Abstract
Atrial fibrillation (AF) is the most common arrhythmia in routine clinical practice. Despite many years of research, its mechanisms still are not well understood, thus reducing the effectiveness of AF treatments. Nowadays, pulmonary vein isolation by catheter ablation is the treatment of choice for AF resistant either to pharmacological or electrical cardioversion. However, given that long-term recurrences are common, an appropriate patient selection before the procedure is of paramount relevance in the improvement of AF catheter ablation outcome. The present work studies how several spectral features of the atrial activity (AA) from a single lead of the surface electrocardiogram (ECG) can become potential pre-ablation predictors of long-term (>2 months) sinus rhythm maintenance. Among all the analyzed spectral features, results indicated that the most significant single predictor of paroxysmal AF ablation treatment outcome was related to the amplitude of the first harmonic of the dominant frequency, providing sensitivity (Se), specificity (Sp) and accuracy (Ac) values of 90%, 42.86 and 77.78%, respectively. On the other hand, the AA harmonic structure was the most significant single predictor for persistent AF, with Se, Sp and Ac values of 100%, 54.55 and 77.27%, respectively. A logistic regression analysis, mainly based on spectral amplitudes as well as on the harmonic structure of the AA, provided a higher predictive ability both for paroxysmal AF (Se = 100%, Sp = 57.14% and Ac = 88.89%) and persistent AF (Se = 90.91%, Sp = 72.73 and Ac = 81.82%). In conclusion, the study of key AA spectral features from the surface ECG can provide a significant preoperative prognosis of AF catheter ablation outcome at long-term follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common clinically relevant heart rhythm disorder, with a current prevalence in the developed world about 12% in the elderly.33 Over 6 million people in Europe and 3 million people in the USA suffer currently from this arrhythmia.60 It is also expected that its prevalence will double in the next 50 years as the population ages.22 AF-related symptoms and morbidity are, moreover, responsible for frequent visits to the physician and hospitalizations leading to substantial and rising costs.52 At least 15% of the healthcare budget in cardiac diseases is spent on the management of AF.7 Moreover, this arrhythmia is associated with a five-fold risk of stroke and a three-fold incidence of congestive heart failure, provoking that AF patients have twice the risk of death than a healthy person of the same age.62 These facts turn AF as a major health challenge in the developed world, where its medical, social and economic aspects are all set to worsen in the forthcoming decades.60
AF has been traditionally described in terms of the duration of its episodes.33 Paroxysmal AF is often the first manifestation of the arrhythmia, with episodes that terminate spontaneously or without intervention in less than 1 week. In persistent AF, the episodes last for more than 7 days and intervention is required for its termination. If the arrhythmia persists for more than a year, then it is named longstanding persistent AF. Finally, the term permanent AF is used when patient and clinician make a joint decision to stop more attempts of sinus rhythm (SR) restoration and/or maintenance.33 Characterization of AF patients by the duration of their episodes is of clinical relevance because outcomes of therapy, such as catheter ablation, are better for paroxysmal AF than for persistent AF.5,10
This arrhythmia leads to persistent changes in the atrial structure and function,43 which promotes its perpetuation.25 As a consequence, early restoration of SR in patients with paroxysmal or persistent AF is a therapeutic goal. The first step in the rhythm control strategy is generally pharmacological and/or electrical cardioversion.48,50 When these therapies are unsuccessful or not recommended, alternative approaches such as radio-frequency catheter ablation (CA) of pulmonary veins63 as well as the surgical treatment of AF in patients requiring open-heart surgery are used.13 In fact, CA is nowadays considered the first-line strategy for the treatment of AF,45 where the use of a standardized anatomical approach of circumferential or antral pulmonary vein isolation (PVI) is the most common procedure.10,63 However, this strategy is only focused on thoracic vein arrhythmogenicity, thus being more effective in paroxysmal AF than in persistent AF.5,45 Therefore, CA of persistent AF has traditionally required extended procedures with additional linear lesions along the left atrial roof, mitral annulus and other atrial structures24,46 or either targeting complex fractionated atrial electrograms (CFAEs), defined as fractionated short cycle length and low-amplitude atrial signals,41 or mapping and ablating localized reentrant sources driving AF.42
The success rates of CA have improved dramatically over the past 15 years, now achieving values of 80% and above.19 Nevertheless, AF recurrence is still as high as 50%, specially in persistent AF patients, thus requiring redo procedures with similar uncertain result.23 Furthermore, AF ablation is one of the most complex interventional procedures with important associated risks.45 Recent studies estimating predictors of inpatient complications and 30-day readmissions reported that 5% of patients had periprocedural complications, and almost 10% were rehospitalized within 30 days.51 These complications resulted in a total mortality rate of 0.24%, which is quite high if one considers that AF itself is not a life-threatening arrhythmia.63 Taking into consideration the significant long-term AF recurrence after CA as well as its associated risks, a proper selection of patients undergoing the procedure would be exceptionally useful for the physicians.
The development of methods based on analyzing the atrial activity (AA) frequency content for the long-term prediction of CA outcome has been addressed by several previous works. Regarding invasive methods, they have been mainly based on computing the dominant frequency (DF) of atrial electrograms or its inverse, named as the atrial fibrillation cycle length (AFCL). Recent studies have reported that the mean DF or AFCL and their decreases after PVI, obtained from different atrial structures like the left atrial appendage or the coronary sinus (CS), are promising predictors of long-term SR maintenance after CA.18,66 Moreover, the existence of a left to right atrial gradient in the DF has also been reported as a significant predictor of CA outcome in patients with persistent AF.4 However, the main drawback of these methods relies on their requirement of invasive recordings, which can only be accessed at the time of the procedure. Obviously, this would imply an unnecessary risk for those patients in whom ablation was finally unsuccessful.19,38
In contrast to these studies, the present work addresses the analysis of key AA spectral features obtained from the surface lead \(V_1\) to predict long-term ablation outcome. Within this context, although previous studies reported that the DF obtained from surface recordings has been unpowered to predict ablation outcome,20,49 many other works have suggested its prediction capabilities.38,64 Hence, the main goal of this work is to complement DF analysis with other interesting AA spectral features, which are here analyzed for the first time in the context of CA. More precisely, this work presents the first application studying the harmonic structure of AF from a single surface lead able to provide a significant prediction ability of CA outcome in patients with paroxysmal as well as with persistent AF.
