Abstract
Background
Direct comparisons of combined (C-ABL) and non-combined (NC-ABL) endo-epicardial ventricular tachycardia (VT) ablation outcomes are scarce. We aimed to investigate the long-term clinical efficacy and safety of these 2 strategies in ischemic heart disease (IHD) and non-ischemic cardiomyopathy (NICM) populations.
Methods
Multicentric observational registry included 316 consecutive patients who underwent catheter ablation for drug-resistant VT between January 2008 and July 2019. Primary and secondary efficacy endpoints were defined as VT-free survival and all-cause death after ablation. Safety outcomes were defined by 30-day mortality and procedure-related complications.
Results
Most of the patients were male (85%), with IHD (67%) and mean age of 63 ± 13 years. During a mean follow-up of 3 ± 2 years, 117 (37%) patients had VT recurrence and 73 (23%) died. Multivariate survival analysis identified electrical storm (ES) at presentation, IHD, left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) functional class III / IV, and C-ABL as independent predictors of VT recurrence. In 135 patients undergoing repeated procedures, only C-ABL and ES were independent predictors of relapse. The identified independent predictors of mortality were C-ABL, ES, LVEF, age, and NYHA class III / IV. C-ABL survival benefit was only seen in patients with a previous ablation (P for interaction = 0.04). Mortality at 30 days was similar between NC-ABL and C-ABL (4% vs. 2%, respectively, P = 0.777), as was complication rate (10.3% vs. 15.1%, respectively, P = 0.336).
Conclusion
A combined or sequential endo-epicardial VT ablation strategy was associated with lower VT recurrence and lower all-cause death in IHD and NICM patients undergoing repeated procedures. Both approaches seemed equally safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Patients with ischemic (IHD) and non-ischemic (NICM) heart disease and reduced left ventricular ejection fraction are at increased risk of ventricular tachycardia (VTs) or sudden cardiac death [1]. Implantable cardioverter-defibrillators (ICDs) are indicated in these patients and have shown to reduce mortality [1]. However, some studies suggest that ICD shocks reduce the quality of life and may be linked to increased mortality, particularly after an electrical storm (ES) [2]. VT catheter ablation is an invasive treatment modality for antiarrhythmic drug-resistant VT that reduces arrhythmic episodes, improves quality of life and survival in patients with ES [3]. Current guidelines for VT ablation [1, 3] recommend epicardial catheter ablation for NICM patients after an initial unsuccessful endocardial catheter ablation procedure or as first intention when there is a clinical or imaging suspicion of an epicardial channel. Epicardial ablation’s role is not as established in the IHD population, although some studies show potential for a combined endo-epicardial approach (C-ABL) [4] vs. a non-combined approach (NC-ABL). Nonetheless, complex arrhythmia substrates and potential life-threating procedure complications increase the technical difficulty of the epicardial approach [5]. Direct comparisons of C-ABL and NC-ABL outcomes are limited by patient characteristics, follow-up duration, protocols heterogeneity, and scarcity of randomized trials [4, 5]. We aim to investigate the long-term clinical outcomes of these 2 strategies in the IHD and NICM populations. To overcome said limitations, a propensity score-matched sensitivity analysis was performed.
2 Methods
2.1 Population
This study included all consecutive patients with drug-resistant VT undergoing catheter ablation in two different institutions, between January 2008 and July 2019. Only patients with scar-related VT etiology of either IHD or NICM etiology were included (other cardiomyopathy causes as Chagas disease, sarcoidosis, arrhythmogenic cardiomyopathy, hypertrophic cardiomyopathy, and acute myocarditis were excluded). ES was defined as the occurrence of ≥ 3 episodes of VT or ventricular fibrillation during a 24-h period resulting in an appropriate ICD therapy.
