Abstract
The number of device implantation procedures has increased in adult patients with congenital heart disease (ACHD). Despite significant improvements in materials and implantation techniques, these patients are exposed to higher risk of device related complications than general population. Herein, we describe our single tertiary referral center experience on transvenous pacemaker (PM) implantation and follow-up in adult patients with moderate and complex congenital heart disease (CHD) as limited data are available on long-term outcome. We considered all adults with moderate and complex CHD aged more than 16 years who underwent transvenous single-chamber and dual-chamber PM implant for sinus node dysfunction or atrioventricular block between January 2013 to December 2022 at our Unit. Seventy-one ACHD patients were included in the study (mean age 38.6 ± 15.2 years, 64% with moderate CHD, 36% with complex CHD). Among 32 patients implanted with a dual chamber PM (DDD PM), 4 devices were reprogrammed in VDD mode, 3 in VVI and 2 in AAI mode during follow-up because of lead dysfunction or permanent atrial arrhythmia. In addition, 26 patients had a single chamber PM (AAI or VVI PM) and 13 patients had single-lead pacing system with a free-floating atrial electrode pair (VDD PM). Just one of 13 single-lead VDD PM was reprogrammed in VVI mode due to a low atrial sensing. In DDD PM group, 10 re-interventions were needed due to lead dysfunction (8 cases) and lead-related infective endocarditis (2 cases). Only 3 patients in the single-lead PM group developed lead dysfunction with 2 re-interventions needed, but no infective endocarditis was reported. The rate of long-term complications is high in moderate and complex ACHD with transvenous PM devices, and it is mainly lead-related. In our experience, the less leads implanted, the less complications will occur. Considering the heterogeneity of the ACHD population, transvenous single-chamber or dual-chamber PM device implantation should always be tailored on the single patient, balancing risks and benefits in this complex population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prevalence of congenital heart disease (CHD) is increasing worldwide [1, 2]. Nowadays, more than 90% of children with CHD survive into adulthood thanks to medical, surgical, and technological evolutions over the past decades [1, 2]. As a result, the number of adult congenital heart disease (ACHD) patients in the European Union exceeds for the first time that of children [2, 3]. Anyway, CHD is a lifelong condition and some complications, such as heart failure or arrhythmias, may affect ACHD patients [2]. In particular, bradyarrhythmia disorders such as sinus node dysfunction (SND) and/or atrioventricular block (AVB) may occur, as direct consequence of congenitally impaired (displaced or malformed) sinus node or AV conduction system and/or corrective surgery/interventional procedures [4, 5]. Because of heterogeneity of the ACHD population and the increased risk of device-related complications in these patients [6, 7], PM implantation should be performed in centers with a multidisciplinary team and wide expertise in CHD-related device therapy [8,9,10,11,12]. However, limited data are available on long-term outcomes of transvenous PM devices in adult patients with moderate and complex CHD.
The aim of this single-center study is to describe our clinical experience on transvenous PM implantation in our moderate and complex ACHD population, focusing on the indications for device insertion, type of devices implanted, rate of complications, device re-programming and re-interventions during follow-up.
Materials and Methods
We conducted an observational, retrospective study on transvenous PM implantation and follow-up in adult patients with moderate or complex CHD referred to our division between January 2013 and December 2022. Data were prospectively collected and retrospectively analyzed using medical records and device registry database of our unit. The study received approval from the local ethics committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments. Informed consent was obtained from all patients or their guardians.
Inclusion/exclusion Criteria
Inclusion criteria consisted of:
-
a.
(a) aged ≥ 16 years,
-
b.
(b) presence of moderate or complex CHD, according to the latest international guidelines,
-
c.
(c) indication for transvenous PM implantation, according to the latest international guidelines [10, 11].
Patients with simple CHD, patients with other types of devices (epicardial or leadless PM, transvenous or epicardial implantable cardioverter defibrillator or cardiac resynchronization therapy devices) or those aged <16 years were excluded.
Implantation Procedure
All procedures were performed in the electrophysiology/cardiac pacing laboratory, by a single team of six electrophysiologists of the ACHD Unit with the support of the manufacturer’s technicians, under fluoroscopic guidance.