Materials and Methods
Study Population
The subjects of this study were drug-refractory 27 paroxysmal and 22 persistent AF patients who underwent first-time radio-frequency CA. Self-terminating AF episodes lasting <48 h were defined as paroxysmal, whereas sustained AF lasting >1 week before the procedure or requiring external cardioversion to restore SR was defined as persistent AF. The clinical baseline characteristics of these patients are shown in Table 1.
Ablation Protocol
Before the study was performed, the protocol was approved by the Institution Research and Ethics Committee and all the patients gave informed written consent. All antiarrhythmic drug therapy except amiodarone was withheld >5 half lives before the study. After the appropriate catheters were in place and sustained AF was induced in paroxysmal AF patients, 3D reconstruction of the atrial chambers and real-time DF determination were obtained by using a CARTO\(^{\copyright }\) navigation system. Circumferential PVI was firstly performed in all the patients. Thereafter, sites showing DFs that were at least 20% higher than their surrounding points were identified as points of maximal DF and those with the highest frequency were targeted by creating a circumferential set of lesions. The procedure continued by ablating the following points of maximal DF, as described by Atienza et al.4 Whenever AF terminated during ablation, reinduction was attempted. AF was considered inducible when persisted for >10 min. If AF was reinduced, ablation was continued until AF became noinducible or until all the identified atrial points of maximal DF were ablated. In case of AF was still present, SR was restored by electrical cardioversion.
All the patients were followed-up 3 months after the ablation procedure and every 3–6 months thereafter. A blanking period of 2 months was considered as appropriate to define recurrences of AF. Procedural success was defined as a lack of AF recurrence in the absence of antiarrhythmic medication. After a mean of 12 ± 7 months of follow-up, 20 (74%) paroxysmal and 11 (50%) persistent AF patients were free-from-AF.
Data Analysis and Preprocessing
In addition to internal bipolar electrograms located at the right atrial appendage, the distal coronary sinus and the pulmonary veins left atrial junctions, a standard 12-lead electrocardiogram (ECG) was continuously recorded on a CardioLab\(^{\copyright }\) system during the entire ablation procedure with a sampling rate of 977 Hz and 16-bit resolution. Lead \(V_1\) was selected for the analysis because it provided the highest AA amplitude from the surface ECG.47 Hence, a 10 s-length segment preceding the procedure was analyzed for each patient. The ECG segments were preprocessed to remove typical interferences by using a forward/backward filtering strategy. Hence, a high-pass filter of 0.5 Hz cut-off frequency was applied to remove base-line wander, next a low-pass filter of 70 Hz cut-off frequency reduced high frequency muscle noise and, finally, adaptive notch filtering at 50 Hz removed powerline interference.54
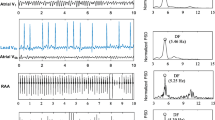
On the other hand, reliable AA analysis requires that the ventricular activity has been first cancelled.8 Although a variety of different techniques exist for this purpose, an adaptive QRST cancelation method was used to remove the ventricular activity and then get the AA signal represented by the fibrillatory (f) waves.1 This method avoids spikes caused by discontinuities between QRST complexes and the subsequent TQ intervals by using an improved approach different from traditional low-pass filtering. It is based on two steps1: (i) synthesizing a ventricular cancellation template that minimizes differences in the edges with resect to the QRST complex under cancellation and (ii) reducing the remaining residual differences by Gaussian fitting. As an example, Fig. 1 shows the AA signals obtained from the same ECG interval both with and without this softening approach of sudden transitions.
Example of an ECG ready for QRST cancellation (a) together with its corresponding AA signals obtained by considering a softening approach of discontinuities between QRST complexes and subsequent TQ intervals1 (b) and by traditional low-pass filtering (c). Note the presence of larger spikes and wave distortion in the latter case.
Spectral Characterization of the Atrial Activity
With the aim to characterize the surface AA in the frequency domain after QRST cancellation, the power spectral density (PSD) was obtained using the Welch Periodogram.59 A Hamming window of 4096 points in length, a 50% overlapping between adjacent windowed sections and a 10,240-points Fast Fourier Transform (FFT) were used as computational parameters,2 thus providing a spectral resolution of 0.1 Hz. Although some authors have considered 3–12 Hz as the typical AA spectral range,40,56 other ones have reported that DF’s higher than 9Hz are unusual.12,55,61 Thus, the AA PSD was normalized by the power contained within this last frequency range in order to reduce the effect of spectral content unrelated to f waves. As a consequence, the AA PSD was defined as
The frequency with the highest amplitude within the 3–9 Hz range was then selected as the DF, referred to as\(f_0\). Furthermore, visual inspection avoided mistaken selection of DF’s caused by the presence of harmonics. For a more accurate spectral analysis, further parameters related to \(f_0\) were also computed. Thus, its first harmonic \(f_1\) together with the PSD values for both frequencies, i.e., \(P_{AA}(f_0)\) and \(P_{AA}(f_1)\), were considered in the study. It should be noted that \(P_{AA}(f_0)\) has previously proven to be able to discern among paroxysmal and longstanding persistent atrial electrograms recorded on the pulmonary veins.12 On the other hand, given that previous works have considered that the locations of the DF harmonics may differ slightly from the expected ones, i.e., \((m+1)\cdot f_0\) for the m-th harmonic,56 the frequency \(f_1\) was searched within a 2 Hz window centered on \(2f_0\).
Other two features taken into account were the 3 dB bandwidth of the DF and its power. The DF bandwidth was defined as \(b_0=f_h-f_l\), where \(f_h\) and \(f_l\) were the closest frequency values to \(f_0\) fulfilling the sequel conditions
The power of \(b_0\) was computed as:
Previous works have suggested that the presence of harmonics in \(f_0\), quantified by an index named harmonic exponential decay, could contain useful electrophysiological information related to AF organization.27,57 Thereby, in this work, the logarithmic ratio between the amplitude of \(f_0\) and its first harmonic, i.e.,
was also included in the study.