2.2 Catheter ablation protocol
All patients underwent the procedure under arterial blood pressure and O2 saturation monitoring. Conscious sedation or general anesthesia was used according to the operator's discretion and with anesthesiologist support. Antiarrhythmic drugs (AAD) were stopped at least 48 h before the procedure when applicable. The choice of the VT ablation access (endocardial vs epicardial vs combined) was decided according to the etiology, previous VT ablation site and imaging information regarding scar localization. Systemic anticoagulation with intravenous heparin targeted a minimum activation clotting time of 300 s during each left ventricular endocardial ablation, with protamine reversal (1 mg / 100U) at the end of the procedure. Systemic anticoagulation was also reversed with protamine before percutaneous epicardial access when necessary. The patients under oral anticoagulation would withhold the drug 24 to 48 h before an epicardial ablation, accordingly with renal function and/or INR level (if under vitamin k antagonist). Anticoagulation was resumed 24 h after the procedure in the absence of hemorrhagic complications. Epicardial access was obtained through the subxiphoid space under fluoroscopic guidance as previously described by Sosa et al [6]. A multipolar mapping catheter was placed in the coronary sinus via femoral vein, and standard transvenous multipolar catheters were placed into the appropriate cardiac chambers under study. Left ventricular mapping was performed via the retrograde aortic or transseptal route. Endocardial and epicardial electroanatomical mapping was obtained with the CARTO® (Biosense Webster, Diamond Bar, CA, USA), the EnSite NavX® (St Jude Medical, St Paul, MN, USA), or with the Rhythmia® (Boston Scientific, Marlborough, Massachusetts, USA) systems and included activation and voltage mapping acquired in sinus rhythm, ventricular pacing or during hemodynamically stable VT. Intracardiac signals were filtered at 30 to 500 Hz. Normal tissue was defined by a voltage threshold greater than 1.5 mV, while dense scare as tissue under a threshold of 0.5 mV in the bipolar substrate map. A first attempt to induce the clinical VT was systematically performed at the beginning of each procedure. Activation and entrainment-mapping techniques were performed in all hemodynamically tolerated VTs, and if not possible, ablation was guided by substrate, pace mapping and identification of delayed and fractionated potentials. Coronary angiography was performed when deemed necessary before epicardial radiofrequency applications. Epicardial phrenic nerve capture was identified by bipolar pacing from the ablation catheter. Radiofrequency (RF) was delivered with a 3.5-mm open irrigated tip catheter in power control mode using a Stockert generator, with power set to 30 ─ 50 W and irrigation set to 17 ─ 30 ml/min. The procedure was deemed as successful if no VT was induced with a standard stimulation protocol of up to 3 extrastimuli at a 200-ms cycle length or effective refractory period in 2 different sites. Incomplete success was defined by inducibility other non-clinical VTs, and the ablation was deemed unsuccessful if clinical VT as still inducible at the end of the procedure.
2.3 Study endpoints
The primary efficacy endpoint was defined as VT recurrence, which included any ICD appropriate therapy (antitachycardia pacing or shock) or a documented sustained VT not detected by the ICD. The secondary efficacy endpoint was defined as all-cause death. The safety outcomes were defined as 30-day mortality and procedure-related complications.
2.4 Follow-up
The follow-up protocol comprised outpatient visits on the 1st, 3rd, 6th, and 12th months’ post-ablation, followed by regular assessments according to the assistant physician’s discretion. Patient’s data and outcomes were collected from electronic and physical medical records. If said records lacked all the required information, a structured telephonic interview was conducted. All patients lacking an ICD underwent implantation before discharge. After the ablation, all ICDs were programmed with at least one zone of detection slower than the clinical VT.
2.5 Statistical analysis
Normally and non-normally distributed variables were expressed as mean and median, respectively. Differences between groups were assessed using independent samples t-test and Chi-square test for continuous and categorical variables, respectively. Proportional-hazards Cox regression was used to identify predictors of time to VT or death. Variables with a P-value (P) ≤ 0.10 in univariate analysis were entered simultaneously in the multivariate regression model and deemed as statistically significant if P < 0.05. Multicollinearity was excluded by assessing Pearson’s correlation coefficient between pairs of continuous variables. Kaplan–Meier curves were used to report VT-free survival for the NC-ABL and C-ABL groups, while differences in their survival curves were assessed with the log-rank test. Annual relapse rates were obtained by dividing the total number of first events by the total number of person-years of follow-up for each group. The propensity score (PS) for an individual is the probability of receiving a particular treatment based on a particular set of individual covariates [7]. A PS matching was assessed for the ablation strategy (C-ABL vs NC-ABL) by multivariable logistic regression, with the inclusion of the covariates identified as independent predictors of VT recurrence and mortality: age; IHD etiology; left ventricular ejection fraction (LVEF); New York Heart Association (NYHA) functional class III / IV; and ES at presentation. The resulting scores were matched in a 1:1 ratio to the best corresponding patient, with a maximal allowable difference of 0.05 (caliper width of 0.05 of the standard deviation of the logit of the PS). Any remaining differences between matched pairs were assessed by standardized difference of the means (level of significance < 0.05). Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 23.0 (SPSS Inc., Chicago, USA) for Windows OS. Statistical significance was set at P < 0.05 (two-sided tailed).