Antibiotic prophylaxis was given to all patients. The more frequent approach for leads insertion was through to the left subclavian or axillary vein; an alternative option was through the left cephalic vein. Right access was required in case of left venous obstruction. The choice between active or passive fixation atrial and ventricular leads was made based on the operator’s preference and underlying anatomy.
Data Collection and Analysis
The following data were collected for each patient: patient demographics, underlying CHD, indication for PM therapy, age at PM implant, type of device implanted, initial programmed pacing mode, change in programmed pacing mode over time, rate of complications and re-interventions, occurrence of arrhythmias during follow-up. The complication rate between dual- and single-chamber PM groups was compared using a “chi-square” test. P value < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS.
Follow-up
After discharge, all patients were scheduled to a regular follow-up at our ACHD outpatient clinic, involving clinical evaluation, ECG and devices interrogation 2–4 weeks after the implant and subsequently every 6–12 months. Additionally, transthoracic echocardiography and Holter monitoring were performed every 12 months to assess the ongoing functional status of the disease.
Results
Patients’ Characteristics
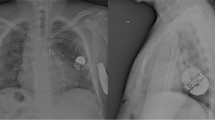
Seventy-one ACHD patients were included in the study. Baseline characteristics of the study cohort are shown in Table 1. Among them, 38 patients (53%) were male and 33 patients (47%) were female. The median age at the time of implant was 38.6 ± 15.2 years (range 16–83). Forty-six patients (64%) had moderate CHD, while 25 patients (36%) were affected by complex CHD (Fig. 1). The most frequent indication for PM implantation was AVB (84.5%), including post-operative AVB (55%) and spontaneous AVB (29.5%), while SND accounted for 15.5%. Thirty-two patients (42%) were implanted with a dual-chamber PM (DDD PM), 26 patients (36%) with a single-chamber PM (AAI PM or VVI PM) (Fig. 2), while 13 patients (18%) had single-lead dual chamber PM with a free-floating atrial electrode pair (VDD PM).
Congenital heart disease distribution in the study population. ToF Tetralogy of Fallot, TGA Transposition of the Great Arteries, ccTGA congenitally corrected Transposition of the Great Arteries, AVSD Atrio-Ventricular Septal Defect, DORV Double Outlet Right Ventricle, CoA Coarctation of the Aorta, FSV Functional Single Ventricle, VSD Ventricular Septal Defect, PVS Pulmonary Valve Stenosis, AVS Aortic Valve Stenosis
Follow-up
At the last clinical follow-up, 36 patients (50.7%) were PM-dependent. The total number of PM generator replacement procedures were 47 and the mean generator longevity was 8 ± 2.2 years, with no differences between dual- and single-chamber PM. Five patients (7%) had a permanent atrial arrhythmia (atrial fibrillation or intra-atrial re-entrant tachycardia).
Of the 32 dual-chamber PM initially programmed to DDD pacing mode, 4 devices (12.5%) were later reprogrammed in VDD mode, 3 devices (9.4%) in VVI mode and 2 devices (6.3%) in AAI mode because of lead failure (4 cases), lead displacement (2 cases) or absence of need for dual-chamber pacing (e.g., no need for atrial pacing after onset of permanent atrial arrhythmia) (3 cases). Therefore, only 23 out of 32 patients (71.8%) were maintained in DDD mode until the last follow-up, but 5/23 (21.7%) showed a percentage of atrial pacing less than 25%. Among single-chamber PM, 25 devices (96.1%) were in VVI pacing mode, and one device (3.9%) in AAI mode. One of 13 single-lead VDD PM (7.7%) was reprogrammed in VVI pacing mode due to a low atrial sensing, while 12 patients (92.3%) maintained VDD mode during follow-up (Table 2).
During the follow-up period, 10/32 patients (28.1%) in the dual-chamber PM group (DDD PM) needed a re-intervention (lead abandonment with addition of a new lead, n = 6; lead extraction and reimplantation, n = 2; lead repositioning, n = 2) due to lead fracture/insulation break (n = 6), lead dislocation (n = 2) or lead-related infective endocarditis (n = 2) (Fig. 3). On the other hand, lead dysfunction occurred only in 3/38 patients (lead fracture/insulation break, n = 1; poor sensing, n = 2) in the single-lead PM group (VVI PM + AAI PM + single-lead VDD PM), with only 2 cases of re-intervention for new lead addition (n = 1) and lead repositioning (n = 1). No cases of infective endocarditis were reported in this group. So, we observed 19/71 (26.7%) device-related late complications, of which 17 were lead-related and 2 were linked to infective endocarditis. (Table 3) (Fig. 4). The number of complications in the dual-chamber PM group was statistically significantly higher compared to the single-chamber PM group (16/32 vs. 3/39, p 0.0001).
