Abstract
As scientific research progresses, there is an increasing understanding of the importance of paternal epigenetics in influencing the health and developmental path of offspring. Prior to conception, the environmental exposures and lifestyle choices of fathers can significantly influence the epigenetic state of sperm, including DNA methylation and histone changes, among other factors. These alterations in epigenetic patterns have the potential for transgenerational transmission potential and may exert profound effects on the biological characteristics of descendants. Paternal epigenetic changes not only affect the regulation of gene expression patterns in offspring but also increase the risk to certain diseases. It is crucial to comprehend the conditions that fathers are exposed to before conception and the potential outcomes of these conditions. This understanding is essential for assessing personal reproductive decisions and anticipating health risks for future generations. This review article systematically summarizes and analyzes current research findings regarding how paternal pre-pregnancy exposures influence offspring as well as elucidates underlying mechanisms, aiming to provide a comprehensive perspective for an enhanced understanding of the impact that paternal factors have on offspring health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
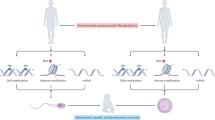
Epigenetics is a rapidly evolving field within molecular biology that focuses on understanding how non-DNA sequence alterations influence gene expression and contribute to phenotypic diversity and genetic variability. These alterations include DNA methylation, histone modifications, and non-coding RNAs (ncRNAs), which together form a complex regulatory network affecting gene expression. DNA methylation is a pivotal modification process in epigenetics whereby a methyl group is added to the 5′ carbon atom of cytosine, typically occurring at CpG islands. DNA methylation controls gene transcription and expression, thereby affecting gene activity [1]. The genomes of mammalian sperm cells are over 90% methylated, the highest of any cell type. This is linked to routine semen parameters [2]. Chemical modifications of histone tail amino acid residues include acetylation, methylation, and phosphorylation. By changing chromatin structure and dynamics, these changes affect gene accessibility and expression [3]. ncRNAs regulate gene expression in many ways. They create complementary pairs with mRNAs to govern stability and translation or modify chromatin modification to affect gene activity. Additionally, ncRNAs can form regulatory complexes with other proteins or RNA molecules to affect gene expression [4]. Epigenetic alterations not only impact gene expression within an individual at various stages of development but also have the capacity to transmit information to the next generation through germ cells, referred to as intergenerational epigenetic inheritance. In some cases, these changes can also affect subsequent generations, which is referred to as transgenerational epigenetic inheritance [5, 6]. Paternal epigenetics involves how paternal spermatozoa pass on key epigenetic markers to relay environmental information to their immediate offspring (intergenerational inheritance) and potentially to subsequent generations (transgenerational inheritance) [7]. Sperm epigenetic markers are affected by the father’s lifestyle, food, stress, and environmental contaminants. These environmental factors may impact sperm epigenetics and future offspring's biology. This epigenetic effect may produce “pre-adaptation”, which improves the fitness of the offspring, but it may also produce a large number of pathological disease states [8]. According to existing studies, such epigenetic effects are dynamic and complex, varying by sex and developmental stage. Paternal epigenetic effects in animals are expressed differently in offspring of different sexes and ages, and offspring of animals exposed to some substances may show resistance to others (see Section 3.5 of this review). Thus, in-depth analysis of the effects of paternal prenatal exposure on offspring is critical to understanding epigenetic effects.
Resetting epigenetic marks: the journey from gametes to embryo
Epigenetic reprogramming is a critical process during gametogenesis (the formation of sperm and eggs) and early embryonic development. This process involves the erasure and re-establishment of epigenetic marks to ensure the correct gene expression patterns necessary for development. Parental origin affects gene expression in imprinted genes. Germ cells undergo reprogramming of these genes to meet embryonic needs and restore their epigenetic state at the right time.
