Abstract
Background
Continuous wavelet transform (CWT) analysis is a frequency analysis to detect areas of stable high-frequent activity (stable pseudo frequency [sPF]) during atrial fibrillation (AF). As previously reported, patients with the highest sPF area in pulmonary veins (PV) showed better short-term outcomes after PV isolation (PVI). This study sought to evaluate the efficacy of CWT analysis in predicting the long-term (2 years) outcomes after PVI. We also combined the left atrial (LA) voltage map with CWT analysis to further predict the outcome.
Methods
Persistent AF patients (n = 109, age 65 ± 10) underwent a CWT analysis at PVs and 8 LA sites during AF for pre-PVI analysis. After PVI during AF, CWT analysis was performed again in the LA as post-PVI analysis and was compared with pre-PVI analysis. A sinus voltage map of LA was created after cardioversion.
Results
Seventy patients had the highest sPF within PVs (PV-dominant group), while 39 patients had the highest sPF outside PVs (LA-dominant group). The global frequency in the LA showed a significant decrease after PVI only in PV-dominant group (6.55 ± 0.27 to 6.43 ± 0.37, P < 0.01). AF-free survival was better in PV-dominant group than LA-dominant group at 2-year follow-up (87.1% vs. 64.3%, P < 0.002). This trend was recognized throughout all degrees of low voltage area in the LA (LA-LVA), and AF-free survival was well predicted by combining CWT analysis and LA-LVA.
Conclusions
By combining CWT analysis and sinus LA-LVA, the long-term AF-free survival after PVI was well stratified and predicted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein isolation (PVI) is an effective procedure for paroxysmal atrial fibrillation (AF)[1]. For patients with persistent AF, left atrial (LA) posterior wall isolation[2] or low voltage area (LVA) ablation[3, 4] are often empirically applied to improve the outcomes.
Studies have suggested that localized AF drivers maintain AF, which are limited regions of fast and repetitive conduction as Jalife et al. described[5, 6]. This localized AF driver is often accompanied by intramural fibrosis, as shown in a study using ex vivo 3D functional and structural imaging[7] as well as late gadolinium-enhanced human cardiac MRI[8]. In human atria, left atrial LVA overlaps the site of high-frequency[9], which is thought to be why AF drivers exist outside PVs in patients with fibrosis in the LA.
We previously reported the benefit of continuous wavelet transform (CWT) analysis over Fast Fourier Transform (FFT) analysis in detecting stable high-frequency sites by compensating for the low temporal resolution in FFT analysis[10,11,12]. The 1-year result after PVI was better in patients with the highest frequency in PVs when analyzed by CWT, while FFT could not detect the highest frequency sites appropriately[10].
This study evaluated the efficacy of combining the information from the CWT analysis and the LA voltage map in predicting the long-term (2 years) outcomes after PVI. We also evaluated the change of local frequency after PVI to examine the effect of PVI on each location of LA, including the LA posterior wall.
2 Methods
2.1 Study population
We included consecutive patients with persistent AF who underwent initial AF ablation at Hiratsuka Kyosai Hospital from April 2016 to March 2018. Patients who underwent any previous AF ablation or cardiac surgery were excluded from this study. Patients were classified as persistent AF by the ACC/AHA/ESC guideline[13]. Data collection, analysis, and the reported measures were approved by the Institutional Review Board.
2.2 Continuous Wavelet Transform (CWT) analysis
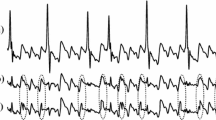
The previous report described the concept of CWT analysis in detail[10]. Bipolar electrograms (EGMs) were recorded during AF on the recording system (Cardio Lab, GE Healthcare, Chicago, IL, USA) using a close-bipolar mapping catheter for 30 seconds at each site. They were bandpass filtered from 30 to 500 Hz. The sampling rate was 977 Hz. The raw bipolar EGM data were exported from Cardio Lab and analyzed for CWT analysis using customized CWT analysis software offline. The continuous change of frequency was measured by applying the mother wavelet to the rectified raw wave signal by continuously compressing and expanding the mother wavelet for 30 seconds. Then, the continuous movement of the dominant frequency for 30 seconds was depicted as a color spectrogram (Fig. 1A). The most dominant frequency (pseudo frequency [PF]) was colored in red in the spectrogram.
