Abstract
Background
Inappropriate sinus tachycardia (IST) often causes palpitations, dyspnea, and exercise intolerance, that are generally treated with beta blockers and non-dihydropyridine calcium-channel antagonists. Ivabradine, a selective inhibitor of cardiac pacemaker If current, has recently emerged as an effective and safe alternative to conventional drugs for IST.
Methods
We performed a systematic overview of clinical studies on the therapeutic yield of ivabradine in patients with inappropriate sinus tachycardia, published in MEDLINE database from January 2000 to March 2015.
Results
Overall, five case reports were found, all showing efficacy of ivabradine in subjects affected by IST. Eight non-randomized clinical studies demonstrated short- and medium-term safety and efficacy of ivabradine administration in IST, also in adjunction to or in comparison with metoprolol. One double-blind randomized crossover study also showed that ivabradine is superior to placebo for heart rate (HR) reduction and symptoms control in patients affected by IST.
Conclusions
Ivabradine is effective and safe in short- and medium-term treatment of IST. However, long-term follow-up studies and randomized studies comparing ivabradine with beta blockers are still lacking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Inappropriate sinus tachycardia (IST) is a non-paroxysmal arrhythmia, characterized by a persistent high sinus heart rate (HR) and/or an exaggerated HR in response to minimal exertion, secondary neither to medical disorders nor mental or physical stress [1, 2]. Generally, patients with IST have resting daytime sinus rate of more than 100 beats per minute and average 24-h Holter ECG HR of more than 90 beats per minute, in the absence of conditions commonly known to increase HR [1, 2]. Multiple mechanisms have been proposed to explain the pathophysiology of IST, including sympatho-parasympathetic imbalance, β-adrenergic receptor super-sensitivity, β-adrenergic receptor auto-antibodies, autonomic neuropathy, and intrinsic abnormality in the sinus node [1, 2].
IST can be responsible for palpitations, chest pain, dyspnea, and dizziness, that are generally treated with HR-lowering agents such as beta blockers and non-dihydropiridinic calcium-channel antagonists [3]. These drugs, however, are often insufficient or not well tolerated because of side effects, that often limit the administered dose. Rarely, invasive procedures such as radiofrequency modification of the sinus node are performed in the most resistant cases [4].
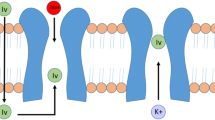
Ivabradine is a selective and dose-dependent inhibitor of the mixed inward Na+/K+ funny (If) current mediated by the HCN4 channels localized in the sinoatrial node [5]. Ivabradine is characterized by a use-dependent effect, due to a slowly progressing block accumulation during repetitive channel activation/deactivation cycles, that is responsible for a higher HR reduction during sinus tachycardia and a minimal effect during normal HR. It also has a neutral inotropic effect and does not affect the atrioventricular and intraventricular conduction times and ventricular repolarization, appearing to be particularly safe [5]. Ivabradine is approved for patients with stable angina due to its ability to reduce anginal symptoms and to increase exercise tolerance by reducing heart rate [6]. For patients with heart failure, it has been demonstrated to reduce hospitalization [7]. However, due to its efficacy and its favorable profile, ivabradine has been used in various different clinical contexts, including IST.
The aim of this study was to perform a systematic overview of clinical studies on the therapeutic yield of ivabradine in patients with IST.
2 Methods
A MEDLINE-search with the terms “inappropriate sinus tachycardia” and a systematic overview of clinical studies on treatment of IST with ivabradine was done.
Prospective clinical studies and case reports of ivabradine administration in IST [1] were included in the review. Moreover, clinical studies that met all of the following specified criteria were considered:
-
1)
Publication before February 2015 in peer-reviewed journals indexed in MEDLINE, in English language, involving human subjects affected by IST, defined as resting daytime sinus rate of more than 100 beats per minute and/or average 24-h Holter ECG HR of more than 90 beats per minute, in the absence of conditions commonly known for increasing HR [1];
-
2)
Study protocol: at least 7 days of ivabradine administration for control of IST-related symptoms, regardless of eventual concomitant anti-arrhythmic drugs intake;
-
3)
HR reduction after ivabradine administration as a specified endpoint, assessed with rest ECG and/or Holter ECG.