The development of primordial germ cells (PGCs) is a precisely regulated journey involving complex epigenetic reprogramming. The developmental journey in mice begins at embryonic day (E)7.25 with the first appearance of small clusters of approximately 40 cells in the proximal ectoderm, which express key transcriptional regulators and mark the initiation of PGCs [9]. From E9.5 to E10.5, PGCs began to express ten-eleven translocation protein (TET)1 and TET2 enzymes, and their expression levels peaked from E10.5 to E11.5, providing key catalytic roles for the DNA demethylation process [10]. The level of 5-methylcytosine (5mC) in mouse PGCs gradually decreased from E9.5 to E11.5 until it was almost undetectable at E11.5, while at the same time, the global enrichment of 5-hydroxymethylcytosine (5hmC) in PGCs indicated a genome-wide 5mC to 5hmC conversion [10]. Mouse PGCs migrate through the posterior endoderm to reach the gonadal primordium between E10.5 and E11.5, a process that delays the removal of methylation in specific regions, including differentially methylated regions (DMRs) of imprinted genes and CpG islands (CGIs) on the X chromosome [9]. The methylation level of PGCs in male mice decreases to 14% at E13.5 and then begins to show significant re-methylation at E16.5, where the methylation level increases to approximately 50% [9]. Methylation changes at the E16.5 stage prepare PGCs to enter the gonads and undergo subsequent stages of the spermatogenesis process. Notably, despite the extensive demethylation that PGCs undergo, a few transposable and repeat elements remain methylated in PGCs, and these loci may be critical for germ cell development and function [9]. For example, endogenous retroviral A-particle (IAP) elements, which are a particularly potent source of insertional mutagenesis in mice, are resistant to DNA demethylation during reprogramming and can mostly remain methylated [10,11,12], thus preserving their epigenetic marks. Sites that escape demethylation tend to be adjacent to IAP elements or telomeric regions [10, 13].
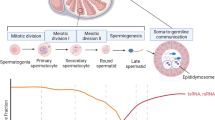
At the stage of sperm maturation, chromatin composition gradually shifts from a nucleosome-based state to a protamine-based state [14]. Protamines are small, arginine-rich, sperm-specific structural proteins that encapsulate 90–95% of the mammalian sperm genome and are essential for sperm chromatin compression, stability, and the subsequent fertilization process. A rodent-specific lysine residue, K49, is acetylated on protamine 1 (P1), a modification that is acquired early in spermatogenesis and remains present in mature spermatozoa [15]. In fertilized eggs, if the lysine at position 49 of P1 is alanine substitution (P1(K49A)), it results in premature decompression of the male pronucleus, altered DNA replication patterns, and arrested embryonic development. In addition, in vitro experiments have shown that the P1(K49A) mutation reduces the binding affinity of protamines for DNA and alters the dynamics of DNA compression and decompression [15]. These changes may affect the stability of sperm chromatin and the efficiency of chromatin reprogramming in fertilized eggs.
Post-fertilization, the parental genome undergoes another round of DNA demethylation through both active and passive mechanisms [16, 17]. In human preimplantation embryos, DNA methylation levels progressively decline from the time of fertilization until the blastocyst stage, with the lowest level observed at approximately 43%. Notably, the paternal and maternal genomes are dramatically different in epigenetic marks and chromatin structure and the paternal genome undergoes more rapid and extensive demethylation compared to the maternal genome [18, 19]. It is observed in mammals that active demethylation of the parental genome involves the conversion of 5mC to 5hmC by TET enzymes and other subsequent reactions that culminate in demethylation [5, 17]. This process is essential for the removal of unwanted epigenetic marks from the paternal genome to ensure smooth embryonic development. In contrast, demethylation of the maternal genome exhibits a more passive feature, whereby newly synthesized DNA strands fail to be remethylated during the early embryonic developmental stages after the formation of a fertilized egg due to the restriction or absence of DNA methylase (DNMT) activity, thus reducing the overall methylation level [20, 21]. During mouse embryogenesis, numerous genes marked by altered histones or DNA methylation in sperm may not be expressed in pre-implantation embryos but could be expressed at later stages of embryonic development [22].
Genetic reprogramming is fundamental to ensuring that genetic information is accurately transmitted and expressed in germ cells and early embryos. However, the transmission of genetic information does not occur in a vacuum; environmental factors also play a key role. It is becoming increasingly apparent that fathers’ pre-pregnancy lifestyle and environmental exposures may influence the health of their offspring through epigenetic alterations. Therefore, we will next review and summarize the research on the effects of paternal pre-pregnancy exposures on offspring, with aiming to provide a scientific basis for understanding the associations between paternal pre-pregnancy exposures and offspring health, as well as providing theoretical support for future prevention strategies and interventions. The conceptual schema of our study is depicted in Fig. 1.
