M, et al. The impact of changing antiseptic skin preparation agent used for cardiac implantable electronic device (CIED) procedures on the risk of infection. Pacing Clin Electrophysiol. 2015;38(2):240–6.
Loveday HP, et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86(Suppl 1):S1–70.
Chen HC, et al. Bundled preparation of skin antisepsis decreases the risk of cardiac implantable electronic device-related infection. Europace. 2016;18(6):858–67.
Wistrand C, et al. The effect of preheated versus room-temperature skin disinfection on bacterial colonization during pacemaker device implantation: a randomized controlled non-inferiority trial. Antimicrob Resist Infect Control. 2015;4:44.
Makki D, et al. Lifting incise drapes off the skin during wound closure can cause contamination. J Perioper Pract. 2015;25(5):112–4.
Grove GL, Eyberg CI. Comparison of two preoperative skin antiseptic preparations and resultant surgical incise drape adhesion to skin in healthy volunteers. J Bone Joint Surg Am. 2012;94(13):1187–92.
Kozon I, et al. Risk factors of cardiac device infection: glove contamination during device procedures. Am J Infect Control. 2017;45(8):866–71.
Nichols CI, Vose JG, Mittal S. Incidence and costs related to lead damage occurring within the first year after a cardiac implantable electronic device replacement procedure. J Am Heart Assoc. 2016:5(2).
Kirkfeldt RE, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35(18):1186–94.
Krahn AD, et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4(2):136–42.
Kypta A, et al. An electrical plasma surgery tool for device replacement—retrospective evaluation of complications and economic evaluation of costs and resource use. Pacing Clin Electrophysiol. 2015;38(1):28–34.
Diemberger I, et al. Detect long-term complications after ICD replacement (DECODE): rationale and study design of a Multicenter Italian Registry. Clin Cardiol. 2015;38(10):577–84.
Lim KK, et al. Effects of electrocautery on transvenous lead insulation materials. J Cardiovasc Electrophysiol. 2009;20(4):429–35.
Kypta A, et al. Economic assessment of traditional surgical intervention versus use of a new innovative radiofrequency based surgical system in device replacements. PLoS One. 2018;13(3):e0192587.
Wasserlauf J, et al. Avoiding damage to transvenous leads—a comparison of electrocautery techniques and two insulated electrocautery blades. Pacing Clin Electrophysiol. 2018;41(12):1593–9.
Kaya E, Totzeck M, Rassaf T. Pulsed electron avalanche knife (PEAK) PlasmaBlade in pacemaker and defibrillator procedures. Eur J Med Res. 2017;22(1):49.
Servello D, Bona AR, Zekaj E. Is capsulectomy a feasible and useful measure in internal pulse generator replacement procedures? A technical note on the use of the PEAK PlasmaBlade(TM). Acta Neurochir. 2016;158(6):1165–8.
Diemberger I, et al. From lead management to implanted patient management: indications to lead extraction in pacemaker and cardioverter-defibrillator systems. Expert Rev Med Devices. 2011;8(2):235–55.
Gleva MJ, Poole JE. Prevention of cardiac implantable electronic device infections: update and evaluation of the potential role for capsulectomy or the antibiotic pouch. J Atr Fibrillation. 2017;9(5):1540.
Kleemann T, et al. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace. 2010;12(1):58–63.
Lakkireddy D, et al. IMpact of pocKet rEvision on the rate of InfecTion and other CompLications in patients rEquiring pocket mAnipulation for generator replacement and/or lead replacement or revisioN (MAKE IT CLEAN): a prospective randomized study. Heart Rhythm. 2015;12(5):950–6.
Steckiewicz R, et al. Morphometric parameters of cardiac implantable electronic device (CIED) pocket walls observed on device replacement. Folia Morphol (Warsz). 2017;
Biefer HR, et al. Generator pocket adhesions of cardiac leads: classification and correlation with transvenous lead extraction results. Pacing Clin Electrophysiol. 2013;36(9):1111–6.