Discussion
ACHD patients are lifelong at higher risk of requiring PM implantation than the general population, primarily due to both anatomic features and previous cardiac surgery or interventional procedures [8, 9]. Post-operative high-degree AVB is estimated to occur in 1–3% of patients undergoing surgery for CHD, especially in patients who underwent ventricular septal defect closure, left ventricle outflow tract obstruction correction, Tetralogy of Fallot repair and left sided valve surgery [5, 7,8,9, 13, 14]. Sinus node dysfunction (SND) may result from cardiac surgery, such as atrial switch operation (Mustard or Senning procedures), atrial septal defects repair and Fontan correction [15,16,17,18,19]. ACHD patients with post-operative SND or high degree/complete AVB are considered at a higher risk of sudden cardiac death (SCD). Therefore, broader indications for PM implantation compared to patients with structurally normal hearts have been suggested [8,9,10,11].
Several issues require attention prior to the implantation procedure in this population. PM implantation in ACHD population may be challenging due to anatomic constraints, technical difficulties related to the underlying CHD, previous cardiac surgery or interventional procedures, complex systemic venous anatomy, presence of severe valve regurgitation, presence of intracardiac devices [2, 8, 9]. The presence of residual intra-cardiac shunts or baffle leaks increases systemic thromboembolic risk, as well as lead placement in systemic right or left ventricle. Moreover, in case of narrowed or occluded intracardiac baffles, prior or simultaneous recanalization and stenting are necessary. Attention is also needed to the absence of direct vascular access to cardiac chambers or to central venous obstruction. Epicardial lead placement, with the necessity of a thoracotomy, should be alternatively considered in these cases, especially if patients are needed of other cardiac surgery.
The rate of peri-procedural and long-term complications in ACHD patients is higher than in general populations [20,21,22,23,24]. A retrospective study on 234 ACHD patients reported a rate of peri-procedural complications of 10.9% (two times greater than the general population) and long-term complication of 35% [20]. Walker et al., in their observational study reported 27% of lead-related complications [21]. In the Royal Brompton Hospital’s registry, 44 out of 238 (18.5%) ACHD patients required ≥ 1 re-intervention due to device related complications [22]; lead failure was the most common cause of device-related complication needing re-intervention. A single-centre study reported 50 systems (24%) dysfunction among 208 endocavitary device recipients with CHD (PM or ICD); the most common reason was lead-related issues (70%), while infection (22%) was the second one [23]. In line with these data, in our study, 19 out of 71 (26.7%) device-related late complications were identified, of which 17 were lead-related and 2 were linked to infective endocarditis.
Our experience suggests that the choice of dual-chamber PM is not always accompanied by a real clinical benefit and it is weighted by an increased risk of long-term complications. In fact, late complications have affected DDD PM more frequently than single-lead PM in our study. Moreover, at the last clinical follow-up, among 32 patients with dual-chamber PM, 9 devices were reprogrammed in single-chamber mode because of lead failure/displacement or absence of need for dual-chamber pacing, without developing significant worsening of clinical status or signs/symptoms of hemodynamic impairment owing to suboptimal AV synchrony or AV dyssynchrony and pacemaker syndrome. Interestingly, a significant number of patients in DDD pacing mode (21.7%) required a low percentage of atrial pacing (less than 25%).
Therefore, in selected ACHD patients with bradyarrhythmia, it could be considered as an alternative option to perform an initial implantation of a transvenous single-chamber PM (AAI or VVI PM), and then upgrade to a dual-chamber device if needed, in order to minimize lead-related complications during follow-up. Anyway, the best therapeutic strategy and the choice of PM type should be assessed case-by-case, balancing risks and benefits.
Conclusions
Despite significant improvements in technologies and clinical expertise on PM therapy, the rate of long-term complications remains high in moderate and complex ACHD, primarily due to lead-related issues. The fewer leads implanted, the lower the likelihood of complications. Given the heterogeneity of the ACHD population, the implantation of transvenous single-chamber or dual-chamber PM device implantation should always be tailored on the single patient, carefully balancing risks and benefits in this complex population (Fig. 5).