Concept of CWT analysis. A The basic concept of CWT analysis. During the analysis, the mother wavelet was transformed over time (t) to select the part of the signal to be analyzed using b then dilated or contracted using a. The scale was converted to a PF to use a scale variable and center frequency of the mother wavelet. x(t), the signal to be analyzed; Ψ(t), mother wavelet (Morlet wavelet in this study); a, scale variable; b, time shift variable. Δ, sampling period. B The bipolar EGMs recorded for 30 seconds were acquired and analyzed. The upper row shows the rectified raw wave signal. The middle row shows the CWT analysis result as a color spectrogram in which the dominant PF is colored in red. The lower row shows the PF time plot and its simple moving average plot. Finally, the CV was calculated from the mean PF and standard deviation of PF. C The left panel shows a PF with a high CV, which means temporally unstable. The right panel shows a PF with low CV, which means temporally stable. A PF with CV ≤ 10 was defined as a temporary stable PF (sPF) in this study. CWT, continuous wavelet transform; PF, pseudo-frequency; CV, coefficient of variation
We also created a dominant PF time plot and a simple moving average plot (Fig. 1B lower). The coefficient of variation (CV), the statistical value of dispersion, was then calculated using the standard deviation and the mean value of PF. A low CV at a given time length indicates less dispersion of the PF, which means the PF is temporally stable (right panel of Fig. 1C). On the contrary, a high CV indicates a large dispersion of the PF, which means the PF is unstable (left panel of Fig. 1C). Same as the previous study[10], the site with the highest PF and CV ≤ 10 was defined as a temporally stable PF (sPF) in this study, which could be considered the site driving AF. The detailed method of CWT analysis was described in the Supplemental.
2.3 EGM sampling and catheter ablation
Antiarrhythmic drugs were stopped at least five half-lives before the procedure. All PVs (left superior PV: LSPV, left inferior PV: LIPV, right superior PV: RSPV, and right inferior PV: RIPV) were mapped using a 20-pole close-bipolar (2-mm inter-electrode distance) ring catheter (15 mm, 17.5 mm, 20 mm) at respective ostia. If left or right common PV ostium (LCPV or RCPV) was present, a larger ring catheter (25 mm or 30 mm) was used if needed for better catheter contact. Local EGM during AF in the LA body was recorded using a high-density mapping catheter (Pentaray, Biosense Webster, Diamond Bar, CA). EGM was recorded using closer bipolar electrode pairs (2-mm inter-electrode space). The mapping sites of LA were the following eight sites: left atrial appendage (LAA) ostium (anterior part of the LAA ostium), anterior wall, high septum wall, low septum wall, roof, posterior wall, low posterior wall, and lateral wall (see sampling sites in Fig. 2). We tried to minimize any QRS signal in the EGM recording when mapping the area is close to the mitral annulus. Patients were divided into PV-dominant or LA-dominant groups according to the location of the highest sPF.
Summary of procedure: EGM sampling, PVI, and LA voltage map. Circles on LA images show the sites of EGM sampling. Green circles (1–4): LSPV, LIPV, RSPV, and RIPV. Red circles (1–8): LAA ostium, anterior, high septum, low septum, roof, posterior, low posterior, and lateral wall. CWT, continuous wavelet transform; LSPV and LIPV, left superior and inferior pulmonary vein; RSPV and RIPV, right superior and inferior pulmonary vein; LAA, left atrial appendage; LVA, low voltage area; DC, direct current; sPF, stable pseudo frequency
After EGM recording for CWT analysis, antral PVI was performed during AF using an open-irrigated radiofrequency (RF) ablation catheter with a contact force sensor and Carto 3 mapping system (Biosense Webster, Diamond Bar, CA). RF energy was applied in a point-by-point fashion with a temperature limit of 40 °C at 30–35 W power setting at each site. On the LA posterior wall close to the esophagus, the power was reduced to 20–25 W. The endpoint of the PVI was the disappearance of PV electrograms seen on a circular mapping catheter during AF. Then, samplings of EGMs were repeated at the same eight sites of LA as described above if AF continued after PVI. If AF converted to sinus rhythm spontaneously during PVI, post-PVI EGM sampling was aborted.
