Abstract
The full potential linearized augmented plane wave based on density functional theory is carried out to study the magnetism and electronic structures of group IA elements (K, Rb, Cs)-doped zinc blende ZnX (X = S, Se). Total energy calculations indicate that the ferromagnetic phase is always energetically more stable than the non-magnetic phase at equilibrium volume. All doped systems are half-metallic ferromagnets with gap in the majority spin channel. The energy band gap decreases on increasing the size of dopant atom from K to Cs. The magnetic moments are mainly carried by anions (S or Se) surrounding the dopant atom. The intrinsic ferromagnetism is originated from the partly filled anionic p orbitals, which is different from classical magnetic materials containing transition metal (TM) atoms. The absence of TM atoms makes these alloys interesting candidates for the study of half-metallic p electron ferromagnetism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spintronics, or spin-based electronics, which offers fascinating opportunities for a new generation of devices by exploiting the spin of electrons as well as their charge [1, 2] has attracted much attention over the past two decades. Half-metallic ferromagnets (HMFs), where one of the two spin channels is semiconducting (either spin-up or spin-down) and the other is metallic, are the most desirable components for the high-performance spintronic devices as they provide nearly 100% spin polarization at the Fermi level (EF). In particular, diluted magnetic semiconductors (DMSs), fabricated by doping conventional compounds semiconductors with magnetic elements have triggered more research [3,4,5,6] aiming at finding materials that are HMFs. For practical device applications, the search for DMS exhibiting the ferromagnetism at or above room temperature (RT) is required. Since Dietl et al. [7] theoretically predicted that RT ferromagnetism could exist in GaN- and ZnO-based DMSs, extensive experimental and theoretical studies have been directed toward wide band gap semiconductors III-V and II-VI in searching for RT ferromagnetic DMS materials [8,9,10,11,12,13,14,15]. However, the literature shows that transition metals (TM) find lower solubility in III-V semiconductors, when compared to II-VI semiconductors [16]. In this respect, TM-doped II-VI (such as ZnO, ZnS, ZnSe, ZnTe, CdS, CdSe, etc.) compounds have remained a focus of great attention by various research groups. For instance, Sambasivam et al. found ferromagnetism at RT in Co/fe-doped ZnS [17, 18]. Lakshmi et al. studied magnetism in Mn: ZnS nanocrystalline and found room temperature ferromagnetism [19]. In the experimental study for Co-, Cr- and V-doped ZnO [20], the magnetization measurements showed that all the samples exhibited RT ferromagnetism. Also, the ferromagnetism behavior was observed experimentally in Cr-doped ZnTe [21, 22]. Soundararajan et al. [21] revealed ferromagnetism in Zn1−x Crx Te (x = 0.05) alloy powder with Curie temperature (TC) much greater than RT. Hou et al. [22] obtained TC of 365 K for Cr concentration > 0.18.
Although a large amount of the literature has been reported on the magnetism of TMs-doped DMSs, experimental works have produced inconsistent results and the mechanism of ferromagnetism of such systems is still under active debate. This because the TMs dopants often have a clustering tendency or secondary phase [23, 24] which are detrimental to applications of DMS. To overcome this drawback, a new class of DMS with intrinsic ferromagnetism behavior has been discovered through doping of non-magnetic atoms into the host semiconductors. This phenomenon is known in the literature with various names as d0 ferromagnetism, p-electron ferromagnetism or sp-electron ferromagnetism. Several theoretical investigations have shown that appropriate non-magnetic elements substitutions in II-VI semiconductors like C-doped ZnO [25] and CdS [26], B/C/N-doped BeO [27] and CaO [28], Cu-doped ZnO [29], as well as K/Cu-doped MgS [30] can induce intrinsic ferromagnetism. On the other hand, experimental observation of RT ferromagnetism in Cu-, N- and C-doped ZnO [31,32,33] confirmed these theoretical predictions.