Spencker S, et al. Comparison of skin adhesive and absorbable intracutaneous suture for the implantation of cardiac rhythm devices. Europace. 2011;13(3):416–20.
Khodak A, Budzikowski AS. Use of barbed suture for wound closure in electrophysiology device procedures. J Atr Fibrillation. 2017;9(6):1571.
De Maria E. New skin closure system facilitates wound healing after cardiovascular implantable electronic device surgery. World J Clin Cases. 2015;3(8):675–7.
Koerber SM, et al. Noninvasive tissue adhesive for cardiac implantable electronic device pocket closure: the TAPE pilot study. J Interv Card Electrophysiol. 2019;54(2):171–6.
O’Hea BJ, Ho MN, Petrek JA. External compression dressing versus standard dressing after axillary lymphadenectomy. Am J Surg. 1999;177(6):450–3.
Turagam MK, et al. Use of a pocket compression device for the prevention and treatment of pocket hematoma after pacemaker and defibrillator implantation (STOP-HEMATOMA-I). J Interv Card Electrophysiol. 2017;49(2):197–204.
Mounsey JP, et al. Antibiotic prophylaxis in permanent pacemaker implantation: a prospective randomised trial. Br Heart J. 1994;72(4):339–43.
de Oliveira JC, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2(1):29–34.
Da Costa A, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998;97(18):1796–801.
Darouiche R, Mosier M, Voigt J. Antibiotics and antiseptics to prevent infection in cardiac rhythm management device implantation surgery. Pacing Clin Electrophysiol. 2012;35(11):1348–60.
Dwivedi SK, et al. Short-term (48 hours) versus long-term (7 days) antibiotic prophylaxis for permanent pacemaker implantation. Indian Heart J. 2001;53(6):740–2.
Ramsdale DR, et al. Antibiotic prophylaxis for pacemaker implantation: a prospective randomized trial in 302 patients. Pacing Clin Electrophysiol. 1993;16:1138.
Tarakji KG, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019;380(20):1895–905.
Townsend TR, et al. Clinical trial of cefamandole, cefazolin, and cefuroxime for antibiotic prophylaxis in cardiac operations. J Thorac Cardiovasc Surg. 1993;106(4):664–70.
Classen DC, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–6.
Zanetti G, Giardina R, Platt R. Intraoperative redosing of cefazolin and risk for surgical site infection in cardiac surgery. Emerg Infect Dis. 2001;7(5):828–31.
Bolon MK, et al. Glycopeptides are no more effective than beta-lactam agents for prevention of surgical site infection after cardiac surgery: a meta-analysis. Clin Infect Dis. 2004;38(10):1357–63.
Finkelstein R, et al. Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J Thorac Cardiovasc Surg. 2002;123(2):326–32.
Saginur R, Croteau D, Bergeron MG. Comparative efficacy of teicoplanin and cefazolin for cardiac operation prophylaxis in 3027 patients. The ESPRIT Group. J Thorac Cardiovasc Surg. 2000;120(6):1120–30.
Mese B, et al. Efficacy of linezolid, teicoplanin, and vancomycin in prevention of an experimental polytetrafluoroethylene graft infection model caused by methicillin-resistant Staphylococcus aureus. Med Sci Monit. 2015;21:909–14.
Byrne CJ, et al. Population pharmacokinetics of teicoplanin and attainment of pharmacokinetic/pharmacodynamic targets in adult patients with haematological malignancy. Clin Microbiol Infect. 2017;23(9):674e7–674e13.
Mini E, Nobili S, Periti P. Does surgical prophylaxis with teicoplanin constitute a therapeutic advance? J Chemother. 2000;12(Suppl 5):40–55.
White RW, et al. Antimicrobial regime for cardiac surgery: the safety and effectiveness of short-course flucloxacillin (or teicoplanin) and gentamicin-based prophylaxis. J Card Surg. 2013;28(5):512–6.
Rein MF, Westervelt FB, Sande MA. Pharmacodynamics of cefazolin in the presence of normal and impaired renal function. Antimicrob Agents Chemother. 1973;4(3):366–71.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9.