2.4 Left atrial voltage map
After post-PVI EGM sampling, patients were converted to sinus rhythm by direct current (DC) cardioversion. A high-density voltage map was created using a Pentaray catheter to discover any LVA in the LA body. Mapping was performed during pacing from the right atrial appendage (RAA), slightly faster than the sinus rhythm (450–500 ms). Data acquisition was automated using the distance from the premade anatomical shell of ≤ 5 mm to avoid poor contact with the Pentaray catheter.
Voltage criteria were defined as follows; Dense LVA as ≤ 0.5 mV, mild LVA as > 0.5 mv and ≤ 1.0 mV, and healthy myocardium as > 1.0 mv. These voltage criteria were derived from the previous report14, which is similar to our study. Patients were classified into three groups as follows: (1) healthy group. patients without LVA ≤ 1.0 mV in the LA body, (2) mild LVA group, patients with mild LVA (> 0.5 mv and ≤ 1.0 mV) but without dense LVA (≤ 0.5 mV) in the LA body, and (3) dense LVA group, patient with dense LVA (≤ 0.5 mV) in the LA body. Figure 2 schematically summarizes the entire procedure.
2.5 Patient follow-up
Antiarrhythmic medication was discontinued after ablation. In cases of symptomatic recurrence, antiarrhythmic medication was administered per the doctor’s decision only during the 3 months of the blanking period. Patients were followed up at 2 weeks, 1 month, and every 3 months. ECG and 24-h Holter monitor were performed every 3-month follow-up until 1 year and every 6 months. Any AF or atrial tachycardia (AT) lasting 30 seconds or more beyond 3 months was considered a recurrence. When recurrence was suspected from symptoms or pulse check, a 24-h Holter monitor was performed. Patients were followed up for at least 1 year and further followed up if possible.
2.6 Statistics and analysis
All analyses were performed using Stata 17.0 (StataCorp LLC). Normally distributed continuous variables were presented as mean ± SD. In cases of a non-normal distribution, a median (interquartile range) was given. Categorical variables were expressed as the number and percentage of patients. Changes between pre-PVI and post-PVI average PF were tested by paired t-test. AF/AT-free survival was estimated by the Kaplan–Meier method and compared between the PV-dominant group versus LA-dominant group. A univariate and multivariate logistic regression analysis was used to identify risk factors for AF recurrence. Results with a 2-sided P-value of < 0.05 were considered statistically significant.
3 Results
3.1 Patient characteristics: PV-dominant group vs. LA-dominant group
A total of 109 patients with persistent AF (81 male, 65 ± 10 years) were enrolled. A total of 120 points (40 from PVs and 80 from LA) were acquired in each patient (if common PV ostium was not present) from close-bipolar local EGMs during AF and processed for pre-PVI CWT analysis. The highest sPF was found inside PVs in 70 patients (PV-dominant group) and in the LA in 39 patients (LA-dominant group). Clinical characteristics comparing PV-dominant and LA-dominant group are shown in Table 1. PV-dominant group exhibited a higher average frequency of PVs than the LA-dominant group in all PVs (LSPV; 6.75 ± 0.56 Hz vs. 6.26 ± 0.49 Hz, LIPV; 6.51 ± 0.64 Hz vs. 6.17 ± 0.54 Hz, RSPV; 6.57 ± 0.56 Hz vs. 6.32 ± 0.44, RIPV; 6.66 ± 0.59 Hz vs. 6.3 ± 0.45 Hz, P < 0.01 for all sites. A left common PV ostium was classified as LSPV, and a right common PV ostium was classified as RSPV).
All PVs were successfully isolated during AF after pre-PVI CWT analysis. Three patients (all from PV-dominant group) had a spontaneous conversion to sinus rhythm during PVI, and the post-PVI CWT analysis was aborted. The other patients successfully converted to sinus rhythm by DC cardioversion after post-PVI CWT analysis. No PV-LA reconnection was observed after DC cardioversion.