During last decade, the binary II–VI chalcogenides ZnS and ZnSe have received enormous research interests in the field of high performance optoelectronic devices due to their wide band gap. The both compounds crystallize in zinc blende structure (ZB) at an ambient pressure but can be also prepared with wurtzite structure. Though the ZnS/Se compounds are in the same family and have the same stable phase, their electronic properties (mainly band gap) are different from each other. Bulk zinc sulfide (ZnS), with a larger band gap of 3.68 eV [34], is an important candidate for ultraviolet light-emitting diodes (LEDs), solar cells, optical sensors, photocatalytic devices, etc. [35,36,37,38], whereas bulk zinc selenide (ZnSe), which has a band gap of ~ 2.8 eV, playing a striking role for application in energy upconversion [39]. Moreover, besides the mentioned properties of ZnS/Se compounds, new functionality can be added to these materials by means of introducing magnetic degree of freedom [40, 41].
These works motivated us to study the possibility of HM ferromagnetism in ZnX (X = S, Se) with group IA elements (K, Rb, Cs) as dopants, searching for further HMFs that do not contain TM elements. Therefore, in the present paper we investigate the electronic and magnetic properties of these alloys in their ordered ZB structure by using first-principles density functional calculations. The roles played by alkali dopants and chalcogen atoms in ferromagnetic properties are discussed in order to establish the DMSs characteristics. The paper is structured as follows. The methodology is described in Sect. 2, while results and discussions are presented in Sect. 3. Finally, the concluding remarks are drawn in Sect. 4.
2 Computational method
The present calculations are performed by using the accurate full potential linearized augmented plane wave (FP-LAPW) method as incorporated in the wien2K package [42] within the framework of density functional theory (DFT) [43]. The generalized gradient approximation (GGA) parameterized by Perdew, Burke and Ernzerhof (PBE) [44] is used for the exchange and correlation potential.
The un-doped cells ZnS and ZnSe have zinc blende (B3) structure with space group of 216 \(\left( {F\overline{4}3m} \right)\), where the Zn atom is located at (0, 0, 0) and S/Se atom at (0.25, 0.25, 0.25) position (Fig. 1a). To simulate our doped systems ZnMX (M = K, Rb, Cs; X = S, Se), we have constructed cubic supercell Zn8X8 with 2 × 2 × 2 dimension, which contains 8 Zn and 8 (S/Se)atoms (Fig. 1b). For 12.5% doping concentration of M atom, the Zn atom positioned at (0, 0, 0) in supercell Zn8X8 is replaced by M atom forming the ternary alloy Zn0.875M0.125X, as shown in Fig. 1c.
To reach an appropriate degree of convergence, the cutoff parameter RMT Kmax is taken as 8.0, where RMT is the smallest muffin-tin radius and Kmax is the maximum modulus for the reciprocal lattice vectors. Inside the atomic spheres, the maximum value of angular momentum for the wave function expansion is l = 10. The fully relativistic and scalar relativistic approach is used for core and valence electrons, respectively. Muffin-tin radii (RMT) are chosen to be 2.0 bohr for S, 2.2 bohr for K, 2.4 bohr for Cs and 2.3 bohr for other atoms. For geometry optimization and physical property calculation, the Brillouin zone is sampled by Monkhorst–Pack mesh [45] of 5 × 5 × 5 k-points. In our calculations, the atomic coordinates are fully relaxed until the maximum force acted on each atom is smaller than 2.10−3Ry/a.u., and the self-consistent convergence of the total energy is set to 10–5 Ry/cell.