Proposed flowchart for clinical decision between single- and dual-chamber PM in moderate and complex CHD patients*. PM Pacemaker; CHD Congenital Heart Disease; AVB Atrio-Ventricular Block; SND Sinus Node Dysfunction; CRT Cardiac Resynchronization Therapy. * In this complex population the choice between single- and dual-chamber PM should always be tailored on the single patient, carefully balancing risks and benefits. ** VDD-PM should be preferred in patient who are at high risk of infection
Data Availability
No datasets were generated or analysed during the current study.
References
Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, Keavney BD (2019) Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 48(2):455–463. https://doi.org/10.1093/ije/dyz009PMID: 30783674; PMCID: PMC6469300
Barracano R, Ciriello GD, Sarubbi B (2023) Pharmacological therapy in adult congenital heart disease with coronary artery disease and atrial fibrillation. Int J Cardiol Congenital Heart Disease 12:2666–6685. https://doi.org/10.1016/j.ijcchd.2023.100446
Brida M, Gatzoulis MA (2019) Adult congenital heart disease: past, present and future. Acta Paediatr 108(10):1757–1764. https://doi.org/10.1111/apa.14921Epub 2019 Aug 18. PMID: 31254360
Anderson RH, Ho SY (1991) The disposition of the conduction tissues in congenitally malformed hearts with reference to their embryological development. J Perinat Med 19(Suppl 1):201–206
Limongelli G, Ducceschi V, D’Andrea A, Renzulli A, Sarubbi B, De Feo M, Cerasuolo F, Calabrò R, Cotrufo M (2003) Risk factors for pacemaker implantation following aortic valve replacement: a single centre experience. Heart 89(8):901–904. https://doi.org/10.1136/heart.89.8.901PMID: 12860869; PMCID: PMC1767749
Sarubbi B, Ciriello GD, Papaccioli G et al (2023) Combined subcutaneous implantable cardioverter defibrillator and pacemaker devices in complex congenital heart disease. A single-center experienced based study. Authorea January 30. https://doi.org/10.22541/au.167507359.94643473/v1
Li W, Sarubbi B, Somerville J (2000) Iatrogenic ventricular tachycardia from endocardial pacemaker late after repair of tetralogy of Fallot. Pacing Clin Electrophysiol.;23(12):2131-4. https://doi.org/10.1111/j.1540-8159.2000.tb00789.x. PMID: 11202260
Hernández-Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, Chessa M, Combes N, Dagres N, Diller G, Ernst S, Giamberti A, Hebe J, Janousek J, Kriebel T, Moltedo J, Moreno J, Peinado R, Pison L, Rosenthal E, Skinner JR, Zeppenfeld K, ESC Scientific Document Group (2018);. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace.;20(11):1719–1753. https://doi.org/10.1093/europace/eux380. PMID: 29579186
Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller G-P, Kluin BlungJ, Irene M, Lang F, Meijboom P, Moons, Barbara JM, Mulder E, Oechslin JW, Roos-Hesselink M, Schwerzmann L, Sondergaard K, Zeppenfeld, February, ESC Scientific Document Group (2021), 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD), European Heart Journal, Volume 42, Issue 6, 7 Pages 563–645, https://doi.org/10.1093/eurheartj/ehaa554
Glikson M, Nielsen JC, Kronborg MB et al (2021) 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 42(35):3427–3520
Stout KK, Daniels CJ, Aboulhosn JA et al (2019) 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. J Am Coll Cardiol 73(12):1494–1563
Cecchin DG, Halpern (2017) Cardiac arrhythmias in adults with congenital heart disease: pacemakers, implantable cardiac defibrillators, and cardiac resynchronization therapy devices. Card Electrophysiol Clin 9:319–328
Lin A, Mahle WT, Frias PA, Fischbach PS, Kogon BE, Kanter KR et al (2010) Early and delayed atrioventricular conduction block after routine surgery for congenital heart disease. J Thorac Cardiovasc Surg 140:158–160
Sarubbi B, Mercurio B, Ducceschi V, Russo MG, Calabrò R (2004) A multisite atrioventricular block. Ital Heart J 5(1):64–68 PMID: 15080584