Randomized controlled trials comparing ivabradine versus placebo or versus beta blockers in IST treatment, with either open-label, blind or crossover designs, were included. From each article, the following outcome measures were considered: mean, maximal and minimal Holter ECG HR, ECG stress test-maximal HR, total exercise time, and metabolic equivalents (METS); adverse event occurrence secondary to ivabradine administration; and IST-related symptoms reduction after ivabradine treatment.
3 Summary of studies
Case reports of ivabradine use in IST
Some case reports have been published regarding the use of ivabradine in IST, all demonstrating its short- and long-term effectiveness and safety, in men and women of various age [8–12] (Table 1). Almost all patients were unsuccessfully treated with or presented contra-indications to beta blockers and/or other anti-arrhythmic drugs. Studies have demonstrated that ivabradine reduces HR, normalizes functional parameters, including stress test ECG, and improves symptoms and quality of life, without significant side effects (Table 1).
Non-randomized clinical studies of ivabradine in IST
The encouraging results of previous case reports were followed by several prospective studies evaluating the safety and efficacy of ivabradine in subjects affected by IST (Table 2). The first one [13] evaluated 13 patients affected by IST and demonstrated ivabradine to cause a reduction in Holter ECG mean HR of about 20 % of basal value. In one patient, ivabradine was added to metoprolol, allowing for a better HR control and attenuation of side effects secondary to the beta blocker with no marked sinus bradycardia.
A second trial [14], including ten women affected by IST resistant to beta blockers, class IC anti-arrhythmic drugs, or calcium-channel antagonists (CCA), demonstrated that ivabradine, alone or in combination with other drugs, reduced the average HR of about 12 %. Ivabradine did not lower the minimal Holter ECG HR, likely due to its use-dependent effect. Only three out of ten patients reported self-limiting phosphenes (objective visual sensations occurring in the absence of retinal stimulation), which did not lead to drug discontinuation.
In a further study [15], including 24 patients, 6-month treatment with ivabradine caused an 18 % reduction in Holter mean HR and an improvement of symptoms, assessed by the SF-36 Health Survey quality of life questionnaire. Notably, after 1 year, among ten patients who were asked to stop ivabradine, only two remained on IST criteria, suggesting an eventual long-term drug-related remodeling effect on HR.
Another small but well-designed study by Kaplinsky et al. [16], enrolling four IST women affected by IST complaining of effort intolerance, dyspnea, and palpitations, demonstrated that ivabradine improved Holter ECG mean HR of about 24 % of baseline level after 3 months. Resting ECG HR also showed a progressive decrease, with a dose-dependent effect. ECG stress test showed longer total exercise time, together with a greater intensity of effort, probably thanks to an improvement in the cardiac chronotropic reserve.
A further study conducted by our group [17], involving 18 patients suffering IST, evaluated at baseline and after 1, 3, and 6 months of ivabradine treatment, showed a progressive reduction in the maximal, average, and minimal HR values on Holter ECG. However, after 6 months, the minimal HR lowering was not statistically significant, suggesting a safe profile of ivabradine. A strong correlation was observed between pretreatment mean HR and reduction at Holter ECG at the end of follow-up [17], as justified by ivabradine use-dependent effect. The stress test also revealed a significant decrease of maximal HR in comparison to the pretreatment value, together with an improvement of stress tolerance, as proved by the progressive increase of the maximal load of exercise reached (Table 2). Notably, after 6 months of treatment, no patient complained of symptoms. In adjunction, ivabradine was safe, since it provoked only early manifesting and mostly reversible phosphenes.
Non-randomized studies comparing ivabradine and beta blockers in IST
Two non-randomized controlled studies were aimed at comparing ivabradine versus beta blockers for IST treatment (Table 2). The first one [18] compared administration of ivabradine versus metoprolol succinate (mean dose, 157 ± 38 mg/day) in 20 subjects. The study protocol included metoprolol administration for 1 month, followed by Holter ECG and treadmill test. Thereafter, metoprolol was interrupted and replaced by ivabradine treatment for 1 month, followed again by Holter ECG and treadmill test. The study demonstrated that ivabradine was superior to metoprolol in reducing Holter ECG daily HR, in increasing the duration of treadmill exercise test and in controlling symptoms, probably due to the scarce tolerance to the beta blocker among patients suffering IST. The target dose of metoprolol was indeed reached in only 50 % of them. Notably, only metoprolol caused episodes of nocturnal sinus bradycardia.