Finally, a simple index summarizing the whole spectral content of the PSD is the median frequency, referred to as \(f_m\). This index is defined as the frequency that contains 50% of the whole PSD.29 Having the typical AA frequency band in mind, the median frequency considered in the present study was estimated from the condition
As a graphical summary, Fig. 2 displays a typical ECG interval in AF together with the extracted AA and the computed spectral features.
Statistical Analysis and Classification of Patients
Continuous variables are reported as mean ± standard deviation (Std.). Both Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess normality of distributions, whereas homocedasticity was verified with Levene’s test. All the parameters met equal variance assumption, but only \(f_0\), \(f_1\), \(\gamma \) and \(f_m\) provided a normal distribution. Thus, for these parameters, a t-Student’s test was used to determine whether there was any statistical significant difference between patients with and without AF recurrence after catheter ablation. In addition, a non-parametric Kolmogorov-Smirnov test was used for the remaining non-normal metrics. A two-tailed value of \(p<0.05\) was considered as statistically significant.
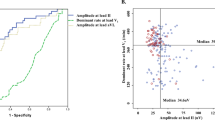
The value of each single parameter providing maximum discrimination between groups of patients, that is, the optimum threshold, was obtained by means of a receiver operating characteristic (ROC) curve.37 The ROC is a graphical representation of the tradeoffs between sensitivity and specificity. Sensitivity was considered as the proportion of patients free-from-AF at the follow-up end, whereas specificity was the percentage of patients in whom AF relapsed. The total rate of patients precisely classified was referred to as diagnostic accuracy. The value providing the highest accuracy was selected as optimum threshold. The area under the ROC curve (AUC) was also computed as a straightforward summary of classification performance. Thus, a perfect discrimination would yield an AUC of 1, while the worst possible classification would provide an AUC of 0.5.
Due to the lack of normality for a considerable number of variables and given that a Doornik-Hansen Omnibus multivariate normality test also showed a deviation from normality, a logistic regression (LR) analysis was performed with the objective of improving the classification of patients with and without AF recurrence after the follow-up end. This analysis mainly obtains a function that assigns a feature vector \(\mathbf {x}=[x_1,\ldots ,x_d]\) to one of c possible categories \(\{w_1,\ldots ,w_c\}\).6,28 Considering that input patterns in the present study must be classified into one of two mutually excluding categories, the probability density for w can be modeled by a Bernoulli distribution.35 Thus, if \(\alpha \) represents the a posteriori probability for class \(w_1\), \(1-\alpha \) is the probability for \(w_2\). Within this context, \(\alpha \) is enough for classification, because the classifier could assign an input vector to the class with the highest a posteriori probability. However, optimal classification was here obtained by determining the threshold \(\alpha \) making use of a ROC curve. In all LR models, \(\alpha \) was assumed to be depending on a linear combination of several inputs, the dependence taking the following form35:
which is called logistic function.34 The maximum likelihood criterion was used to optimize the components of vector \(\mathbf {\beta }=[\beta _0,\ldots ,\beta _d]\) from the original data.34,35 Moreover, single variables with \(p<0.2\) were included in the LR model or removed in a stepwise manner, such as in Yoshida et al.64 Thus, at each step, the hypothesis that the entered or eliminated term significantly changes the prediction is tested from the logarithmic ratio of the current likelihood function value vs. the previous one. When the relative improvement of the logarithmic likelihood is negligibly small, the algorithm deems the solution attained. In this way, the LR model reaches optimal performance.
Finally, given that the number of analyzed patients was notably limited for a statistical validation approach with separated learning and test sets, a leave-one-out cross-validation strategy was applied to the classification of patients with each single parameter and with the obtained LR models. In this approach, the index under study is validated by the same number of iterations as available patients. Thus, in each iteration all the data except one are used for training and the model is tested on the remaining single observation. In this way, unbiased and generalized estimates of the metric’s discriminant ability are obtained.36
Results
Mean and Std. values of the analyzed parameters before catheter ablation are shown in Tables 2 and 3 for paroxysmal and persistent AF, respectively. As can be observed in Table 2 for paroxysmal AF, none of the single parameters provided statistically significant differences between patients with and without AF recurrence. Although accuracy values higher than 70% were observed for some single features, such as \(P_{AA}(f_1)\) and \(P_{AA}(b_0)\), the AUC reported poorer classification performance. Moreover, in these cases a notable unbalance between sensitivity and specificity was noticed. Thus, specificity values lower than 60% were obtained for that cases. Also, less than 70% of the cross-validated grouped cases were correctly classified for all the parameters. On the contrary, a LR model based on three pre-ablation features, such as
achieved a significant prediction of the ablation result before it is attempted. In this case, accuracy was 88.89%, although specificity was also reduced in comparison with sensitivity, its value being lower than 60%, such as Table 2 shows.
Regarding persistent AF, \(\gamma \) was the only significant single predictor of the long-term ablation outcome (\(p=0.031\)), see Table 3. For this spectral feature, a considerably high accuracy of 77.27% was noticed, but sensitivity (100%) and specificity (54.55%) were notably unbalanced, such as in paroxysmal AF. A slightly higher discriminant power was achieved by the following LR model based on the baseline values of \(f_1\) and \(f_m\):
Thus, with this model, an accuracy of 81.82% was observed, being more balanced sensitivity (90.91%) and specificity (72.73%) than for \(\gamma \). It should also be noted that more than 75% of the cross-validated grouped cases were correctly identified for \(\gamma \) as well as for the obtained LR model in Eq. (9). Finally, in both cases the AUC also reported values that were in agreement both with the diagnostic accuracy and the cross-validation strategy.
Discussion
Main Findings
An optimal selection of patients undergoing CA could be reached by predicting the procedure outcome before it is attempted.5 This aspect would be fundamentally useful for persistent AF, in which the ablation effectiveness rate is more reduced than for paroxysmal AF.19 However, as will be next discussed, very few studies have paid attention to this aspect by analyzing preoperatively key AA spectral features from the surface ECG. Firstly, some comments on the results have to be made.