3 Results
3.1 Population
Baseline characteristics are presented in Table 1. In a population of 316 patients, most were male (N = 267, 85%) and had IHD (N = 195, 67%) and mean age was 63 ± 13 years. Mean LVEF was 34 ± 11%. One hundred twenty-seven (40%) patients were in either NYHA class III / IV, and 84 (27%) patients had ES at presentation. During a mean follow-up of 3 ± 2 years, 117 (37%) patients had VT recurrence and 73 (23%) patients died (all but one of cardiovascular origin).
3.2 Catheter ablation procedure
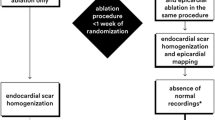
The clinical VT was of right bundle branch block-like (RBBB) morphology in 254 patients (80%), and mean procedure duration was 4 ± 2 h. Ninety-four (30%) patients underwent LV ablation through a transseptal approach and 179 (57%) through retro-aortic route. Epicardial ablation was performed in 61 (19%) patients, and 53 (17%) patients were subject to a combined or sequential endocardial and epicardial ablation (C-ABL), either simultaneously or at different procedures (Fig. 1). In this group, the second procedure was performed after a median 162 days [interquartile range 41—526 days]. Channels, scar, and late potentials were identified and targeted for RF ablation in all 61 epicardial ablation patients. Twelve patients were treated with a C-ABL in the first procedure, with the remaining having 1 previous ablation. All patients in the C-ABL group underwent epicardial ablation as the last procedure. One hundred and five (33%) patients underwent more than one ablation. The mean number of ablations was 1 ± 1, from 1 to 4 procedures, and most of the patients were only treated with one ablation (N = 211, 67%). Six (2%) patients underwent 3 or 4 procedures, and only 1 patient underwent more than 1 epicardial ablation. Complete success was achieved in 83% of the patients, with partial success in 14% and unsuccess in 3% of the ablations. The mean duration of hospital stay was of 12 ± 11 days.
3.3 Primary efficacy endpoint
Multivariate Cox survival analysis identified ES at presentation, NYHA class III / IV, LVEF, IHD, and C-ABL as in independent predictors of VT relapse (Table 2). In sub-group analysis, the VT-free survival improvement was only statistically significant in patients with a previous ablation (P for interaction = 0.003). This finding was consistent in both IHD and NICM patients (P for interaction = 0.170). In patients undergoing 2 or more procedures C-ABL (HR = 0.36, 95% CI 0.17 ─ 0 0.80, P = 0.011), and ES (HR = 2.42, 95% CI 1.24 ─ 4.70, P = 0.009) were the only independent predictors of arrhythmia recurrence.
3.4 Secondary efficacy endpoint
The independent predictors of mortality identified by regression analysis were ES at presentation, NYHA class III / IV, age, LVEF, IHD, and C-ABL (Table 3).
In sub-group analysis, the survival improvement with a combined or sequential strategy was only statistically significant in patients undergoing a redo procedure (P for interaction = 0.04). As in the primary efficacy endpoint, this finding was consistent in both IHD and NICM patients (P for interaction = 0.231).
3.5 Safety Outcomes
Mortality at 30 days after index procedure was similar between NC-ABL and C-ABL (10 vs. 1 for NC-ABL and C-ABL, respectively, P = 0.777). The complication rate was not different between both groups (10.3% vs. 15.1%, respectively, P = 0.336). In sub-group analysis, the C-ABL group had more pericardial effusions (2% vs 9%, P = 0.014) and right ventricular punctures (1% vs 7%, P = 0.017). There were 2 strokes in the NC-ABL group and none in the C-ABL group (P = 1.000). Two patients in the NC-ABL group developed complete heart block vs. 1 in the C-ABL group (P = 1.000). One-hundred and twelve patients had prior coronary artery bypass graft surgery, from whose 6 underwent subxiphoid epicardial ablation. In this subgroup, there was 1 with pericardial effusion.