3.2 The site of high-frequency activation
Figure 3A describes the location of the highest sPF of both groups. The common site in PV-dominant group was left side PVs (LCPV, LSPV, and LIPV). In LA-dominant group, LAA ostium, high septum, and LA roof exhibited the highest sPF. Notably, only two patients in LA-dominant group exhibited the highest sPF at the LA posterior wall, where we often perform empirical ablation with PVI.
CWT analysis: Sites of highest sPF pre-PVI. A Location of highest sPF in the PV-dominant group and the LA-dominant group. B The number of PV-dominant and LA-dominant by voltage criteria. LSPV and LIPV, left superior and inferior pulmonary vein; RSPV and RIPV, right superior and inferior pulmonary vein; LAA, left atrial appendage; LCPV, left common PV; RCPV, right common PV; PLSVC, persistent left superior vena cava; LVA, low voltage area; sPF, stable pseudo frequency
3.3 LVA classification by LA voltage map
Patients were classified into three groups from the voltage map of LA after cardioversion (mean mapping points 1724 ± 895) as follows; Healthy (n = 45), Mild LVA (n = 44), and Dense LVA (n = 20). As shown in Fig. 3B, the Healthy group predominantly consisted of the PV-dominant group (40 of 45 patients) compared with Mild LVA and Dense LVA groups (P < 0.01).
3.4 Changes of frequency in the LA by PVI: PV-dominant group vs. LA-dominant group
This analysis excluded three patients (all in the PV-dominant group) with AF termination during PVI. Figure 4 shows the average frequency during AF at each mapping site and the entire LA for both pre-PVI (blue bar) and post-PVI (orange bar), which is also summarized in Table 2. The average frequency of each mapping site and the average frequency of the whole LA were higher in PV-dominant group than LA-dominant group. The high septum, roof, and posterior wall showed a high frequency before PVI in PV-dominant group. However, the frequency at the sites significantly decreased after PVI (6.72 ± 0.27 to 6.57 ± 0.35 Hz, 6.73 ± 0.34 to 6.50 ± 0.41 Hz, and 6.70 ± 0.35 to 6.52 ± 0.39 Hz, respectively P < 0.01). These sites showed a significant susceptibility to PVI. As a result, the average frequency of the whole LA decreased (6.55 ± 0.27 to 6.43 ± 0.37 Hz, P < 0.01) (Table 2, Fig. 4A). On the other hand, in LA-dominant group, the roof was the only location that showed a significant decrease in frequency after PVI. The average frequency of the whole LA did not change after PVI (Table 2, Fig, 4B).
Comparison of average frequency; pre-PVI vs. post-PVI. Comparison of average frequency (pre-PVI vs. post-PVI) at each site of LA and whole LA. A PV-dominant group. B LA-dominant group. Blue bar: pre-PVI. Orange bar: post-PVI. LA, left atrium; PVI, pulmonary vein isolation; LAA, left atrial appendage
3.5 AF/AT-free survival after a single ablation procedure
Patients were followed up for 23.7 ± 8.4 months after ablation, and 22 patients had an AF recurrence during the follow-up. Figure 5A shows a Kaplan–Meier plot depicting the AF/AT-free survival comparing PV-dominant group and LA-dominant group after a single ablation. AF/AT-free survival at 1 year was 92.9% for PV-dominant group and 79.5% for LA-dominant group, respectively (P = 0.002, PV-dominant vs. LA-dominant). At 2 years of follow-up, the survival rate decreased to 87.1% in PV-dominant group and 64.3% in LA-dominant group (P = 0.002, PV-dominant vs. LA-dominant).
Kaplan–Meier survival analysis of 2-year follow-up: PV-dominant vs. LA-dominant. A Kaplan–Meier plot depicting the AF/AT free survival comparing PV-dominant group (red line) and LA-dominant group (blue line). B AF/AT recurrence in each voltage group comparing PV-dominant vs. LA-dominant. PV, pulmonary vein; LA, left atrium; AF, atrial fibrillation; AT, atrial tachycardia; LVA, low voltage area
A recurrence of AF/AT was recognized in 4 patients (3 AF and one AT) in the healthy group (n = 45), 8 patients (7 AF and one AT) in the mild LVA group (n = 44), and 10 patients (9 AF and one AT) in the dense LVA group (n = 20) after 23.7 ± 8.4 months of follow-up.