Pre-pregnancy paternal exposures: a blueprint for offspring developmental outcomes
Cigarette smoke
Extensive research has demonstrated the negative effects of tobacco smoking throughout pregnancy and early infancy on both the mother and child [23,24,25]. Subsequently, the researchers found that paternal smoking before conception was also strongly associated with offspring health [26, 27]. An epigenome-wide association study in humans found a link between paternal cigarette smoking and altered DNA methylation patterns in offspring, independent of maternal smoking [28]. One notable finding was the differential methylation of the Catenin Alpha 2 gene, which is implicated in central nervous system (CNS) functions and psychiatric disorders [29]. The idea that paternal exposure to cigarette smoke may influence the prevalence of psychiatric disorders in offspring through epigenetic effects which is supported by another human study reporting that offspring of men who smoke have a higher risk of developing attention deficit hyperactivity disorder (ADHD) and schizophrenia [30]. This suggests that epigenetic inheritance may affect psychiatric disorders in offspring. Prenatal paternal smoking and maternal smoking have different profiles on chromosomes 7 and 15, and fathers who start smoking in early adolescence have a particularly strong effect on DNA methylation in their offspring, an effect that may be associated with the development of diseases such as asthma, obesity, and poor lung function in their offspring [28, 31,32,33,34,35]. Notably, even passive exposure to cigarette smoke in adolescent males in human studies is associated with an increased likelihood of non-allergic asthma in their offspring at the age of 7 years [36]. The dose of prenatal paternal cigarette smoke exposure was positively associated with the level of methylation of CG loci in the DNA of the offspring’s cord blood, including the LMO2 and IL10 genes, which may be relevant to childhood asthma [37]. Sexual dimorphism has been shown in the genetic effects of paternal pre-pregnancy exposure on offspring, and smokers’ male offspring are at higher risk of obesity than female offspring [27, 38, 39]. However, fathers who start smoking after the age of 15 years are associated with increased obesity in their offspring of both sexes [33]. There are results of different statements in human studies about the correlation between preconception smoking and offspring cancer risk. A French study found an association between paternal smoking before pregnancy and brain cancer in offspring under 5 years of age [40]. This is contrary to earlier studies that found no correlation between parental smoking and offspring cancer risk [41, 42]. These conflicting results could be attributed to various factors such as differences in sample sizes, methods of smoking behavior quantification, and the control of confounding factors. A more critical examination of these studies suggests that the timing and intensity of smoking, as well as genetic predispositions, could play significant roles in mediating these effects. Overall, while there is evidence supporting a link between paternal smoking and certain health risks in offspring, the variability in findings indicates a need for more standardized research methodologies to draw definitive conclusions.
Elevated levels of DNA methylation and reduced chromatin condensation in spermatozoa of human smokers [43] imply that the chromatin structure becomes more open, potentially increasing the accessibility of genes and making otherwise tightly regulated genes more susceptible to the effects of transcription factors and other regulatory proteins. The observation that smoking exposure induces DNA methylation changes in spermatozoa and is reversible in mice, and the similar effects of cigarette exposure observed in wild-type and Nrf2 knockout mice [44] may indicate that reactive oxygen species (ROS) leading to DNA damage and lipid peroxidation affects not only the quality of spermatozoa but possibly also their epigenetic status. Pre-pregnancy cigarette exposure in mouse fathers affects miRNAs in their sperm [45]. miRNAs are important molecules that regulate gene expression, and smoking exposure may alter the expression of specific miRNAs (e.g., genes critical for early embryonic developmental regulation) in sperm, thereby affecting the development and health of the offspring. Although DNA methylation and miRNA changes in spermatozoa may be reversible to some extent, whether they can be fully restored to the pre-exposure state and whether these changes can be transmitted across generations remain unknown, and more research is needed to elucidate the stability and persistence of these epigenetic marks across multiple generations.