3 Results and discussions
The stability of the materials is one step forward to get insight into their physical properties. In this paper, the stability of studied DMS compounds has been estimated from the variation of total energy with respect to cell volume in both non-magnetic (NM) and ferromagnetic (FM) states. The equilibrium lattice constant (a), bulk modulus (B), its pressure derivative (Bʹ) and the total energy difference between NM and FM states \(\left( {\Delta E = E_{{{\text{NM}}}} - E_{{{\text{FM}}}} } \right)\) at their equilibrium lattice constants are listed in Table 1. These predicted results have been obtained by fitting the calculated total energies as function of volume using Murnaghan’s equation of state (EOS) [46]. This EOS describes the relationship between the variables (E, V) and it is given by
where \(V_{0}\) is volume at zero pressure (i.e., equilibrium volume), \(V\) is the volume at pressure \(P\) and \(E_{0}\) is the energy corresponding to the \(V_{0}\). The \(B\) and \(B^{\prime}\) can be calculated from the following formula:
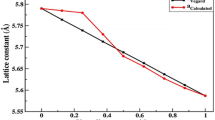
Our calculated lattice parameters increased from K- to Cs-doped ZnS/Se, which can be attributed to the increase in atomic radii. For all the six compounds, the FM state is more favorable in energy than the corresponding NM state. To evaluate the structural stability, the cohesive energy is introduced to make easy a comparison of stability of compounds. The cohesive energy of a solid is defined as the energy required to decompose it into single atoms, which is a measure of bonds strength. We calculated the cohesive energy of each compound using relation [47]
where \(E_{{{\text{coh}}}}^{{\text{ZnMS/Se}}}\) refers to the total energy of the ZnMS/Se compounds, \(E_{{{\text{iso}}}}^{{{\text{Zn}}}}\), \(E_{{{\text{iso}}}}^{{\text{M}}}\) and \(E_{{{\text{iso}}}}^{{\text{S/Se}}}\) are the energies of an isolated Zn, M and S/Se atoms, respectively. l, m and n are the number of each atom in the unit cell. The cohesive energy versus volume curves under FM order are displayed in Fig. 2. For the sake of simplicity, we have presented the cohesive energy plots for only FM ground state. Figure 2 shows that absolute value of cohesive energy decreases as the doped elements range from K to Cs, which suggest that the stability of the alloys decreases with increasing dopant size which is consistent with decreasing bulks modulus given in Table 1.
The calculated total magnetic moment in all investigated compounds is exactly 1.00 μB, which are typical HM ferromagnets. It is well known that the HM ferromagnets with large magnetic moment are expected to present large stray magnetic fields, and thus lead to considerable energy losses in spintronic devices applications [48]. Thus, to this respect, a very interesting case is HM magnets which possess small values of magnetic moment. The total and atomic resolved magnetic moments of the alkali-doped ZnX (X = S, Se) at their equilibrium constants are summarized in Table 2. As indicated in the results, the total magnetic moment is principally contributed by doping atom M, its nearest neighboring X atoms and the interstitial region. However, the other farther X atoms and Zn atoms have vanishing moments. Summing up the magnetic moments, we found more than 62% of all magnetic moments are restricted within MX4 tetrahedron in the doped systems. To obtain the visual impression of the magnetic property of the studied alloys, we have selected Rb-doped ZnS as an example for analysis of its three dimensional (3D) isosurface of spin charge density. This is shown in Fig. 3, where the spin density is localized mainly on the nearest neighboring S atoms, which derives from the p orbital. Although the magnetic moment induced by Rb atoms is small, it activates the p electrons of its connecting S atoms which contribute chiefly to the total moment. Thus alkali metals (K, Rb and Cs) behave as spin polarizers in host matrices.
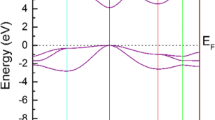
The spin-polarized electronic structures of ZnMS/Se systems have been calculated. In the following, we concentrate the discussion on the computational electronic structure of ZnRbS/Se for which the magnetic stability is highest, while ZnKS/Se and ZnCsS/Se have similar behavior. Figure 4 shows the spin-polarized band structure of ZnRbS/Se alloys for minority spin (spin-down) and majority spin (spin-up) configurations along high-symmetry directions in the first Brillouin zone. The minority spin channels are metallic whereas the majority spin channels keep semiconductor behavior with an indirect energy gap (Eg) of about 1.18 eV and 0.4 eV for ZnRbS and ZnRbSe, respectively. Therefore, these systems are HMFs, leading to 100% carrier spin polarization at the Fermi level \(\left( {E_{f} } \right)\). This is opposite to the properties of HMFs CrS [49] and V- and Cr-doped zinc chalcogenides [50], where the majority spin electrons exhibit metallic character and the energy gap is at the minority spin channel. The bands close to EF in the whole Brillouin zone for both compounds and both spin channels are flat and nearly dispersionless. The main contributor to the flat band is chalcogen p-states. It has been argued by other authors [51, 52] that the mechanism leading to this phenomenon is an important condition for stability of HM ferromagnetism. Above flat band, the lower conduction band is shifted toward the Fermi level as we move along the IA column from K to Cs, and hence, the band gap decreases as shown in Fig. 5. Band gap is also found to decrease as one goes from S to Se atom.