With regard to paroxysmal AF, none of the analyzed single parameters was able to significantly predict the ablation outcome before it is attempted, see Table 2. This observation has been reported previously in other works for the DF. In fact, Chang et al. reported that the DF itself was not sufficient to accurately identify critical drivers of AF nor to predict CA clinical outcome in paroxysmal AF patients.11 On the other hand, a LR analysis provided a model based on three characteristics related to the AA harmonic structure with ability to predict long-term AF recurrence after ablation, see Eq. (8). Within this model, the two more significant features were \(P_{AA}(f_0)\) and \(\gamma \), because their associated coefficients presented considerably lower values than the corresponding to \(P_{AA} (f_1)\). Both parameters indicated that stronger harmonics of \(f_0\) were present in patients with higher probability of SR maintenance. This result suggests that patients with a more organized paroxysmal AF have a lower probability of AF recurrence after ablation. To this respect, a similar observation was reported by Yoshida et al.66 in a previous work in which complexity of AF was studied from bipolar electrograms recorded at the coronary sinus (CS) with two indexes based on how often depolarizations change polarity and direction.
As for persistent AF, although \(\gamma \) was able to predict ablation outcome, none of the metrics \(f_0\), \(f_1\) or \(f_m\) provided to be statistically significant harbingers of the procedure result, see Table 3. Indeed, results from these indices agreed with other previous works.11,20,64,66 Nevertheless, an appropriate combination of \(f_1\) and \(f_m\) in the LR model of Eq. (9) provided to be a significant pre-ablation predictor of persistent AF recurrence at long term follow-up. Within this model, those patients presenting higher values of \(f_1\) as well as of \(f_m\) and, therefore, a stronger harmonic content, presented a higher rate of SR maintenance. This finding is in agreement with the results reported by Dyer et al.,16 in which the spectral organization, defined as the ratio of power in the harmonics to the power in the DF, was computed. They found that increased spectral organization after CA was predictive of AF recurrence with Se of 80% and Sp of 100% over 15 patients. However, they did not specify the type of AF ablated, paroxysmal or persistent, nor the follow-up time analyzed. In addition, the stronger limitation of this study was its requirement of post-ablation recordings to compute the spectral organization.16 Similarly, Garibaldi et al. reported that CA would be more feasible in those patients with higher spectral concentration, which measures how the spectral energy is concentrated around the DF.20 They reported a statistically significant CA outcome prediction with an AUC of 0.89. Nonetheless, the follow-up time was only 4 months onwards.20
Although with a lower diagnostic accuracy than the LR model of Eq. (9), the single parameter \(\gamma \) was able to predict significantly AF recurrence after ablation of persistent AF patients, see Table 3. This parameter could be related to the presence or absence of harmonics of the DF, such as similar parameters proposed in previous works.16,20,57 Thus, when strong harmonics are present in the AA signal, low values of \(\gamma \) should be computed. Contrarily, high values should indicate a wider frequency content.57 As a consequence, the obtained outcomes suggest that those persistent AF patients with higher probability of SR maintenance after ablation presented stronger harmonics, whereas those with higher probability of AF recurrence had a broader spectral content. This observation has been reported elsewhere by several works in which SR restoration in persistent AF was attempted by ablation, ECV or antiarrhythmic drug treatments. Thus, the presence of stronger harmonics in the AA signal has been associated with procedural AF termination during PVI58 as well as SR maintenance after CA.16 Furthermore, the existence of an AA harmonic structure has been also associated to SR maintenance after internal17 and external27 ECV as well as in use of antiarrhythmic drugs.31,32 Therefore, the agreement between these studies suggests that the harmonic structure of the surface AA signal could be a powerful outcome predictor of any AF treatment. In addition, given that a more organized AF has been associated with stronger harmonics in previous works,27 it could be considered that patients with more organized fibrillatory activity present a higher probability to respond successfully to any treatment, including ablation.
Previous Studies
Apart from traditional clinical predictors,5,38 during past years many studies have attempted to predict CA long-term outcome by analyzing spectral properties of the atrial fibrillatory waves both invasively or via the surface ECG. Regarding invasive studies, one of the first approaches was introduced by Takahashi et al.58 They reported that a decrease in the DF during ablation, together with an increase in the organization index (OI), defined as the ratio of the area under the DF and its harmonics to the total spectral power, was associated with termination of AF during limited ablation, thus allowing to monitor the effects of CA as well as to anticipate the extent of the procedure.58 Later, Yoshida et al. reported that a decrease in DF after antral PVI in patients with persistent AF was correlated with efficacy of CA, thus identifying patients who may require additional ablation beyond antral PVI.66 Atienza et al. reported that the presence of a pre-ablation left-to-right atrium gradient of the DF was predictive of a better clinical outcome in patients with persistent AF.4 Chang et al. observed that only indices related with the regularity of the atrial electrograms, complexity and fractionation indices, were associated with paroxysmal AF ablation outcome, but no indices related to the frequency spectrum, specially the DF, were found significant.11 Contrarily, other authors have reported the relevance of the DF by studying its inverse through the AFCL. To this respect, Di Marco and coworkers, reported that the AFCL was able to predict restoration of SR after CA of paroxysmal as well as of persistent AF.14 One possible key aspect of this interesting result could be the application of recurrent plots to the AFCL time series.14 However, other studies have reported a weak correlation between DF and AFCL after PVI in persistent AF patients undergoing CA.53 Finally, Gal et al. recently reported that the existence of a correlation between the AFCL and electrograms fractionation may predict CA outcome at long-term.18 Nevertheless, the most relevant drawback of all the aforementioned studies is the requirement of invasive recordings. As a consequence, they are unable to provide any preoperative information and can only be computed at the time of the procedure or retrospectively, thus impairing optimal selection of patients undergoing CA.