The same authors [19] also compared the efficacy and safety of a month’s treatment with metoprolol alone or in combination with ivabradine in 20 patients (Table 2). Both regimens were safe and effective. However, Holter ECG mean HR during daily activity was significantly better lowered by ivabradine + metoprolol as compared to monotherapy [19]. Moreover, the combined treatment yielded a significant improvement in exercise capacity, as assessed by treadmill stress test, and a significant reduction in IST-related symptoms [19]. The latter were evaluated by means of the European Heart Rhythm Association score [20], that is commonly adopted to assess AF-related symptoms and was previously demonstrated to be useful for assessment of symptoms in subjects affected by IST by the same authors [18].
Randomized clinical studies of ivabradine in IST
A double-blind, placebo-controlled crossover study [21] was recently published, enrolling 21 patients affected by IST, who were firstly randomized to receive 6-week treatment with ivabradine or placebo, and secondarily, after a washout period, to cross over for an additional 6 weeks. When compared to placebo, ivabradine caused a significant reduction of mean, maximal, and minimal Holter ECG HR and of maximal HR during stress ECG. Moreover, it led to a significant increase in exercise performance with no cardiovascular side effects and to resolution of symptoms in almost half of patients.
A further randomized study (www.clinicaltrial.org; NCT01657136), comparing the safety and effectiveness of ivabradine versus bisoprolol in consecutive patients affected by IST, is currently ongoing in our center [22]. Preliminary results, about 24 subjects, revealed that both drugs effectively reduced Holter ECG mean HR at 3 months with respect to baseline level. However, ivabradine alone also lowered maximal and minimal Holter ECG HR and maximal HR at stress test. A complete resolution of IST-related symptoms occurred in about 90 and 75 % of cases with ivabradine and bisoprolol, respectively. Impaired vision (mostly phosphenes) occurred in 5.3 % of patients with ivabradine, while bisoprolol caused hypotension in 25 % of cases. The higher efficacy of ivabradine in comparison to bisoprolol observed in our study is probably explained by the scarce tolerance to the beta blocker, whose mean dose administered was relatively low (2 ± 1 mg/day).
4 Discussion
Overall, case reports and clinical studies on ivabradine administration in patients affected by IST demonstrated that ivabradine, although is still an off-label therapy:
-
1)
is effective in reducing HR at rest and during effort, which are reflected by mean and maximal Holter ECG, respectively.
-
2)
successfully controls IST-related symptoms, also in patients resistant to beta blockers and CCA.
-
3)
seems to be superior to metoprolol in limiting exaggerate HR increase during effort and in attenuating IST-related symptoms.
-
4)
is safe and does not cause any significant side effect, including excessive sinus bradycardia, even though its HR-lowering effect is progressive over time, as revealed by slight decrease of minimal Holter ECG HR.
-
5)
is safe and effective in association with metoprolol to treat resistant IST.
-
6)
in some subjects, may induce HR normalization after long-lasting administration, persisting also after drug discontinuation.
Despite characterized by a generally good prognosis, the management of patients affected by IST may be challenging. Indeed, IST-related symptoms are often interpreted as a marker of anxiety, with consequent delay in the correct diagnosis of the arrhythmia. Moreover, drugs commonly employed in IST, such as beta blockers and CCA, often cause hypotension and fatigue, with limited effectiveness eventually needing dose reduction.
Ivabradine effects on symptoms and clinical outcomes
Clinical studies on ivabradine administration in IST, although limited by designs, small sample sizes, and short follow-ups, reveal that this drug is effective in terms of HR lowering, exercise capacity improvement, and symptoms reduction, also in patients unresponsive to beta blockers and anti-arrhythmic drugs [8–19, 21, 22]. Moreover, ivabradine has demonstrated to be safe with no significant side effects [8–19, 21, 22].
Ivabradine effects on the sinus node, heart rate, and hemodynamics
The possible explanation of ivabradine’s high efficacy in the treatment of IST may be found in its specific action mechanism. The drug, indeed, directly inhibits the If current in the sinoatrial node and causes a reduction of the steepness of phase 4 of action potential, with consequent HR lowering [5]. Beta blockers and anti-arrhythmic drugs, conversely, regulate the If current only indirectly [5].