3.6 PS matching sensitivity analysis
A PS was used to match patients in a 1:1 fashion for NC-ABL vs C-ABL groups accordingly to all variables identified as independent predictors of VT recurrence and mortality. The PS utilized the covariates: ES at presentation, LVEF, NYHA class III / IV, IHD, and age. The PS matched two groups of 43 patients. NC-ABL and C-ABL patients had a mean age of 60 ± 14 and. 61 ± 12 years, being 86% and 77% of male sex and 72% being redo procedures in both groups, respectively. Both groups presented with well-matched baseline characteristics (Table 4), except for atrial fibrillation, with a higher incidence in the NC-ABL group (N = 10, 23% vs N = 2, 5%, P = 0.026). Only 2 patients underwent epicardial ablation at index procedure in the NC-ABL group, while 37 (86%) patients were treated with an epicardial ablation, and 6 (14%) patients had a previous epicardial ablation in the C-ABL group (P < 0.001). During a mean follow-up of 3 ± 2 years, 27 (63%) NC-ABL patients had VT recurrence and 10 (23%) in the C-ABL group (P = 0.003). The yearly rates of VT recurrence were 34% /y ear for NC-ABL vs. 11%/year for C-ABL (P = 0.003). Multivariate survival analysis in the PS population identified only C-ABL (HR = 0.42, 95% CI 0.20 ─ 0.88, P = 0.023) as an independent predictor of VT relapse (Fig. 2) in both IHD and NICM (P for interaction = 0.110), but only in patients with a previous endocardial ablation (P for interaction = 0.03).
3.7 Safety Outcomes in the PS population
Mortality at 30 days was similar between NC-ABL and C-ABL (2 vs. 1 for NC-ABL and C-ABL, respectively, P = 0.501). The complication rate was not different between both groups (9.3% vs. 14.0%, respectively, P = 0.738) (Table 4). There was 1 pericardial effusion in the NC-ABL group vs 4 in the C-ABL group (P = 0.167), and in the C-ABL group occurred 2 right ventricle punctures. In the NC-ABL group, 1 patient needed pericardiocentesis after cardiac tamponade. No further surgical treatment was required. In the C-ABL group, 1 patient underwent subxiphoid pericardiocentesis after the procedure, one patient underwent surgical repair due to VD free wall laceration and uncontrollable pericardial hemorrhage, and the remaining 2 patients were left with pericardial drainage after hemopericardium was identified during epicardial ablation. Two patients in the NC-ABL group developed complete heart block vs. 1 in the C-ABL group (P = 0.567) [Table 4].
4 Discussion
This study documents the long-term outcomes of a combined or sequential endo-epicardial strategy for VT ablation in IHD and NICM patients. In our multicentric real-world analysis, a C-ABL approach was associated with increased overall survival and VT-free survival in patients undergoing a redo procedure. A direct comparison of clinical outcomes between a NC-ABL vs. a C-ABL strategy has been limited by heterogeneous patient characteristics and lack of randomized clinical trials [4, 5]. In our study, ES at presentation, LVEF, NYHA class III / IV, IHD, and C-ABL have been identified as independent predictors of VT-free survival, cardiovascular mortality, and all-cause mortality. Patients with previous myocardial infarction and severely depressed left ventricular ejection fraction are at increased risk of VT and sudden cardiac death [1, 3], and international guidelines recommend an ICD [1]. However, recurrent VT and ES are associated with increased mortality even in patients with such device [2]. This may be related to a progressive deterioration of cardiac function from frequent shocks, chronic low-cardiac output, and AAD therapy toxicity [8]. Several studies have shown the superiority of catheter ablation for VT treatment when compared to AADs, with a success rate of 51% to 67% [9]. The VANISH randomized controlled trial [10] showed that in patients with IHD and an ICD with VT despite AAD therapy, VT ablation had a lower rate of the composite primary outcome of death, ventricular tachycardia storm, or appropriate ICD therapy when compared to AAD therapy escalation. An endocardial approach frequently does not eliminate all reentrant circuits in IHD patients, which may lead to relapse [11]. Additionally, the development and widespread use of early reperfusion therapies led to a higher number of patients with non-transmural necrosis and heterogeneous infarcted tissues. These scars may have multiple slow conduction channels with epicardial exit sites [12], providing the rationale for a C-ABL approach. Current guidelines have an IIB class recommendation for the epicardial ablation in IHD after a failed endocardial ablation [1, 3], but there is an important knowledge gap. Di Biase et al. [4] showed that extensive scar homogenization with a combined endocardial and epicardial approach in a first ablation procedure was linked to increased VT-freedom in patients with ES. One possible explanation for their findings is a greater amount and complexity of the arrhythmic substrate in IHD with ES