The overall AF recurrence in each voltage group was 4/45 (8.9%) in the healthy group, 8/44 (18.2%) in the mild LVA group, and 10/20 (50%) in the dense LVA group in this study. However, if patients have PV-dominant sPF, the AF recurrence was significantly less [3/40 (7.5%) in the healthy group, 2/21 (9.5%) in the mild LVA group, and 2/9 (22.2%) in the dense LVA group] compared with patients with LA-dominant sPF [1/5 (20%) in the healthy group, 6/23 (26.1%) in the mild LVA group, and 8/11 (72.7%) in the dense LVA group. The AF recurrence rate over 2 years after a single ablation was well stratified by adding the information on the location of sPF over the degree of LVA (Fig. 5B).
3.6 Univariate and multivariate logistic regression analysis for predictors of AF recurrence
The univariate and the multivariate logistic regression analyses were used to determine predictors of AF recurrence (Table 3). AF duration, degree of LVA (healthy, mild, and dense), and LA-dominant sPF were selected for multivariate analysis among the variables listed. By multivariate logistic regression analysis, LA-dominant sPF was determined as the strongest predictor of AF recurrence (HR 3.409, 95% CI 1.156–10.714, p = 0.026).
3.7 Findings during redo procedure; recurrence of PV-LA connection
Of 22 patients with AF recurrence, 5 patients in the PV-dominant group and 4 patients in the LA-dominant group underwent a redo procedure. The recurrence of PV-LA conduction was observed in 4 patients in the PV-dominant group and one patient in the LA-dominant group.
3.8 Case example 1: a patient in PV-dominant group with a healthy LA myocardium
Supplemental Fig. 1 shows a case example of a patient in the PV-dominant group with a healthy LA myocardium. A stable and high-frequent activity was noted inside the LSPV, which showed the highest sPF (7.5 Hz, CV 9.33) (Supplemental Fig. 1A). After PVI, the average frequency decreased significantly in all LA sites and the whole LA frequency (from 6.84 to 6.65 Hz) (Supplemental Fig. 1B). This patient had a healthy LA myocardium (Supplemental Fig. 1C). No recurrence of AF was observed during 26 months of follow-up without antiarrhythmic medication.
3.9 Case example 2: patient in LA-dominant group with mild LVA
Supplemental Fig. 2 shows a case example of a patient in the LA-dominant group with a mild LVA in the LA. The highest sPF was located at the anterior part of the LAA ostium by CWT analysis (6.4 Hz, CV 8.5) (Supplemental Fig. 2A), where mild LVA (> 0.5mv and ≤ 1.0 mV) was present (Supplemental Fig. 2B). The highest sPF in PVs (LSPV, PF 6.12 Hz, and CV 5.29) was lower than the sPF at LAA ostium. The average frequency of the whole LA did not significantly decrease after PVI (from 6.32 to 6.29 Hz). The highest sPF at the base of LAA was co-localized at the area of mild LVA, where no LVA was noted below 0.5 mv but was noted at 0.5–1.0 mV. The patient had an AF recurrence 4 months after the procedure.
4 Discussion
4.1 Major findings from this study
Major findings from this study are summarized as follow: (1) By pre-PVI analysis, the average frequency of each site of LA as well as the average frequency of whole LA- in the PV-dominant group was higher than in the LA-dominant group (Fig. 4). (2) High septum, roof, and posterior wall were high sPF sites by pre-PVI analysis in the PV-dominant group. However, the frequency at these sites (including LA posterior wall) decreased significantly after PVI (Fig. 4A). (3) In the LA-dominant group, only two patients showed the highest sPF at LA posterior wall, where empirical isolation is often performed (Fig. 3). The average frequency in the whole LA did not decrease by PVI in the LA-dominant group (Fig. 4B). (4) Patients in the PV-dominant group had significantly fewer AF/AT recurrences than in the LA-dominant group. The long-term AF-free survival was well stratified and predicted by combining the information from the CWT analysis and the degree of LVA.