Bratzler DW, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
Krahn AD, et al. Prevention of Arrhythmia Device Infection Trial: The PADIT Trial. J Am Coll Cardiol. 2018;72(24):3098–109.
Lakkireddy D, et al. The impact of povidone-iodine pocket irrigation use on pacemaker and defibrillator infections. Pacing Clin Electrophysiol. 2005;28(8):789–94.
Raad I, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med. 1997;127(4):267–74.
Darouiche RO, et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med. 1999;340(1):1–8.
Zabramski JM, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98(4):725–30.
Illingworth BL, et al. In vivo efficacy of silver-coated (Silzone) infection-resistant polyester fabric against a biofilm-producing bacteria. J Heart Valve Dis. 1998;7(5):524–30.
Block SS, editor. Disinfection, sterilization and preservation, in silver and its compounds. Philadelphia: Lea & Febiger; 1997. p. 395–40.
Dandekar UP, et al. Silzone-coated St. Jude Medical valves: six-year experience in 46 patients. J Heart Valve Dis. 2007;16(1):37–41.
Hansen LK, et al. In vivo model of human pathogen infection and demonstration of efficacy by an antimicrobial pouch for pacing devices. Pacing Clin Electrophysiol. 2009;32(7):898–907.
Hassoun A, et al. Retrospective comparative analysis of cardiovascular implantable electronic device infections with and without the use of antibacterial envelopes. J Hosp Infect. 2017;95(3):286–91.
Koerber SM, et al. Use of antibiotic envelopes to prevent cardiac implantable electronic device infections: a meta-analysis. J Cardiovasc Electrophysiol. 2018;29(4):609–15.
Kay G, et al. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ. 2018;21(3):294–300.
Kusumoto FM, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503–51.
Ahsan SY, et al. A simple infection-control protocol to reduce serious cardiac device infections. Europace. 2014;16(10):1482–9.
Guan G, et al. Application of an infection control protocol (ICP) reduced cardiac device infection (CDI) in low-volume centers. Med Sci Monit. 2018;24:1366–72.
Manolis AS, Melita H. Prevention of cardiac implantable electronic device infections: single operator technique with use of povidone-iodine, double gloving, meticulous aseptic/antiseptic measures and antibiotic prophylaxis. Pacing Clin Electrophysiol. 2017;40(1):26–34.
Connolly SJ, et al. Randomized cluster crossover trials for reliable, efficient, comparative effectiveness testing: design of the Prevention of Arrhythmia Device Infection Trial (PADIT). Can J Cardiol. 2013;29(6):652–8.
Henrikson CA, et al. Antibacterial envelope is associated with low infection rates after implantable cardioverter-defibrillator and cardiac resynchronization therapy device replacement: results of the CITADEL and CENTURION studies. JACC Clin Electrophysiol. 2017;3(10):1158–67.
Valgimigli M, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–60.
Bloom HL, et al. Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope. Pacing Clin Electrophysiol. 2011;34(2):133–42.
Kolek MJ, et al. Use of an antibacterial envelope is associated with reduced cardiac implantable electronic device infections in high-risk patients. Pacing Clin Electrophysiol. 2013;36(3):354–61.
Mittal S, et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm. 2014;11(4):595–601.
Kolek MJ, et al. Efficacy of a bio-absorbable antibacterial envelope to prevent cardiac implantable electronic device infections in high-risk subjects. J Cardiovasc Electrophysiol. 2015;26(10):1111–6.
Tarakji KG, et al. Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial (WRAP-IT). Am Heart J. 2016;180:12–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Diemberger, I., Boriani, G., Deharo, JC. (2020). Prevention of Device Infection: Procedural Aspects, Drugs, and Preventive Tools. In: Diemberger, I., Boriani, G. (eds) Infections of Cardiac Implantable Devices. Springer, Cham. https://doi.org/10.1007/978-3-030-46255-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-46255-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46254-3
Online ISBN: 978-3-030-46255-0
eBook Packages: MedicineMedicine (R0)