Abstract
ZnS nanoparticles were successfully synthesized by reflux under an alkaline medium. The nanoparticles were characterized by using X-Ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The optical properties of ZnS nanoparticles were examined by photoluminescence (PL) spectrum. The result shows that the as-synthesized ZnS nanoparticles had a cubic phase. SEM image shows that ZnS nanoparticles are basically in spherical shape and are homogeneous. The particle size was found to be in the range of 18 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ZnS has attracted much attention owing to its wide applications, including UV-light-emitting diodes, efficient phosphors in flat-panel displays, and photocatalysis. ZnS nanoparticles have the capabilities for applications in areas such as non-linear optical devices and fast optical switches and have been studies extensively [1–5]. Nanostructured ZnS such as nanocrystals, nanowires, and nanobelts exhibits excellent optical and optoelectronic performances, which differ much from the bulk ZnS material due to the three-dimensional electrons and holes confinement in a small volume. The surface of a nanoparticle is more important than the bulk because nanoparticles have larger surface-to volume ratios. Surface atoms are bound by weaker forces because of missing neighbours, which leads to high surface reactivity [6].
ZnS is a direct wide band gap (for the bulk cubic and hexagonal phases of ZnS, E g = 3.68 and 3.80 eV, respectively) semiconductor has also been widely used as a phosphor in luminescent devices due to its emission of in the visible range [7, 8]. Their optical property, due to quantum confinement effect, dramatically changes and, in most cases, improves as compared with their bulk counterparts [9]. ZnS crystal usually exhibits a polymorphism of two phases with different stacking sequences of close-packed planes to each structure: one is the cubic phase with a zinc blende structure (c-ZnS); and the other is the hexagonal phase with a wurtzite structure (h-ZnS) [2, 9]. At atmospheric pressure, c-ZnS is more stable at low temperatures and transforms to h-ZnS only at ≥1,023 °C [9]. Since the inherent crystal structures of ZnS play an important role in its physical and chemical properties, the preparation of ZnS nanocrystals with controllable phase is vital to develop them as building blocks in constructing the future nanoscale optoelectronic devices. We report the synthesis of ZnS nanoparticles without the use of surfactants or stabilizers.
2 Experimental procedure
All the reagents used in our experiment were of analytical purity and were used without further purification. An appropriate amount of zinc chloride (0.1 M), sulphur powder (0.1 M), NaOH (5 M) and deionised water (100 mL) were refluxed together for 5 h under constant stirring. Upon reflux, the product was centrifuged and washed several times with deionised water. The sample was then dried in the oven at 80 °C for 2 h to obtain powders of ZnS nanoparticles which appear white in colour. The powders are highly stable and do not show any coalescence or agglomeration even after several months. The product was characterised using PANanlytical X-Pert PRO X-Ray Diffractometer (XRD) for 2θ range 20–60° using CuKα radiation (λ = 1.5418 Å). Scanning Electron Microscopy (SEM) was performed using JEOL JSM 6400 Scanning Microscope. Transmission electron microscopy (TEM) images were obtained using JEOL-JEM 1011 electron microscope operating at 100 kV. A drop of nanoparticles dispersed in solvent was placed on a copper TEM grid and left to evaporate. Photoluminescence (PL) results were obtained with a Perkin Elmer spectrophotometer.
3 Results and discussions
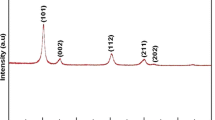
The XRD pattern of Fig. 1 shows three diffraction peaks with 2θ values of 29.09°, 48.16° and 57.88°. These peaks correspond to the 111, 220 and 311 planes of cubic zinc sulphide. The interplaner spacing (d) corresponding to XRD peaks and JCPDS data card have been compared as shown in Table 1. From the XRD analysis, no characteristic peaks of impurity phases were observed. The calculated lattice constant, a = 5.317 Å is very close to the reported value for cubic ZnS (JCPDS card No. 79–0043, a = 5.318 Å). We also observed the typical broadening of the peaks which indicated that the size of the ZnS particles was very small [10]. The average size of the particles was calculated using the Scherrer’s equation given by \( {\rm L} = {k\lambda}/{\beta \cos \theta} \), where L is the average particle size, k is a constant of 0.9, λ is the wavelength of X-ray (1.5418 Å), β is the Full-Widths at Half-Maximum (FWHM) of the (hkl) diffraction peak and θ is the Bragg angle of the (hkl) peak. The average size of the particles prepared using this method was found to be 18.2 nm.