4.2 The gradient of frequency in the LA and a different impact of PVI: PV-dominant vs. LA-dominant
Spatiotemporal organization of the LA frequency after circumferential PVI has been well documented in a previous study using dominant frequency (DF) mapping[15]. However, the frequency change in the LA after PVI depending on the degree of PV activity has not been described well. This study showed the impact of PV activity on the change of LA frequency after PVI by comparing the difference between PV-dominant and LA-dominant group. It is essential to evaluate the degree of PV activity to predict the effect of PVI when ablating persistent AF.
We found a particular frequency gradient in the LA before PVI, especially in the PV-dominant group (Fig. 4A, Table 2). The high septum, the roof, and the posterior wall demonstrated a high-frequency activity, similar to what Kalifa et al. reported previously[16]. These regions were the most susceptible area to PVI, and the high frequency in these regions disappeared after PVI only in PV-dominant group. The proximity to PVs and the susceptibility to PVI of these regions (high septum, roof, and posterior wall) suggest that driver domains were inside PVs in the PV-dominant group. The region mentioned above (high septum, roof, and posterior wall) in the LA-dominant group (Fig. 4B, Table 2) did not show a high frequency before PVI compared with the PV-dominant group. This suggested the low PV activity and the existence of slow conduction areas in the LA in the LA-dominant group.
4.3 Termination of AF during PVI
AF termination during or upon PVI could be well recognized. In this present study, AF terminated in only 3 patients in the PV-dominant group. However, studies have failed to find significant differences in terms of long-term AF-free survival between patients with or without AF termination during PVI[17]. The important observation showing the PV dominance might only be the significant decrease of the global frequency in the LA after PVI, which was well recognized in this present study.
4.4 The importance of mild LVA
The presence of dense LVA (≤ 0.5 mV) in the LA strongly predicts AF recurrence after circumferential PVI[18]. Recently, mid-range LVA (so-called mild LVA in this study) has been found as a factor of AF recurrence (voltage setting varies between studies, 0.4–1.3 mV, or 0.5–1.1 mV) [3, 14]. In this present study, over half of the mild LVA group (23 of 44) consisted of the LA-dominant group, which was significantly larger than the healthy group (5 of 45, P < 0.01 vs. mild LVA). Also, the recurrence rate of AF after PVI was worse than in the healthy group, as shown in Fig. 5B, meaning that some high-frequency domain or substrate exists in the LA in the mild LVA group more than in the healthy group. Supplemental Fig. 2 demonstrates an example of the site of highest frequency within the mild LVA located at the anterior part of the LAA ostium.
4.5 Factors related to AF recurrence: combining CWT analysis and LA voltage map
Based on the multivariate logistic regression analysis, LA-dominant sPF was the only independent predictor of AF recurrence (HR 3.409, 95% CI 1.156–10.714, p = 0.026), while the degree of LVA was not (Table 3).
However, as previously reported in many articles, the degree of LVA is significantly related to AF recurrence after PVI. Considering the limitations of multivariate logistic regression analysis, we combined the information on the location of sPF from CWT analysis and the degree of LVA. By combining the two factors (PV/LA dominance and the residual LA substrate after PVI), the prediction of AF recurrence became more precise, as shown in Fig. 5B.
4.6 Passive activation noted at LA posterior wall
In the PV-dominant group, the average frequency was significantly high at the posterior wall before PVI. As Kalifa et al. mentioned[16], the high-frequency activity at the posterior wall during AF is the rationale for the empirical isolation of the LA posterior wall. However, there was a significant decrease in frequency at the posterior wall after PVI, which suggested the passive activation from PVs in the PV-dominant group. Only two patients in the LA-dominant group exhibited the highest sPF at the posterior wall.
These findings suggested that the posterior wall might be passively activating. This might be why recent studies failed to show the impact of posterior wall isolation[2, 19,20,21]. However, we could not deny the effect of posterior wall isolation completely from these findings since there might be a possible effect in reducing the excitable atrial myocardium by isolating the posterior wall. The effect of posterior wall isolation still needs further investigations by large, randomized control studies.