A further strength of ivabradine relies in its use-dependent effect, with a progressive If current block accumulation during repetitive channel activation/deactivation cycles [5]. Consequently, the faster the sinus tachycardia is, the higher the probability that ivabradine binds to the HCN4 channel. This makes this drug not really a HR-lowering agent, but rather an anti-tachycardic one, with eventual particular benefit in IST forms mostly characterized by daily elevated HR values normalizing during the night [3]. In these cases, indeed, beta blockers or CCA should be avoided because of the risk of nocturnal sinus bradycardia. Moreover, the neutral effect of ivabradine on cardiac inotropism, blood pressure, and atrioventricular and intraventricular conduction times allows for drug dose optimal titration [5]. This gives ivabradine a great advantage over beta blockers, CCA, and other anti-arrhythmic drugs, whose side effects often limit the dose to be safety administered.
Comparison of ivabradine to beta blockers
The superiority of ivabradine over metoprolol in IST treatment, both in terms of HR and symptoms control, has been suggested by a non-randomized study [18], that however is limited by its sub-optimal design, the short follow-up (1 month), and selection bias (patients unresponsive to beta blockers).
Initial results of an ongoing randomized controlled study [22] confirm the superiority of ivabradine over bisoprolol in reducing HR during effort and suggest a higher ability of this drug in improving stress tolerance and symptoms, likely due to its neutral effect on hemodynamics and its favorable impact on cardiac chronotropic reserve [5]. However, to confirm such hypothesis, the conclusion of the study is to be expected. Whenever non-inferiority or even a superiority over bisoprolol (and beta blockers in general) is demonstrated, ivabradine could eventually be considered not only as an alternative, but as the potential first-line treatment for IST. This may be justified by ivabradine’s higher efficacy in attenuating HR during effort, which is particularly diffused among patients affected by IST, and mostly by its high tolerability, that will eventually make this drug a “first choice” in subjects generally complaining of low blood pressure values.
Future directions
An interesting future field of research is represented by the long-term effect of ivabradine in IST, with a particular focus on possible normalization of intrinsic HR over time, persistent even after drug discontinuation, as demonstrated in some patients in a recent study [15]. This phenomenon is not yet clear. However, in a rat model of myocardial infarction, funny current and HCN4 transcription overexpression were demonstrated to be significantly reduced in ventricle tissue by ivabradine, thanks to post-transcriptional expression modulation of the HCN4 gene [23]. This could suggest eventual influence of ivabradine on HCN4 transcription overexpression in patients suffering IST.
5 Conclusions
Published case reports and clinical studies demonstrated that ivabradine is effective in reducing HR at rest and during effort and successfully controlled IST-related symptoms, also in patients resistant to beta blockers and CCA. Moreover, ivabradine is safe, does not cause any significant side effect, including excessive sinus bradycardia, and seems to be superior to metoprolol, both in terms of control of symptoms and of HR during effort. Further randomized trials comparing ivabradine versus beta blockers could possibly confirm this drug as the first-line treatment for IST. Moreover, long-term studies on ivabradine administration in patients affected by IST could add information on its eventual “remodeling effect” on funny current, leading to definitive treatment of this arrhythmia.
Abbreviations
- Bpm:
-
Beats per minute
- CCA:
-
Calcium-channel antagonists
- EF:
-
Ejection fraction
- HF:
-
Heart failure
- HR:
-
Heart rate
- IST:
-
Inappropriate sinus tachycardia
- LV:
-
Left ventricular
- METS:
-
Metabolic equivalents
References
Sheldon, R. S., Grubb, B. P., 2nd, Olshansky, B., Shen, W. K., Calkins, H., Brignole, M., et al. (2015). Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm, 12, e41–e63.
Still, A. M., Raatikainen, P., Ylitalo, A., Kauma, H., Ikäheimo, M., Antero Kesäniemi, Y., et al. (2005). Prevalence, characteristics and natural course of inappropriate sinus tachycardia. Europace, 7, 104–112.
Rubenstein, J. C., Freher, M., Kadish, A., & Goldberger, J. J. (2010). Diurnal heart rate patterns in inappropriate sinus tachycardia. Pacing and Clinical Electrophysiology, 33, 911–919.
Lin, D., Garcia, F., Jacobson, J., Gerstenfeld, E. P., Dixit, S., Verdino, R., et al. (2007). Use of noncontact mapping and saline-cooled ablation catheter for sinus node modification in medically refractory inappropriate sinus tachycardia. Pacing and Clinical Electrophysiology, 30, 236–242.
DiFrancesco, D. (2010). The role of the funny current in pacemaker activity. Circulation Research, 106, 434–446.
Montalescot, G., Sechtem, U., Achenbach, S., Andreotti, F., Arden, C., Budaj, A., et al. (2013). 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. European Heart Journal, 34, 2949–3003.