Figure 2 shows the SEM image of ZnS nanoparticles prepared using this method. The particles are spherical and agglomerated to form larger particles. To verify the morphological scheme obtained from XRD, the data from the transmission electron microscopy (TEM) was studied. Figure 3 shows the agglomeration of ZnS particles prepared under alkaline medium by TEM observation. The tendency to form nanoclusters due to the high surface energy of nanoparticles is an unavoidable phenomenon as observed in the present case [11]. The size of the particle is in the range of 15–20 nm and the individual particle was retained irrespective of the size. But it is hard to determine the exact dimension of particles by only observing the TEM image because the extremely small particles aggregate to secondary particles [12]. It is consistent with the calculated value (about 18 nm) from the FHWM of its XRD peaks using the Scherrer’s Equation. Figure 4 illustrates the temperature dependence emission of ZnS nanoparticles dispersed in toluene with an excitation wavelength of 200 nm. As temperature decreased, the emission intensity increased. The violet emission peak around 385–395 nm (∼3.14 eV) could be attributed to the recombination of electrons from the energy level of sulphur vacancies (neutral donor) with the holes from the valence band [4, 13, 14].
4 Conclusion
ZnS nanoparticles could be successfully prepared under alkaline medium without the use of surfactants or stabilizers. XRD examination showed that the product was ZnS nanoparticles with zinc-blend crystal structure. The SEM and TEM image showed that the particles are spherical and agglomerated to form bigger clusters. The photoluminescence study shows violet emission due to sulphur vacancy. Such ZnS nanoparticles could be used to design advanced semiconductor nanodevices such as optoelectronics and gas sensors.
References
J. Li, Y. Xu, D. Wu, Y. Sun, Solid State Commun. 130(9), 619 (2004)
M. Jayalakshmi, M.M. Rao, J. Power Sources 157, 624 (2006)
R. Maity, U.N. Maiti, M.K. Mitra, K.K. Chattopadhyay, J. Phys. E. 33(1), 104 (2006)
Y. Yang, W. Zhang, Mater. Lett. 58, 3836 (2004)
R. Lv, C. Cao, H. Zhu, Mater. Res. Bull. 39, 1517 (2004)
M. Behboudnia, M.H. Majlesara, B. Khanbabaee, Mater. Sci. Eng. B 122, 160 (2005)
F. Wei, G. Li, Z. Zhang, Mater. Res. Bull. 40, 1402 (2005)
Y.C. Zhang, G.Y. Wang, X.Y. Hu, Q.F. Shi, T. Qiao, Y. Yang, J. Cryst. Growth 284, 554 (2005)
W. Liu, Mater. Lett. 60, 551 (2006)
Y. Li, Y. Ding, Y. Zhang, Y. Qian, J. Phys. Chem. Solids 60, 13 (1999)
M. Jayalakshmi, M.M. Rao, J. Power Sources 157, 626 (2006)
Y. Yin, X. Xu, X. Ge, Y. Lu, Z. Zhang, Radiat. Phys. Chem. 55, 353 (1999)
Y. Zhang, F. Lu, Z. Wang, H. Wang, M. Kong, X. Zhu, K. Zhang, Cryst. Growth Des. 7, 1459 (2007)
S. Kar, S. Biswas, S. Chaudhuri, Nanotechnology 16, 737 (2005)
Acknowledgement
The authors thank the Universiti Tunku Abdul Rahman for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saravanan, N., Teh, G.B., Yap , S.Y.P. et al. Simple synthesis of ZnS nanoparticles in alkaline medium. J Mater Sci: Mater Electron 19, 1206–1208 (2008). https://doi.org/10.1007/s10854-007-9529-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-007-9529-5