4.7 Clinical implication
CWT analysis was feasible in evaluating the contribution of PV activity in maintaining AF. We could reasonably stratify the 2-year outcome after PVI by two factors, the site of highest sPF and the degree of LVA, as shown in Fig. 5B. We may avoid unnecessary additional ablation beyond PVI from this 2-year result. For example, a patient who shows PV dominance with healthy LA myocardium does not need additional ablation with PVI, given the significantly low AF recurrence rate at 2 years.
4.8 Study limitations
We acknowledge the following limitations of this study: (1) This is an observational study investigating the feasibility of predicting and stratifying the long-term outcome after PVI by combining CWT analysis and an LA voltage map. Therefore, we did not evaluate the effect of ablation at the site of high-frequency activity in the LA body in this study. Further studies are needed targeting the high-frequency sites in the LA beyond PVI. (2) Frequency may change after 30 s of each sampling time. However, for practical reasons and according to the previous study[10], we limited the sampling time to 30 s. (3) The intensity of rhythm monitoring post-procedure is not as intense as in other recent studies (e.g., implanted loop recorders, 14 days Holter monitor), though this meets current Heart Rhythm Society recommendations.
5 Conclusion
PV activity analyzed by the CWT analysis significantly impacted the fibrillatory activity of LA after PVI and the outcome after PVI. The long-term result could be well predicted and stratified by combining the information from the CWT analysis and the LA voltage map, by precisely recognizing PV dominance using CWT analysis and recognizing the residual substrate in the LA by voltage map. The activation at the posterior wall might be passive in persistent AF.
Abbreviations
- PV:
-
Pulmonary vein
- PVI:
-
Pulmonary vein isolation
- AF:
-
Atrial fibrillation
- LA:
-
Left atrium
- LVA:
-
Low voltage area
- FFT:
-
Fast Fourier Transform
- CWT:
-
Continuous wavelet transform
- EGM:
-
Electrogram
- PF:
-
Pseudo frequency
- CV:
-
Coefficient of variation
- sPF:
-
Stable pseudo frequency
- LSPV:
-
Left superior pulmonary vein
- LIPV:
-
Left inferior pulmonary vein
- RSPV:
-
Right superior pulmonary vein
- RIPV:
-
Right inferior pulmonary vein
- LCPV:
-
Left common pulmonary vein
- RCPV:
-
Right common pulmonary vein
- LAA:
-
Left atrial appendage
- DC:
-
Direct current
- RAA:
-
Right atrial appendage
- CTI:
-
Cavo-tricuspid isthmus
- AT:
-
Atrial tachycardia
References
Ouyang F, Bansch D, Ernst S, Schaumann A, Hachiya H, Chen M, Chun J, Falk P, Khanedani A, Antz M, Kuck KH. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–6.
Thiyagarajah A, Kadhim K, Lau DH, Emami M, Linz D, Khokhar K, Munawar DA, Mishima R, Malik V, O’Shea C, Mahajan R, Sanders P. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2019;12: e007005.
Yang B, Jiang C, Lin Y, Yang G, Chu H, Cai H, Lu F, Zhan X, Xu J, Wang X, Ching CK, Singh B, Kim YH, Chen M and Investigators* S-S. STABLE-SR (electrophysiological substrate ablation in the left atrium during sinus rhythm) for the treatment of nonparoxysmal atrial fibrillation: a prospective, multicenter randomized clinical trial. Circ Arrhythm Electrophysiol. 2017;10.
Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, Hindricks G, Piorkowski C. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–33.
Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–62.
Seitz J, Bars C, Theodore G, Beurtheret S, Lellouche N, Bremondy M, Ferracci A, Faure J, Penaranda G, Yamazaki M, Avula UM, Curel L, Siame S, Berenfeld O, Pisapia A, Kalifa J. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient-tailored approach. J Am Coll Cardiol. 2017;69:303–21.
Hansen BJ, Zhao J, Li N, Zolotarev A, Zakharkin S, Wang Y, Atwal J, Kalyanasundaram A, Abudulwahed SH, Helfrich KM, Bratasz A, Powell KA, Whitson B, Mohler PJ, Janssen PML, Simonetti OP, Hummel JD, Fedorov VV. Human atrial fibrillation drivers resolved with integrated functional and structural imaging to benefit clinical mapping. JACC Clin Electrophysiol. 2018;4:1501–15.