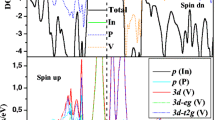
The ternary compound under investigation does not contain TM atoms thus the proposed mechanism of magnetism is different from the Zener’s p–d hybridization [53], the Zener’s double exchange [54], the super-exchange [55] and the Ruderman–Kittel–Kasuya–Yoshida (RKKY) [56] mechanisms. To elucidate the mechanism which may be responsible for observed magnetic behavior in these systems, the total density of states (DOS) and partial DOS of the rubidium atom and one of its four nearest neighboring chalcogen atoms (S, Se) are calculated and illustrated in Figs. 6 and 7. From total DOS (TDOS) plots, we can see that spin splitting near the Fermi level shifts the spin-up states downward and spin-down states upward to lower the total energy of systems. The asymmetrical distributions of TDOS between spin-up and spin-down channels suggest the magnetism of such doped systems (see Figs. 6a and 7a). The partial DOS (PDOS) for each compound shows existing gaps in both spin directions that separate the anion and cation orbitals. The states below the gaps arise exclusively from (S, Se)-p orbitals, while the states above the gaps are of Rb-d orbitals (see Figs. 6b–c and 7b–c). As it is well known that for ZB structure, the tetrahedral crystal field splits the cation d states into threefold degenerate \(t_{2g}\) (dxy, dxz and dyz) and twofold degenerate \(e_{g}\) \(\left( {d_{{x^{2} - y^{2} }} \;{\text{and}}\;d_{{z^{2} }} } \right)\) symmetry states, as presented in Figs. 6d and 7d, while anions p states have \(t_{2g}\) symmetry. Only cation \(t_{2g}\) states can couple with the surrounding anions p orbitals having the same symmetry and create bonding and antibonding hybrid orbitals. The bands around \(E_{f}\) are composed mostly from S-3p/Se-4p states, hybridized slightly with Rb p and \(t_{2g}\) states. These bands lead to an anomalously flat anion p band near the Fermi level, as shown in the previous paragraph, and reflect the bonding states. In contrast to the \(p\) states, most of \(d\) states belonging to \(e_{g}\) and \(t_{2g}\) representations are located at higher conduction energies at about 4–16.5 eV. Hence, these states are not directly involved in formation of spin polarization and ferromagnetism in Rb-doped zinc chalcogenides. It was found in previous study that the origin of HM ferromagnetism in alkali-based beryllium perovskites MBeO3 is attributed to the hole mediated double exchange mechanism through the \(p\)-\(p\) coupling between M and O atoms [57]. In the present case, the exchange splitting of S-3p/Se-4p states around \(E_{f}\) plays the pivotal role in the appearance of the half-metallicity. Thus the magnetic moment is mainly attributed to the (S, Se)-p states, and minor contribution of Rb atom to the total moment results from the hybridization between the (S, Se)-p and Rb p and Rb \(t_{2g}\) states, which is consistent with spin charge density calculation. However, the governing mechanism behind these systems clearly distinguishes them from TM (TM = Mn, Fe, Co, Ni)-doped ZnS/Se [15] where TM d electrons provide the main magnetic moment and the p-d hybridization coupling is responsible for ferromagnetism. We note that in the case of V- and Cr-doped ZnS/Se, the ferromagnetic state is stable via double exchange coupling in which the delocalized antibonding band is partially occupied [50].
4 Conclusion
In conclusion, electronic and ferromagnetic properties of alkali metals (K, Rb and Cs) incorporated ZnS/Se systems have been studied using first-principles calculation. These systems showed 100% spin polarization at Fermi level with gap in the spin-up channel when alkali dopant replaces the Zn site in periodic supercells. The band gap decreases as one goes from K to Cs. In all cases under study, half-metallic ferromagnetism has been observed with an induced magnetic moment of 1.00 μB per dopant atom leading to minimal energy losses in spintronic applications. From the analysis of partial density of states and magnetic moments, the origin of half-metallicity arises from the spin polarization of the chalcogen p-orbitals. Thus alkali metals may be promising non-magnetic dopants for zinc chalcogenides to fabricate dilute magnetic semiconductors free of magnetic precipitates.