With regard to the noninvasive studies addressing CA outcome prediction, the first works were focused on analyzing the AFCL. Thus, Matsuo et al.38 reported that the AFCL computed from the surface ECG was a significant predictor of CA outcome in persistent AF patients after 12 months of follow-up, providing an AUC of 0.88. Other significant parameter able to provide CA outcome prognosis from the surface ECG was found to be the f-wave amplitude. To this respect, Nault and coworkers44 reported that high f-wave amplitude computed from lead \(V_1\) predicted procedural termination of arrhythmia in patients with persistent AF and SR maintenance upon a follow-up of 12 months, providing an AUC of 0.77. However, the f-wave amplitude was manually measured, thus being subject to operator-dependent criteria.
On the other hand, Yoshida et al. found that a decrease larger than 11% in the DF in pre- vs. post-ablation AA computed from lead \(V_1\) was a significant predictor of SR maintenance with an AUC of 0.74.64 In contrast, a decrease smaller than 11% after ablation indicated recurrence very likely, because AF recurred in 80% of the patients.64 This study was performed over 100 persistent AF patients after a mean follow-up period longer than 1 year. However, it requires performing the procedure to compute the DF critical decrease, so nothing can be done with only the preoperative data. In addition, the DF analysis can only be performed over those patients still in AF after CA, which resulted to be only 61% of them.64
Aside from the analysis of the surface ECG spectral features, other studies have also addressed CA outcome prediction by applying different computational techniques to the body surface AA. Thus, to overcome the limitation of estimating manually the f-wave amplitude as in,44 later studies introduced new automated methods, such as computing the root mean square value of the AA,3 or the use of cubic Hermite interpolation.40 This last work introduced by Meo et al. condensed the most representative features of the AA carried by the 12-leads ECG in a reduced-rank approximation index, based on principal component analysis, to provide a preoperative prediction of CA outcome in persistent AF patients.40 Although Meo et al. reported outstanding results, with an AUC of 0.98, this prediction has to be considered with caution because it was performed at very short-term. In fact, the study considered CA effectiveness as AF conversion, either directly to SR or intermediate tachyarrhythmia, exclusively during the procedure or by CA followed by electrical cardioversion.40 Furthermore, those patients who did not experience AF termination after a first procedure underwent a second ablation. This could be the reason why the ablation short-term success rate of this study (83.8%) was so high in comparison with the normal rates of around 50% for persistent AF reported by many studies at long-term.19 Therefore, such a very short-term prognosis of CA outcome could experience remarkable variations after significantly longer periods of follow-up.21 In fact, the same authors introduced later an improved methodology, based on the normalized mean square error of the reduced-rank AA signal approximation by weighted principal component analysis, which reported poorer outcomes with an AUC of 0.91. In this case, the number of analyzed patients was smaller and the follow-up was slightly extended beyond the 3 months blanking period, being 4 months onwards.39 In addition, some patients received antiarrhythmic drugs subsequent to CA and other underwent a second ablation, thus achieving a 65% of AF-free patients at the follow-up end.39 In contrast, many other studies define CA clinical efficacy as freedom from all atrial tachyarrhythmias in the absence of antiarrhythmic drug therapy and after only one ablation procedure.4,11,44,64,65 Indeed, ablation guidelines recommend to report the CA successful outcome as freedom from AF or any other tachyarrhythmia without antiarrhythmic drug therapy assessed from the end of the 3 months blanking period.10
Finally, Di Marco et al. have introduced the study of recurrence patterns of AF from the surface ECG to predict CA outcome both in paroxysmal and persistent AF.15 Although the study was performed with a follow-up of 6 months, they reported that several recurrent plot indices were significant predictors of CA outcome. Thus, the AA entropy of recurrence predicted ablation outcome with an AUC of 0.81 and 0.72 for paroxysmal and persistent AF, respectively.15 Nonetheless, the validation of all the proposed time and frequency features from the ECG, by comparison with internal electrograms, is a pending task for most part of them. Indeed, only some previous studies have tried to validate the surface DF with invasive recordings from different atrial regions, but reporting inconclusive results.30,31,47 Anyway, all these works, regardless of the very dissimilar methodology employed, prove that the standard surface ECG can be considered as a promising tool for mean and long-term outcome prognosis of CA as well as for assisting the proper selection of patients undergoing this procedure.
Limitations
Some limitations merit consideration in the present study. Firstly, the number of analyzed patients with paroxysmal and persistent AF was not very large and, therefore, the presented results must be considered with caution. To this respect, the small sample size of subjects included in the ROC analysis may have overestimated the true sensitivity and specificity.37 Moreover, the small number of patients with paroxysmal AF in whom arrhythmia recurred during the follow-up may have precluded the detection of a single parameter related to the AA harmonic structure as a significant pre-ablation predictor of the procedure outcome. Despite this, it is worth noting that no great differences between accuracies reported by ROC curves, AUC’s and the rates of cross-validated grouped cases correctly classified were observed for those metrics revealing statistically significant predictions as well as for the obtained LR models. Thus, an adequate validation procedure of the proposed indices can be assumed. Anyway, a wider dataset allowing more rigorous statistical analyses should be required in order to provide confidence of the information obtained from the spectral analysis of the surface AA signal.
A second limitation is that spectral analysis was restricted to the lead \(V_1\). However, this lead is readily available during catheter AF ablation without the need for additional instrumentation and reflects the global activation of the atria.9,26,30,31,47 In addition, studies relying on only one surface lead can easily be extended to simpler recording systems, like Holter recorders, able to be performed long before the CA procedure. In contrast, other works have reported that improved prediction outcomes can be achieved by combining the information provided by multi-lead surface ECG recording systems.15,16,20,39,40 Hence, multi-lead and multi-variable analyses considering the proposed spectral indices as well as other relevant time features have to be considered in the future to improve the CA outcome prediction both for paroxysmal and persistent AF.
Conclusions
The study of key spectral features extracted from the atrial activity signal and its harmonic structure from surface lead \(V_1\) has provided clinically useful information to predict the outcome of catheter ablation in patients with paroxysmal and persistent AF. The spectral features have shown different logistic regression models for paroxysmal than for persistent patients. These features have provided ability to assist preoperatively the selection of patients undergoing catheter ablation, thus making it possible to avoid unnecessary interventional risks for those patients in whom ablation would finally be unsuccessful very likely at long-term follow-up. Further studies are required to validate the robustness of these findings on wider databases.