presentation, which can justify an initial combined approach. Izquierdo et al. [13] showed that a first combined endo-epicardial procedural was linked to fewer hospitalizations, but failed to show an increase in survival free of VT. Sarkozy et al. [14] showed that two-thirds of the patients selected for epicardial mapping after a failed endocardial ablation had epicardial arrhythmic substrate, and Acosta et al. [15] showed that an endocardial ablation in patients with transmural MI was associated with an increased risk of recurrence. Tung et al. [16] showed better VT-free survival for IHD patients after a combined endo-epi ablation vs. endocardial only, which can partly be explained by the high number of previous endocardial ablation (80%) in their series. Our analysis showed an increase in VT-free survival and a decrease in all-cause death with a C-ABL in IHD patients with multiple ablations, and to the best of our knowledge, this is the first study to show such survival benefit. A recent meta-analysis [17] was also consistent with our findings, showing a reduction in all-cause mortality and VT-recurrence in IHD. These combined findings suggest that an epicardial ablation is probably the most effective strategy for VT elimination and survival improvement after an initial failed endocardial ablation in IHD. VT arrhythmogenic substrate differs from NICM and IHD patients. An analysis of 445 patients undergoing VT ablation showed that some NICM VT’s critical isthmi cannot be identified in either endo or epicardium, possibly due to mid myocardial location [18]. Current guidelines suggest an initial endocardial ablation or possibly a first epicardial ablation if the arrhythmia has characteristics pointing to an epicardial origin [1, 18], and NICM patients have shown VT-free survival from 41 to 70% at 1-year after the procedure [19]. The worse outcomes of VT ablation in NICM patients appear to be related to intramural or epicardial isthmi [20]. Acute success and VT-freedom after catheter ablation in NICM patients are associated with a reduction in mortality and heart transplantation [19]. To the best of our knowledge, this is also the first study to show an improvement in VT-free survival and reduction of all-cause death with a C-ABL compared to a NC-ABL in NICM patients undergoing more than 1 ablation procedure. The fundamental principle of PS matching is the homogenization of a chosen set of covariates according to a dependent variable, in a pseudo-randomized controlled design [7]. This statistic technique is of particular interest in our study since we are presented with 2 heterogeneous groups with several potential confounding variables. PS matching allows the reduction of the impact of other independent variables in the outcome analysis, strengthening the findings of the initial analysis and allowing a more accurate assessment of the safety outcomes. Serious and potentially life-threating complications can be associated with the subxiphoid epicardial ablation technique [11, 14, 17]. Common procedure-related complications are right ventricular puncture, subxiphoid bleeding, pericardial effusion, cardiac tamponade, coronary arteries lesion, acute myocardial infarction, thoracic artery lesion, complete heart block, phrenic nerve lesion, abdominal organ puncture, and stroke. Our population had a high burden of traditional cardiovascular risk factors (Table 1), and there was a considerable amount of ES at presentation. Although this was a high-risk population, our complication rate was comparable to the current reports in the literature. In this real-world population analysis, the C-ABL strategy had a similar safety profile when compared to the NC-ABL approach, despite a much higher number of epicardial ablations. While we cannot completely exclude the influence of underpowering in the procedural complication rate analysis, the potential benefit of the combined or sequential ablation appeared to outweigh the risks.
4.1 Limitations
Being a retrospective observational analysis, our study lacks the randomization of potential future trials. Also, our research analyzed the populations of two high-volume national reference centers of VT ablation, whose results may not be generalized to all centers. Third, while PS matching was used to assess the robustness of our results, the non-inclusion of other potential confounding variables cannot be guaranteed. Fourth, it was not possible to access detailed information on the ventricular scars (voltage maps or cardiac magnetic resonance imaging) in all patients, and CMRI was not systematically performed. The coronary venous system was also not systematically mapped prior to percutaneous epicardial access. Also, the heterogeneity of the study population may have an impact in the outcomes. At last, the choice of combined or non-combined strategy, and the choice of the epicardial approach in ICM patients, was done at the operator’s discretion, which may create a non-objectifiable bias.