McMurray, J. V., Adamopoulos, S., Anker, S. D., Auricchio, A., Bohm, M., Dickstein, K., et al. (2012). ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. European Heart Journal, 14, 803–869.
Retegui, G., Quintero, M., Ruiz-Borrell, M., & Revello, A. (2009). Ivabradine as a treatment option for inappropriate sinus tachycardia. Revista Española de Cardiología, 62, 577–579.
Celebi, O. O., Canbay, A., Celebi, S., Aydogdu, S., & Diker, E. (2010). Inappropriate sinus tachycardia-successful treatment with ivabradine. Kardiologia Polska, 68, 935–937.
Schulze, V., Steiner, S., Hennersdorf, M., & Strauer, B. E. (2008). Ivabradine as an alternative therapeutic trial in the therapy of inappropriate sinus tachycardia. Cardiology, 110, 206–208.
Weyn, T., Stockman, D., & Degreef, Y. (2011). The use of ivabradine for inappropriate sinus tachycardia. Acta Cardiologica, 66, 259–262.
Wilson, D., & Crook, B. (2009). Ivabradine for inappropriate sinus tachycardia. British Journal of Cardiology, 16, 151–152.
Rakovec, P. (2009). Treatment of inappropriate sinus tachycardia with ivabradine. Wiener Klinische Wochenschrift, 121, 715–718.
Zellerhoff, S., Hinterseer, M., Felix Krull, B., Schulze-Bahr, E., Fabritz, L., Breithardt, G., et al. (2010). Ivabradine in patients with inappropriate sinus tachycardia. Naunyn-Schmiedebergs Arch Pharmacol, 382, 483–486.
Benezet-Mazuecos, J., Rubio, J. M., Farrè, J., Quiňones, M. A., Pepa, S. B., & Macìa, E. (2013). Long-term outcomes of ivabradine in inappropriate sinus tachycardia patients: appropriate efficacy or inappropriate patients. Pacing and Clinical Electrophysiology, 36, 830–836.
Kaplinsky, E., Comes, F. P., Urondo, L. S., & Ayma, F. P. (2010). Efficacy of ivabradine in four patients with inappropriate sinus tachycardia: a three month-long experience based on electrocardiographic, Holter monitoring, exercise tolerance and quality of life assessment. Cardiology Journal, 17, 166–171.
Calò, L., Rebecchi, M., Sette, A., Martino, A., de Ruvo, E., Sciarra, L., et al. (2010). Efficacy of ivabradine administration in patients affected by inappropriate sinus tachycardia. Heart Rhythm, 7, 1318–1323.
Ptaszynski, P., Kaczmarek, K., Ruta, J., Klingenheben, T., & Wranicz, J. K. (2013). Metoprolol succinate vs. ivabradine in the treatment of inappropriate sinus tachycardia in patients unresponsive to previous pharmacological therapy. Europace, 15, 116–121.
Ptaszynski, P., Kaczmarek, K., Ruta, J., Klingenheben, T., Cygankiewicz, I., & Wranicz, J. K. (2013). Ivabradine in combination with metoprolol succinate in the treatment of inappropriate sinus tachycardia. Journal of Cardiovascular Pharmacology and Therapeutics, 18, 338–344.
Wynn, G. J., Todd, D. M., Webber, M., Bonnett, L., McShane, J., Kirchhof, P., et al. (2014). The European Heart Rhythm Association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace, 16, 965–972.
Cappato, R., Castelvecchio, S., Ricci, C., Bianco, E., Vitali-Serdoz, L., Gnecchi-Ruscone, T., et al. (2012). Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia. Journal of the American College of Cardiology, 60, 1323–1329.
Martino A, Sette A, Rebecchi M, de Ruvo E, Sciarra L, Calo’ L. Ivabradine versus bisoprolol for inappropriate sinus tachycardia. Abstract 14–9. ECAS Congress 2015. April 19, 2015.
Suffredini, S., Stillitano, F., Comini, L., Bouly, M., Brogioni, S., Ceconi, C., et al. (2012). Long-term treatment with ivabradine in post-myocardial infarcted rats counteracts f-channel overexpression. British Journal of Pharmacology, 165, 1457–1466.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Annamaria, M., Lupo, P.P., Foresti, S. et al. Treatment of inappropriate sinus tachycardia with ivabradine. J Interv Card Electrophysiol 46, 47–53 (2016). https://doi.org/10.1007/s10840-015-0066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-015-0066-5