Data availability
Enquiries about data availability should be directed to the authors.
References
Wolf, S.A., Awschalom, D.D., Buhrman, R.A., Daughton, J.M., von Molnar, S., Roukes, M.L., Chtchelkanova, A.Y., Treger, D.M.: Spintronics: a spin-based electronics vision for the future. Science 294, 1488–1495 (2001). https://doi.org/10.1126/science.1065389
Žutić, I., Fabian, J., Das Sarma, S.: Spintronics: fundamentals and applications. Rev. Mod. Phys. 76, 323–410 (2004). https://doi.org/10.1103/RevModPhys.76.323
Zhang, Y., Liu, W., Niu, H.: Half-metallic ferromagnetism in Cr-doped AlP-density functional calculations. Solid State Commun. 145, 590–593 (2008). https://doi.org/10.1016/j.ssc.2007.12.022
Zhao, Y.-H., Zhao, G.-P., Liu, Y., Liu, B.-G.: Structural stability and half-metallicity of the zinc-blende phase of Al1−xCrxAs: density-functional study. Phys. Rev. B 80, 224417 (2009). https://doi.org/10.1103/PhysRevB.80.224417
Sajjad, M., Manzoor, S., Zhang, H.X., Noor, N.A., Alay-e-Abbas, S.M., Shaukat, A., Khenata, R.: The half-metallic ferromagnetism character in Be1−xVxY (Y = Se and Te) alloys: an ab-initio study. J. Magn. Magn. Mater. 379, 63–73 (2015). https://doi.org/10.1016/j.jmmm.2014.11.004
Berber, M., Doumi, B., Mokaddem, A., Mogulkoc, Y., Sayede, A., Tadjer, A.: Investigation of electronic structure and half-metallic ferromagnetic behavior with large half-metallic gap in Sr1−xVxO. J. Comput. Electron 16, 542–547 (2017). https://doi.org/10.1007/s10825-017-1038-z
Dietl, T., Ohno, H., Matsukura, F., Cibert, J., Ferrand, D.: Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science 287, 1019–1022 (2000). https://doi.org/10.1126/science.287.5455.1019
Chitta, V.A., Coaquira, J.A.H., Fernandez, J.R.L., Duarte, C.A., Leite, J.R., Schikora, D., As, D.J., Lischka, K., Abramof, E.: Room temperature ferromagnetism in cubic GaN epilayers implanted with Mn+ ions. Appl. Phys. Lett. 85, 3777–3779 (2004). https://doi.org/10.1063/1.1812590
Liu, H.X., Wu, S.Y., Singh, R.K., Gu, L., Smith, D.J., Newman, N., Dilley, N.R., Montes, L., Simmonds, M.B.: Observation of ferromagnetism above 900 K in Cr– GaN and Cr–AlN Appl. Phys. Lett. 85, 4076–4078 (2004). https://doi.org/10.1063/1.1812581
Sato, K., Dederics, P.H., Katayama-Yoshida, H.: Curie temperatures of III–V diluted magnetic semiconductors calculated from first principles. Europhys. Lett. 61, 403–408 (2003). https://doi.org/10.1209/epl/i2003-00191-8
Granville, S., Ruck, B.J., Preston, A.R.H., Stewart, T., Budde, F., Trodahl, H.J., Bittar, A., Downes, J.E., Ridgway, M.: Electronic properties of (Ga, Mn)N thin films with high Mn content. J. Appl. Phys. 104, 103710 (2008). https://doi.org/10.1063/1.3020536
Singh, J., Kumar, S., Verma, N.K.: Enhancement of room temperature ferromagnetism in Cd1−xNixSe nanoparticles. J. Mater. Sci. Mater. Electron 25, 2267–2272 (2014). https://doi.org/10.1007/s10854-014-1870-x
Fang, W., Liu, Y., Guo, B., Peng, L., Zhong, Y., Zhang, J., Zhao, Z.: Room temperature ferromagnetism and cooling effect in dilute Co-doped ZnS nanoparticles with zinc blende structure. J. Alloys Compd. 584, 240–243 (2014). https://doi.org/10.1016/j.jallcom.2013.08.215