Higuchi K, Cates J, Gardner G, Morris A, Burgon NS, Akoum N, Marrouche NF. The spatial distribution of late gadolinium enhancement of left atrial magnetic resonance imaging in patients with atrial fibrillation. JACC Clin Electrophysiol. 2018;4:49–58.
Kumagai K, Minami K, Sugai Y, Sumiyoshi T, Komaru T. Effect of ablation at high-dominant frequency sites overlapping with low-voltage areas after pulmonary vein isolation of nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2019;30:1850–9.
Kimata A, Yokoyama Y, Aita S, Nakamura H, Higuchi K, Tanaka Y, Nogami A, Hirao K, Aonuma K. Temporally stable frequency mapping using continuous wavelet transform analysis in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:514–22.
Higuchi K, Iwai S, Yokoyama Y, Hirao K. Persistent left superior vena cava as a perpetuator of atrial fibrillation: frequency analysis using continuous wavelet transform analysis. J Cardiovasc Electrophysiol. 2019;30:1701–5.
Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97.
Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–98.
Yagishita A, Sparano D, Cakulev I, Gimbel JR, Phelan T, Mustafa H, De Oliveira S, Mackall J, Arruda M. Identification and electrophysiological characterization of early left atrial structural remodeling as a predictor for atrial fibrillation recurrence after pulmonary vein isolation. J Cardiovasc Electrophysiol. 2017;28:642–50.
Lin YJ, Tai CT, Kao T, Chang SL, Lo LW, Tuan TC, Udyavar AR, Wongcharoen W, Hu YF, Tso HW, Tsai WC, Chang CJ, Ueng KC, Higa S, Chen SA. Spatiotemporal organization of the left atrial substrate after circumferential pulmonary vein isolation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:233–41.
Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–33.
Singh SM, d’Avila A, Kim YH, Aryana A, Mangrum JM, Michaud GF, Dukkipati SR, Barrett CD, Heist EK, Parides MK, Thorpe KE, Reddy VY. Termination of persistent atrial fibrillation during pulmonary vein isolation: insight from the MAGIC-AF trial. Europace. 2017;19:1657–63.
Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–92.
Naga Venkata K. Pothineni AL, David S. Frankel, Gregory E. Supple, Fermin C. Garcia, David Lin, Matthew C. Hyman, Ramanan Kumareswaran, Jeffrey Arkles, Michael Riley, Rajat Deo, Andrew E. Epstein, Saman Nazarian, Robert D. Schaller, David J. Callans, Francis E. Marchlinski, Pasquale Santangeli, Sanjay Dixit,. Impact of left atrial posterior wall isolation on arrhythmia outcomes in patients withatrial fibrillation undergoing repeat ablation. Heart Rhythm O2. 2021.
Lee JM, Shim J, Park J, Yu HT, Kim TH, Park JK, Uhm JS, Kim JB, Joung B, Lee MH, Kim YH, Pak HN, Investigators P-A. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5:1253–61.
Pak HN, Park J, Park JW, Yang SY, Yu HT, Kim TH, Uhm JS, Choi JI, Joung B, Lee MH, Kim YH, Shim J. Electrical posterior box isolation in persistent atrial fibrillation changed to paroxysmal atrial fibrillation: A Multicenter, Prospective Randomized Study. Circ Arrhythm Electrophysiol. 2020;13: e008531.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by ethical committee of Hiratsuka Kyosai Hospital.
Conflict of interest
The software for CWT analysis was provided by Nihon Kohden Medical Company. Dr. Higuchi received lecture honoraria from Biosense Webster, Abbott. Inc., Medtronic, and Biotronik.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Higuchi, K., Iwai, S., Kato, N. et al. The utility of combining continuous wavelet transform analysis and high-density voltage map in predicting the long-term outcomes after ablation of persistent atrial fibrillation. J Interv Card Electrophysiol 66, 405–416 (2023). https://doi.org/10.1007/s10840-022-01337-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01337-3