References
Alcaraz, R., and J. J. Rieta. Adaptive singular value cancellation of ventricular activity in single-lead atrial fibrillation electrocardiograms. Physiol. Meas. 29(12):1351–1369, 2008.
Alcaraz, R., and J. J. Rieta. Non-invasive organization variation assessment in the onset and termination of paroxysmal atrial fibrillation. Comput. Methods Programs Biomed. 93(2):148–154, 2009.
Alcaraz, R., and J. J. Rieta. The application of nonlinear metrics to assess organization differences in short recordings of paroxysmal and persistent atrial fibrillation. Physiol. Meas. 31(1):115–130, 2010.
Atienza, F., J. Almendral, J. Jalife, S. Zlochiver, R. Ploutz-Snyder, E. G. Torrecilla, A. Arenal, J. Kalifa, F. Fernández-Avilés, and O. Berenfeld. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 6(1):33–40, 2009.
Balk, E. M., A. C. Garlitski, A. A. Alsheikh-Ali, T. Terasawa, M. Chung, and S. Ip. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J. Cardiovasc. Electrophysiol. 21(11):1208–1216, 2010.
Bishop, C. M. Neural Networks for Pattern Recognition. Oxford University Press Inc, 1995.
Blomstrom Lundqvist, C., G. Y. H. Lip, and P. Kirchhof. What are the costs of atrial fibrillation? Europace 13 Suppl 2:ii9–12, 2011.
Bollmann, A., D. Husser, L. T. Mainardi, F. Lombardi, P. Langley, A. Murray, J. J. Rieta, J. Millet, S. B. Olsson, M. Stridh, and L. Sornmo. Analysis of surface electrocardiograms in atrial fibrillation: techniques, research, and clinical applications. Europace 8(11):911–926, 2006.
Bollmann, A., N. K. Kanuru, K. K. McTeague, P. F. Walter, D. B. DeLurgio, and J. J. Langberg. Frequency analysis of human atrial fibrillation using the surface electrocardiogram and its response to ibutilide. Am. J. Cardiol. 81(12):1439–1445, 1998.
Calkins, H., K. H. Kuck, R. Cappato, J. Brugada, A. J. Camm, S. A. Chen, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 14(4):528–606, 2012.
Chang, S.-H., M. Ulfarsson, A. Chugh, K. Yoshida, K. Jongnarangsin, T. Crawford, E. Good, F. Pelosi, F. Bogun, F. Morady, and H. Oral. Time- and frequency-domain characteristics of atrial electrograms during sinus rhythm and atrial fibrillation. J. Cardiovasc. Electrophysiol. 22(8):851–857, 2011.
Ciaccio, E. J., A. B. Biviano, W. Whang, A. Gambhir, and H. Garan. Spectral profiles of complex fractionated atrial electrograms are different in longstanding and acute onset atrial fibrillation atrial electrogram spectra. J. Cardiovasc. Electrophysiol. 23(9):971–9, 2012.
Cox, J. L. Surgical treatment of atrial fibrillation: a review. Europace 5 Suppl 1:S20–9, 2004.
Di Marco, L. Y., D. Raine, J. P. Bourke, and P. Langley. Characteristics of atrial fibrillation cycle length predict restoration of sinus rhythm by catheter ablation. Heart Rhythm 10(9):1303–1310, 2013.
Di Marco, L. Y., D. Raine, J. P. Bourke, and P. Langley. Recurring patterns of atrial fibrillation in surface ECG predict restoration of sinus rhythm by catheter ablation. Comput. Biol. Med. 54C:172–179, 2014.
Dyer, J., S. Malakouti, and S. Stavrakis. Measuring spectral organization in atrial fibrillation. In Instrumentation and Measurement Technology Conference (I2MTC), 2015 IEEE International IS - SN - VO - VL -, 2015, pp. 773–778.
Everett, T. H., L. C. Kok, R. H. Vaughn, J. R. Moorman, and D. E. Haines. Frequency domain algorithm for quantifying atrial fibrillation organization to increase defibrillation efficacy. IEEE Trans. Biomed. Eng. 48(9):969–978, 2001.
Gal, P., A. C. Linnenbank, A. Adiyaman, J. J. J. Smit, A. R. Ramdat Misier, P. P. H. M. Delnoy, J. M. T. de Bakker, and A. Elvan. Correlation of atrial fibrillation cycle length and fractionation is associated with atrial fibrillation free survival. Int. J. Cardiol. 187 IS:208–215, 2015.
Ganesan, A. N., N. J. Shipp, A. G. Brooks, P. Kuklik, D. H. Lau, H. S. Lim, T. Sullivan, K. C. Roberts-Thomson, and P. Sanders. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J. Am. Heart. Assoc. 2(2):e004549, 2013.
Garibaldi, M., V. Zarzoso, D. G. Latcu, and N. Saoudi. Predicting catheter ablation outcome in persistent atrial fibrillation using atrial dominant frequency and related spectral features. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference 2012, 2012, pp. 613–616.
Gerstenfeld, E. P. and S. Duggirala. Atrial fibrillation ablation: indications, emerging techniques, and follow-up. Prog. Cardiovas. Dis. 2015.
Gillis, A. M., A. D. Krahn, A. C. Skanes, and S. Nattel. Management of atrial fibrillation in the year 2033: new concepts, tools, and applications leading to personalized medicine. Can. J. Cardiol. 29(10):1141–1146, 2013.
Gorenek, B. and G. Kudaiberdieva. Cost analysis of radiofrequency catheter ablation for atrial fibrillation. Int. J. Cardiol. 167(6):2462–2467, 2013.
Haïssaguerre, M., M. Hocini, P. Sanders, F. Sacher, M. Rotter, Y. Takahashi, T. Rostock, L.-F. Hsu, P. Bordachar, S. Reuter, R. Roudaut, J. Clémenty, and P. Jaïs. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J. Cardiovasc. Electrophysiol. 16(11):1138–47, 2005.