Akhtar, M.S., Malik, M.A., Alghamdi, Y.G., Ahmad, K.S., Riaz, S., Naseem, S.: Chemical bath deposition of Fe-doped ZnS thin films: investigations of their ferromagnetic and half-metallic properties. Mater. Sci. Semicond. Process. 39, 283–291 (2015). https://doi.org/10.1016/j.mssp.2015.05.017
Mahmood, Q., Hassan, M., Noor, N.A.: Systematic study of room-temperature ferromagnetism and the optical response of Zn1−xTMxS/Se (TM = Mn, Fe Co, Ni) ferromagnets: first-principle approach. J. Phys. Condens. Matter 28, 506001 (2016)
Sato, K., Katayama-Yoshida, H.: Ab initio study on the magnetism in ZnO-, ZnS-, ZnSe- and ZnTe-based diluted magnetic semiconductors. Phys. Stat. Sol. B 229, 673–680 (2002). https://doi.org/10.1002/1521-3951(200201)229:2%3c673::AID-PSSB673%3e3.0.CO;2-7
Sambasivam, S., Joseph, D.P., Reddy, D.R., Reddy, B.K., Jayasankar, C.K.: Synthesis and characterization of thiophenol passivated Fe-doped ZnS nanoparticles. Mater. Sci. Eng. B 150, 125–129 (2008). https://doi.org/10.1016/j.mseb.2008.03.009
Sambasivam, S., Joseph, D.P., Lin, J.G., Venkateswaran, C.: Doping induced magnetism in Co–ZnS nanoparticles. J. Solid State Chem. 182, 2598–2601 (2009). https://doi.org/10.1016/j.jssc.2009.07.015
Lakshmi, P.V.B., Raj, K.S., Ramachandran, K.: Synthesis and characterization of nano ZnS doped with Mn. Cryst. Res. Tech. 44, 153–158 (2009). https://doi.org/10.1002/crat.200800271
Ahmed, S.A.: Room-temperature ferromagnetism in Co-, Cr-, and V-doped ZnO diluted magnetic semiconductor. Appl. Phys. A 123, 440 (2017). https://doi.org/10.1007/s00339-017-1058-3
Soundararajan, D., Mangalaraj, D., Nataraj, D., Dorosinskii, L., Kim, K.H.: Magnetic properties of Cr doped ZnTe alloy powder. Mater. Lett. 87, 113–116 (2012). https://doi.org/10.1016/j.matlet.2012.07.042
Hou, X.J., Teo, K.L., Sreenivasan, M.G., Liew, T., Chong, T.C.: MBE growth and properties of Cr-doped ZnTe on GaAs(001). Thin Solid Films 505, 126–128 (2006). https://doi.org/10.1016/j.tsf.2005.10.024
Park, J.H., Kim, M.G., Jang, H.M., Ryu, S., Kim, Y.M.: Co-metal clustering as the origin of ferromagnetism in Co-doped ZnO thin films. Appl. Phys. Lett. 84, 1338–1340 (2004). https://doi.org/10.1063/1.1650915
Kaspar, T.C., Droubay, T., Heald, S.M., Engelhard, M.H., Nachimuthu, P., Chambers, S.A.: Hidden ferromagnetic secondary phases in cobalt-doped ZnO epitaxial thin films. Phys. Rev. B 77, 201303 (2008). https://doi.org/10.1103/PhysRevB.77.201303
Zheng, F.-B., Zhang, C.-W., Wang, P.-J., Luan, H.-X.: Tuning the electronic and magnetic properties of carbon-doped ZnO nanosheets: first-principles prediction. J. Appl. Phys. 111, 044329 (2012). https://doi.org/10.1063/1.3688233
Pan, H., Feng, Y.P., Wu, Q.Y., Huang, Z.G., Lin, J.: Magnetic properties of carbon doped CdS: a first-principles and Monte Carlo study. Phys. Rev. B 77, 125211 (2008). https://doi.org/10.1103/PhysRevB.77.125211
Hua, P., Sha, Z., Shen, L.F.: Electronic structures and magnetic couplings of B-, C-, and N-doped BeO. Chin. Phys. B 22, 047504 (2013). https://doi.org/10.1088/1674-1056/22/4/047504
Kenmochi, K., Seike, M., Sato, K., Yanase, A., Katayama-Yoshida, H.: New class of diluted ferromagnetic semiconductors based on CaO without transition metal elements Jpn. J. Appl. Phys. 43, L934–L936 (2004). https://doi.org/10.1143/JJAP.43.L934