5 Conclusion
In patients undergoing repeated VT catheter ablations, a combined endo-epicardial strategy, either in the same or in different procedures, was associated with lower VT recurrence and lower all-cause death. The superiority of a combined vs. non-combined approach was consistent in both ischemic and non-ischemic populations. Both strategies seemed equally safe.
Change history
09 May 2022
Springer Nature’s version of this paper was updated: In the first line of the abstract conclusion, “TV ablation strategy” should instead be “VT ablation strategy".
References
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91–220.
Hohnloser SH, Al-Khalidi HR, Pratt CM, Brum JM, Tatla DS, Tchou P, Dorian P. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J. 2006;27(24):3027–32.
Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. J Arrhythmia. 2019;35(3):323–484.
Di Biase L, Santangeli P, Burkhardt DJ, Bai R, Mohanty P, Carbucicchio C, et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60(2):132–41.
Berruezo A, Acosta J, Fernández-Armenta J, Pedrote A, Barrera A, Arana-Rueda E, et al. Safety, long-term outcomes and predictors of recurrence after first-line combined endoepicardial ventricular tachycardia substrate ablation in arrhythmogenic cardiomyopathy. Impact of arrhythmic substrate distribution pattern. A prospective multicentre study. Europace. 2017;19(4):607-616.
Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7(6):531–6.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009–17.
Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118(25):2773–82.
Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med. 2016;375(2):111–21.
Schmidt B, Chun KRJ, Baensch D, Antz M, Koektuerk B, Tilz RR, et al. H. Catheter ablation for ventricular tachycardia after failed endocardial ablation: epicardial substrate or inappropriate endocardial ablation? Heart Rhythm. 2010;7(12):1746–52.
Schreieck J, Zrenner B, Deisenhofer I, Schmitt C. Rescue ablation of electrical storm in patients with ischemic cardiomyopathy: a potential-guided ablation approach by modifying substrate of intractable, unmappable ventricular tachycardias. Heart Rhythm. 2005;2(1):10–4.
Izquierdo M, Sánchez-Gómez JM, Ferrero de Loma-Osorio A, Martínez A, Bellver A, Peláez A, et al. Endo-epicardial versus only-endocardial ablation as a first line strategy for the treatment of ventricular tachycardia in patients with ischemic heart disease. Circ Arrhythm Electrophysiol. 2015;8(4):882–9.
Sarkozy A, Tokuda M, Tedrow UB, Sieria J, Michaud GF, Couper GS, John R, Stevenson WG. Epicardial ablation of ventricular tachycardia in ischemic heart disease. Circ Arrhythm Electrophysiol. 2013;6(6):1115–22.
Acosta J, Fernández-Armenta J, Penela D, Andreu D, Borras R, Vassanelli F, et al. Infarct transmurality as a criterion for first-line endo-epicardial substrate-guided ventricular tachycardia ablation in ischemic cardiomyopathy. Heart Rhythm. 2016;13(1):85–95.
Tung R, Michowitz Y, Yu R, Mathuria N, Vaseghi M, Buch E, et al. Epicardial ablation of ventricular tachycardia: an institutional experience of safety and efficacy. Heart Rhythm. 2013;10(4):490–8.
Romero J, Cerrud-Rodriguez RC, Di Biase L, Diaz JC, Alviz I, Grupposo V, et al. Combined Endocardial-Epicardial Versus Endocardial Catheter Ablation Alone for Ventricular Tachycardia in Structural Heart Disease: A Systematic Review and Meta-Analysis. JACC Clin Electrophysiol. 2019;5(1):13–24.
Shirai Y, Liang JJ, Santangeli P, Arkles JS, Schaller RD, Supple GE, et al. Comparison of the Ventricular Tachycardia Circuit Between Patients With Ischemic and Nonischemic Cardiomyopathies. Circ Arrhythm Electrophysiol. 2019;12(7):e007249.
Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K, et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12(9):1997–2007.
Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch E, et al. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55(21):2355–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The patients signed an informed consent both for the procedure and publication of any relevant data.
Conflict of interest
The authors report no financial relationships or conflicts of interest regarding the content herein.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matos, D., Adragão, P., Pisani, C. et al. Outcomes of a combined vs non-combined endo-epicardial ventricular tachycardia ablation strategy. J Interv Card Electrophysiol 66, 87–94 (2023). https://doi.org/10.1007/s10840-022-01175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01175-3