Broberg CS, van Dissel A, Minnier J, Aboulhosn J, Kauling RM, Ginde S, Krieger EV, Rodriguez F 3rd, Gupta T, Shah S, John AS, Cotts T, Kay WA, Kuo M, Dwight C, Woods P, Nicolarsen J, Sarubbi B, Fusco F, Antonova P, Fernandes S, Grewal J, Cramer J, Khairy P, Gallego P, O’Donnell C, Hannah J, Dellborg M, Rodriguez-Monserrate CP, Muhll IV, Pylypchuk S, Magalski A, Han F, Lubert AM, Kay J, Yeung E, Roos-Hesselink J, Baker D, Celermajer DS, Burchill LJ, Wilson WM, Wong J, Kutty S, Opotowsky AR (2022) Long-Term Outcomes After Atrial Switch Operation for Transposition of the Great Arteries. J Am Coll Cardiol.;80(10):951–963. https://doi.org/10.1016/j.jacc.2022.06.020. PMID: 36049802
Greenwood RD, Rosenthal A, Sloss LJ, LaCorte M, Nadas AS (1975) Sick sinus syndrome after surgery for congenital heart disease. Circulation.;52(2):208 – 13. https://doi.org/10.1161/01.cir.52.2.208. PMID: 1149204
Murphy JG, Gersh BJ, McGoon MD et al (1990) Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med 323(24):1645–1650
Cohen MI, Bridges ND, Gaynor JW et al (2000) Modifications to the cavopulmonary anastomosis do not eliminate early sinus node dysfunction. J Thorac Cardiovasc Surg 120:891–901
Peters NS, Somerville J (1992) Arrhythmias after the Fontan procedure. Br Heart J 68(2):199–204
Opić P, van Kranenburg M, Yap SC et al (2013) Complications of pacemaker therapy in adults with congenital heart disease: a multicenter study. Int J Cardiol 168(4):3212–3216
Walker F, Siu SC, Woods S, Cameron DA, Webb GD, Harris L (2004) Long-term outcomes of cardiac pacing in adults with congenital heart disease. J Am Coll Cardiol 43:1894–1901. https://doi.org/10.1016/j.jacc.2003.12.044
Midha D, Chen Z, Jones DG et al (2017) Pacing in congenital heart disease - A four-decade experience in a single tertiary centre. Int J Cardiol 241:177–181
Bowman HC, Shannon KM, Biniwale R, Moore JP (2021) Cardiac implantable device outcomes and lead survival in adult congenital heart disease. Int J Cardiol 324:52–59. https://doi.org/10.1016/j.ijcard.2020.09.027Epub 2020 Sep 15. PMID: 32941867
Silvetti MS, Drago F, Grutter G, De Santis A, Di Ciommo V, Ravà L (2006) Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients. Europace.;8(7):530-6. https://doi.org/10.1093/europace/eul062. PMID: 16798767
Acknowledgements
Special thanks to the nursing staff of the Adult Congenital Heart Disease Unit, and specially to the head nurs,e Mrs. Assunta Carandente, for their essential contribution and support in maintaining a high-quality standard of care for our complex patients. Additionally, we express our gratitude to Dr. Gabriella Piccolo, Dr. Nadia Puzone, Dr. Cecilia Spinelli Barrile and Dr. Tiziana Varriale, the data manager and research assistants, for their efforts in data collection and analysis, as well as their support in remote control monitoring.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
GP, FLR and BS contributed to write, review, and image editing. NG, AO and GDC contributed to data collection and review. AC, DC, ER and MP contributed to production of tables/figures and review. All authors approved the manuscript text.
Corresponding author
Ethics declarations
Competing Interests
The authors have no financial or proprietary interests in any material discussed in this article.
Ethics Approval and Consent
The study received approval from the local ethics committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments. Informed consent was obtained from all patients or their guardians.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Papaccioli, G., Rocca, F.L., Ciriello, G.D. et al. Single-Chamber and Dual-Chamber Pacemaker Devices in Adults with Moderate and Complex Congenital Heart Disease: A Single Tertiary Referral Center Experience. Pediatr Cardiol (2024). https://doi.org/10.1007/s00246-024-03444-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00246-024-03444-6