Kang, B.-S., Kim, K.-S., Yu, S.-C., Chae, H.: First-principles study for ferromagnetism of Cu-doped ZnO with carrier doping. J. Solid State Chem. 198, 120–124 (2013). https://doi.org/10.1016/j.jssc.2012.09.041
Adli, W.: Prediction of half-metallic ferromagnetism in Cu- and K-doped MgS: a comparative study. J. Supercond. Novel. Magn. 33, 3107–3112 (2020). https://doi.org/10.1007/s10948-020-05558-3
Khan, Z.A., Ghosh, S.: Robust room temperature ferromagnetism in Cu doped ZnO thin films. Appl. Phys. Lett. 99, 042504 (2011). https://doi.org/10.1063/1.3615714
Yu, C.-F., Lin, T.-J., Sun, S.-J., Chou, H.: Origin of ferromagnetism in nitrogen embedded ZnO : N thin films. J. Phys. D: Appl. Phys. 40, 6497–6500 (2007). https://doi.org/10.1088/0022-3727/40/21/004
Zhou, S., Xu, Q., Potzger, K., Talut, G., Grötzschel, R., Fassbender, J., Vinnichenko, M., Grenzer, J., Helm, M., Hochmuth, H., Lorenz, M., Grundmann, M., Schmidt, H.: Room temperature ferromagnetism in carbon-implanted ZnO. Appl. Phys. Lett. 93, 232507 (2008). https://doi.org/10.1063/1.3048076
Saravanan, N., Teh, G.B., Yap, S.Y.P., Cheong, K.M.: Simple synthesis of ZnS nanoparticles in alkaline medium. J. Mater. Sci. Mater. Electron. 19, 1206–1208 (2008). https://doi.org/10.1007/s10854-007-9529-5
Fang, X., Bando, Y., Gautam, U.K., Zhai, T., Zeng, H., Xu, X., Liao, M., Golberg, D.: ZnO and ZnS nanostructures: ultraviolet-light emitters, lasers, and sensors. Crit. Rev. Solid State Mater. Sci 34, 190–223 (2009). https://doi.org/10.1080/10408430903245393
Samadpour, M., Dehghani, M., Parand, P., Najafi, M.N., Parvazian, E.: Photovoltaic performance and electrochemical impedance spectroscopy analysis of CdS/CdSe- sensitized solar cell based on surfactant-modified ZnS treatment. Appl. Phys. A 126, 461 (2020). https://doi.org/10.1007/s00339-020-03652-w
Wang, X., Xie, Z., Huang, H., Liu, Z., Chen, D., Shen, G.: Gas sensors, thermistor and photodetector based on ZnS nanowires. J. Mater. Chem. 22, 6845–6850 (2012). https://doi.org/10.1039/C2JM16523F
Zhao, Q., Xie, Y., Zhang, Z., Bai, X.: Size-selective synthesis of zinc sulfide hierarchical structures and their photocatalytic activity. Cryst. Growth Des. 7, 153–158 (2007). https://doi.org/10.1021/cg060521j
Holzman, J.F., Vermeulen, F.E., Irvine, S.E., Elezzabi, A.Y.: Free-space detection of terahertz radiation using crystalline and polycrystalline ZnSe electro-optic sensors. Appl. Phys. Lett. 81, 2294–2296 (2002). https://doi.org/10.1063/1.1508435
Zhang, C.-W., Yan, S.-S.: First-principles prediction of half-metallic ferromagnetism in Cu-doped ZnS. J. Appl. Phys. 107, 043913 (2010). https://doi.org/10.1063/1.3309771
Long, R., English, N.J.: Magnetic properties of first-row element-doped ZnS semiconductors: a density functional theory investigation. Phys. Rev. B 80, 115212 (2009). https://doi.org/10.1103/PhysRevB.80.115212
Blaha, P., Schwarz, K., Madsen, G.K.H., Kvasnicka, D., Luitz, J.: Wien2k, an augmented plane wave program for Calculating crystal properties. Vienna University of Technology, Vienna (2001)
Hohenberg, P., Kohn, W.: Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964). https://doi.org/10.1103/PhysRev.136.B864
Perdew, J.P., Burke, K., Ernzerhof, M.: Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