Healey, J. S., C. W. Israel, S. J. Connolly, S. H. Hohnloser, G. M. Nair, S. Divakaramenon, A. Capucci, I. C. V. Gelder, C.-P. Lau, M. R. Gold, M. Carlson, E. Themeles, and C. A. Morillo. Relevance of electrical remodeling in human atrial fibrillation: results of the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial mechanisms of atrial fibrillation study. Circ. Arrhythm. Electrophysiol. 5(4):626–631, 2012.
Holm, M., S. Pehrson, M. Ingemansson, L. Sornmo, R. Johansson, L. Sandhall, M. Sunemark, B. Smideberg, C. Olsson, and S. B. Olsson. Non-invasive assessment of the atrial cycle length during atrial fibrillation in man: introducing, validating and illustrating a new ECG method. Cardiovasc. Res. 38(1):69–81, 1998.
Holmqvist, F., M. Stridh, J. E. P. Waktare, A. Roijer, L. Sornmo, P. G. Platonov, and C. J. Meurling. Atrial fibrillation signal organization predicts sinus rhythm maintenance in patients undergoing cardioversion of atrial fibrillation. Europace 8(8):559–565, 2006.
Holmstrom, L., P. Koistinen, J. Laaksonen, and E. Oja. Neural and statistical classifiers-taxonomy and two case studies. IEEE Trans. Neural. Netw. 8(1):5–17, 1997.
Hornero, R., J. Escudero, A. Fernández, J. Poza, and C. Gómez. Spectral and nonlinear analyses of meg background activity in patients with alzheimer’s disease. IEEE Trans. Biomed. Eng. 55(6):1658–65, 2008.
Hsu, N.-W., Y.-J. Lin, C.-T. Tai, T. Kao, S.-L. Chang, W. Wongcharoen, L.-W. Lo, A. R. Udyavar, Y.-F. Hu, H.-W. Tso, Y.-J. Chen, S. Higa, and S.-A. Chen. Frequency analysis of the fibrillatory activity from surface ECG lead V1 and intracardiac recordings: implications for mapping of AF. Europace 10(4):438–43, 2008.
Husser, D., M. Stridh, D. S. Cannom, A. K. Bhandari, M. J. Girsky, S. Kang, L. Sornmo, S. B. Olsson, and A. Bollmann. Validation and clinical application of time-frequency analysis of atrial fibrillation electrocardiograms. J. Cardiovasc. Electrophysiol. 18(1):41–46, 2007.
Husser, D., M. Stridh, L. Sornmo, C. Geller, H. U. Klein, S. B. Olsson, and A. Bollmann. Time-frequency analysis of the surface electrocardiogram for monitoring antiarrhythmic drug effects in atrial fibrillation. Am. J. Cardiol. 95(4):526–528, 2005.
January, C. T., L. S. Wann, J. S. Alpert, H. Calkins, J. E. Cigarroa, J. C. Cleveland, J. B. Conti, P. T. Ellinor, M. D. Ezekowitz, M. E. Field, K. T. Murray, R. L. Sacco, W. G. Stevenson, P. J. Tchou, C. M. Tracy, C. W. Yancy, and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014.
Jian, A. K., R. P. W. Duin, and J. Mao. Statistical pattern recognition: a review. IEEE Trans. Pattern. Anal. Mach. Intell. 22:4–37, 2000.
Jobson, J. D. Applied Multivariate Data Analysis: Categorical and Multivariate Methods. Springer, 1991.
Jung, Y., and H. Jianhua. A k-fold averaging cross-validation procedure. J.Nonparameteric Stat. 27(2):167–179, 2015.
Lasko, T. A., J. G. Bhagwat, K. H. Zou, and L. Ohno-Machado. The use of receiver operating characteristic curves in biomedical informatics. J. Biomed. Inform. 38(5):404–415, 2005.
Matsuo, S., N. Lellouche, M. Wright, M. Bevilacqua, S. Knecht, I. Nault, K.-T. Lim, L. Arantes, M. D. O’Neill, P. G. Platonov, J. Carlson, F. Sacher, M. Hocini, P. Jaïs, and M. Haïssaguerre. Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J. Am. Coll. Cardiol. 54(9):788–795, 2009.
Meo, M., V. Zarzoso, O. Meste, D. G. Latcu, and N. Saoudi. Catheter ablation outcome prediction in persistent atrial fibrillation using weighted principal component analysis. Biomed. Signal Proc. Con. 8(6):958–968, 2013.
Meo, M., V. Zarzoso, O. Meste, D. G. Latcu, and N. Saoudi. Spatial variability of the 12-lead surface ECG as a tool for noninvasive prediction of catheter ablation outcome in persistent atrial fibrillation. IEEE Trans. Biomed. Eng. 60(1):20–7, 2013.
Nademanee, K., J. McKenzie, E. Kosar, M. Schwab, B. Sunsaneewitayakul, T. Vasavakul, C. Khunnawat, and T. Ngarmukos. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 43(11):2044–53, 2004.
Narayan, S. M., D. E. Krummen, K. Shivkumar, P. Clopton, W.-J. Rappel, and J. M. Miller. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial J. Am. Coll. Cardiol. 60(7):628–636, 2012.
Nattel, S., and M. Harada. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J. Am. Coll. Cardiol. 63(22):2335–2345, 2014.
Nault, I., N. Lellouche, S. Matsuo, S. Knecht, M. Wright, K.-T. Lim, F. Sacher, P. Platonov, A. Deplagne, P. Bordachar, N. Derval, M. D. O’Neill, G. J. Klein, M. Hocini, P. Jaïs, J. Clémenty, and M. Haïssaguerre. Clinical value of fibrillatory wave amplitude on surface ECG in patients with persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 26(1):11–19, 2009.
Nishida, K., T. Datino, L. Macle, and S. Nattel. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J. Am. Coll. Cardiol. 64(8):823–831, 2014.
Oral, H., C. Pappone, A. Chugh, E. Good, F. Bogun, F. Pelosi, Jr, E. R. Bates, M. H. Lehmann, G. Vicedomini, G. Augello, E. Agricola, S. Sala, V. Santinelli, and F. Morady. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N. Engl. J. Med. 354(9):934–41, 2006.