Monkhorst, H.J., Pack, J.D.: Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976). https://doi.org/10.1103/PhysRevB.13.5188
Murnaghan, F.D.: The compressibility of media under extreme pressures. Proc. Nat. Acad. Sci. U.S.A. 30, 244–247 (1944). https://doi.org/10.1073/pnas.30.9.244
Fan, S.W., Dong, J.H., Ding, L.J., Wang, Z.L., Yao, K.L.: Half-metallic ferromagnetism in tetrahedrally coordinated compounds MGe (M = Ca, Sr and Ba): Ab initio calculations. Comput. Mater. Sci. 67, 83–87 (2013). https://doi.org/10.1016/j.commatsci.2012.08.026
Gao, G.Y., Yao, K.L.: Half-metallic sp-electron ferromagnets in rocksalt structure: the case of SrC and BaC. Appl. Phys. Lett. 91, 082512 (2007). https://doi.org/10.1063/1.2775081
Yao, K.L., Gao, G.Y., Liu, Z.L., Zhu, L.: Half-metallic ferromagnetism of zinc-blende CrS and CrP: a first-principles pseudopotential study. Solid State Commun. 133, 301–304 (2005). https://doi.org/10.1016/j.ssc.2004.11.016
Sato, K., Katayama-Yoshida, H.: Materials design of transparent and half-metallic ferromagnets in V- or Cr-doped ZnS, ZnSe and ZnTe without P- or N-type doping treatment. Jpn. J. Appl. Phys. 40, L651–L653 (2001). https://doi.org/10.1143/JJAP.40.L651
Yao, K.L., Jiang, J.L., Liu, Z.L., Gao, G.Y.: First principle prediction of half-metallic ferromagnetism in zinc-blende MBi (Ca, Sr, Ba). Phys. Lett. A. 359, 326–329 (2006). https://doi.org/10.1016/j.physleta.2006.06.052
Sieberer, M., Redinger, J., Khmelevskyi, S., Mohn, P.: Ferromagnetism in tetrahedrally coordinated compounds of I/II-V elements: Ab initio calculations. Phys. Rev. B. 73, 024404 (2006). https://doi.org/10.1103/PhysRevB.73.024404
Kanamori, J., Terakura, K.: A general mechanism underlying ferromagnetism in transition metal compounds. J. Phys. Soc. Jpn. 70, 1433–1434 (2001). https://doi.org/10.1143/jpsj.70.1433
Krstajić, P.M., Peeters, F.M., Ivanov, V.A., Fleurov, V., Kikoin, K.: Double-exchange mechanisms for Mn-doped III-V ferromagnetic semiconductors. Phys. Rev. B 70, 195215 (2004). https://doi.org/10.1103/PhysRevB.70.195215
Liu, H., Zhang, J.-M.: Effect of two identical 3d transition-metal atoms M doping (M = V, Cr, Mn, Fe Co, and Ni) on the structural, electronic, and magnetic properties of ZnO Phys. Status Solidi B 254, 1700098 (2017). https://doi.org/10.1002/pssb.201700098
Chen, W.Q., Teo, K.L., Lim, S.T., Jalil, M.B.A., Liew, T., Chong, T.C.: Magnetic and transport behaviors in Ge1−xMnxTe with high Mn composition. Appl. Phys. Lett. 90, 142514 (2007). https://doi.org/10.1063/1.2720353
Mahmood, Q., Ul Haq, B., Yaseen, M., Shahid, A., Laref, A.: Exploring the origin of p- type half-metallic ferromagnetism in beryllium doped alkali based perovskites. Solid State Commun. 299, 113654 (2019). https://doi.org/10.1016/j.ssc.2019.113654
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have not disclosed any competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adli, W., Belbachir, A.H. A first-principles investigation of electronic structure and ferromagnetic properties in alkali metal-doped ZnS/Se semiconductors. J Comput Electron 21, 1061–1069 (2022). https://doi.org/10.1007/s10825-022-01910-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-022-01910-z