Petrutiu, S., A. V. Sahakian, W. Fisher, and S. Swiryn. Manifestation of left atrial events and interatrial frequency gradients in the surface electrocardiogram during atrial fibrillation: contributions from posterior leads. J. Cardiovasc. Electrophysiol. 20(11):1231–6, 2009.
Polovina, M. M., and T. S. Potpara. Recent advances in antiarrhythmic drug treatment of atrial fibrillation. Recent Pat. Cardiovasc. Drug Discov. 8(2):112–126, 2013.
Raine, D., P. Langley, A. Murray, S. S. Furniss, and J. P. Bourke. Surface atrial frequency analysis in patients with atrial fibrillation: assessing the effects of linear left atrial ablation. J. Cardiovasc. Electrophysiol. 16(8):838–44, 2005.
Reiffel, J. A. Cardioversion for atrial fibrillation: treatment options and advances. Pacing Clin. Electrophysiol. 32(8):1073–1084, 2009.
Shah, R. U., J. V. Freeman, D. Shilane, P. J. Wang, A. S. Go, and M. A. Hlatky. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 59(2):143–149, 2012.
Sheikh, A., N. J. Patel, N. Nalluri, K. Agnihotri, J. Spagnola, A. Patel, D. Asti, R. Kanotra, H. Khan, C. Savani, S. Arora, N. Patel, B. Thakkar, N. Patel, D. Pau, A. O. Badheka, A. Deshmukh, M. Kowalski, J. Viles-Gonzalez, and H. Paydak. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog. Cardiovas. Dis. 2015.
Singh, S. M., E. K. Heist, J. S. Koruth, C. D. Barrett, J. N. Ruskin, and M. C. Mansour. The relationship between electrogram cycle length and dominant frequency in patients with persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 20(12):1336–1342, 2009.
Sörnmo, L., and P. Laguna. Bioelectrical Signal Processing in Cardiac and Neurological Applications. Elsevier Academic Press, 2005.
Stridh, M., A. Bollmann, S. B. Olsson, and L. Sörnmo. Detection and feature extraction of atrial tachyarrhythmias. a three stage method of time-frequency analysis. IIEEE Eng. Med. Biol. Mag. 25(6):31–9, 2006.
Stridh, M., D. Husser, A. Bollmann, and L. Sörnmo. Waveform characterization of atrial fibrillation using phase information. IEEE Trans. Biomed. Eng. 56(4):1081–9, 2009.
Stridh, M., L. Sornmo, C. J. Meurling, and S. B. Olsson. Sequential characterization of atrial tachyarrhythmias based on ECG time-frequency analysis. IEEE Trans. Biomed. Eng. 51(1):100–114, 2004.
Takahashi, Y., P. Sanders, P. Jaïs, M. Hocini, R. Dubois, M. Rotter, T. Rostock, C. J. Nalliah, F. Sacher, J. Clémenty, and M. Haïssaguerre. Organization of frequency spectra of atrial fibrillation: relevance to radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 17(4):382–388, 2006.
Welch, P. D. Use of fast Fourier transform for estimation of power spectra: a method based on time averaging over short modified periodograms. IEEE Trans. Audio Electroacustics 15(2):70–73, 1967.
Woods, C. E., and J. Olgin. Atrial fibrillation therapy now and in the future: drugs, biologicals, and ablation. Circ. Res. 114(9):1532–1546, 2014.
Xi, Q., A. V. Sahakian, and S. Swiryn. The effect of qrs cancellation on atrial fibrillatory wave signal characteristics in the surface electrocardiogram. J. Electrocardiol. 36(3):243–9, 2003.
Yiin, G. S., D. P. Howard, N. L. Paul, Z. Mehta, and P. M. Rothwell. Incidence, outcome and future projections of atrial fibrillation-related stroke and systemic embolism at age \(>=\)80 years: 10-year results of a population-based study. J. Neurol. Neurosurg. Psychiatry 84(11):e2, 2013.
Yoshida, K., and K. Aonuma. Catheter ablation of atrial fibrillation: past, present, and future directions. J. Arrhythm. 28(2):83–90, 2012.
Yoshida, K., A. Chugh, E. Good, T. Crawford, J. Myles, S. Veerareddy, S. Billakanty, W. S. Wong, M. Ebinger, F. Pelosi, K. Jongnarangsin, F. Bogun, F. Morady, and H. Oral. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm 7(3):295–302, 2010.
Yoshida, K., A. Chugh, M. Ulfarsson, E. Good, M. Kuhne, T. Crawford, J. F. Sarrazin, N. Chalfoun, D. Wells, W. Boonyapisit, S. Veerareddy, S. Billakanty, W. S. Wong, K. Jongnarangsin, F. Pelosi, Jr., F. Bogun, F. Morady, and H. Oral. Relationship between the spectral characteristics of atrial fibrillation and atrial tachycardias that occur after catheter ablation of atrial fibrillation. Heart Rhythm 6(1):11–7, 2009.
Yoshida, K., M. Ulfarsson, H. Tada, A. Chugh, E. Good, M. Kühne, T. Crawford, J. F. Sarrazin, N. Chalfoun, D. Wells, K. Jongnarangsin, F. Pelosi, F. Bogun, F. Morady, and H. Oral. Complex electrograms within the coronary sinus: time- and frequency-domain characteristics, effects of antral pulmonary vein isolation, and relationship to clinical outcome in patients with paroxysmal and persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 19(10):1017–1023, 2008.
Acknowledgments
Research funded by Grants TEC2014-52250-R from the Spanish Ministry of Economy and Competitiveness and PPII-2014-026-P from Junta de Comunidades de Castilla-La Mancha.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Nathalie Virag oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Alcaraz, R., Hornero, F. & Rieta, J.J. Electrocardiographic Spectral Features for Long-Term Outcome Prognosis of Atrial Fibrillation Catheter Ablation. Ann Biomed Eng 44, 3307–3318 (2016). https://doi.org/10.1007/s10439-016-1641-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1641-3