As societal awareness of the health risks associated with traditional cigarettes grows, e-cigarettes have gained popularity among young people as an alternative [46]. However, the emergence of e-cigarettes has sparked concerns regarding their potential health impacts. While e-cigarettes have been shown to potentially decrease the risk of cardiovascular events compared to traditional cigarettes [47], reproductive genetics studies have not been completed in time to assess if e-cigarette smoke will damage future generations. The main harmful ingredient in e-cigarettes is nicotine, and results from animal studies suggest that paternal exposure to nicotine may have neurodevelopmental and metabolic effects on the offspring, such as an increase in nicotine demand [48] and spontaneous locomotor activity in animal offspring, whereas significant deficits in reversal learning ability and attention were observed [49, 50]. Additionally, there is an elevated risk for ADHD [51]. Male offspring also exhibited significant deficits in brain monoamine concentration and content and significantly reduced dopamine receptor mRNA expression [50], but the liver of the offspring appeared to be better able to clear nicotine [52]. This may suggest that nicotine exposure in animal fathers may affect the metabolic capacity of the offspring to become better resistant to nicotine, but it is not clear whether e-cigarettes would have a similar effect in humans. Table 1 outlines the effects of pre-pregnancy cigarette smoke and nicotine on offspring.
Body mass index (BMI)
Studies from humans have shown that pre-pregnancy paternal obesity affects imprinted genes reprogramming during spermatogenesis [53]. After controlling for various maternal and neonatal factors, DNA methylation levels in cord blood leukocytes of neonates of obese fathers remained associated with paternal obesity [54]. Some studies have reported that paternal BMI has different correlations with DNA methylation depending on the sex of the child. However, after correction for multiple testing, none of the observed correlations remained significant [55, 56]. Despite this, the potential influence of paternal obesity on offspring methylation patterns warrants further investigation, as other studies have suggested significant effects at specific sexes and at specific gene loci [55]. This may explain the sex specificity of paternal obesity producing epigenetic effects observed in multiple studies. For example, it has been observed in female mouse offspring that paternal overweight may affect onset mammary development and increase breast cancer risk by altering miRNA expression profiles in offspring mammary tissue [57]. Meanwhile, in humans even though an increased risk of asthma was observed in all offspring children, the risk was more pronounced in female offspring [58, 59], and reduced lung function in offspring adult males was not observed in females [60]. Pre-pregnancy BMI in human fathers was positively associated with the growth rate of WHO BMI z-scores (zBMI), mean zBMI, and weight status in offspring. However, this association was not significant in female offspring [61, 62].
Even after controlling for diabetes, smoking, alcohol, and cardiovascular disease, paternal early-onset obesity is associated with higher serum alanine transaminase levels in children. This elevation is not due to the offspring’s BMI [63]. This indicates that paternal obesity may impact the liver health of offspring through non-genetic factors, as elevated alanine transaminase levels persist even in offspring of normal weight [63]. A correlation was observed between an increase in the father’s pre-pregnancy BMI and a corresponding increase in the birth weight of his offspring. The observed increase was 10.7 g per unit increase in the father’s BMI [64], indicating the potential impact of paternal obesity on the early growth and development of the offspring. Concurrently, fathers who were obese in the six months prior to pregnancy demonstrated an increasing prevalence of asthma, hand-foot-mouth disease, anemia, dental caries, and obesity in their adolescent offspring [65]. In comparison, the prevalence of anemia in adolescents in the offspring was higher in the offspring of underweight fathers [65]. This reminds us that a father’s BMI impact on offspring health risks is multifaceted.
Excessive BMI in men causes an increase in the level of hypocondensed chromatin and a decrease in the level of decondensed chromatin in their spermatozoa, and this may affect normal expression of genes in the spermatozoa [66]. These abnormal states of chromatin can affect the DNA repair process in the sperm nucleus, resulting in damaged DNA not repaired in time, which may cause the accumulation of mutations in genetic information. And these mutations may adversely affect the development of embryos after fertilization, thus increasing the risk of birth defects. Sperm from obese men exhibited elevated ROS levels and significant telomere length shortening [67], and sperm proteomic analysis showed significant differences in protein abundance [68], with changes in proteins involved in oxidative stress response and DNA damage repair mechanisms. Reduced telomere length may contribute to sperm aging, which may affect the early development of the embryo after fertilization and, at the same time, may lead to the accumulation of DNA damage, which may affect the genomic stability and developmental potential of the embryo. The increased expression of oxidative stress-related proteins may reflect an adaptive response of sperm to elevated levels of ROS, which may result in oxidative damage to sperm DNA, increasing the risk of mutations that may affect the health and disease susceptibility of the offspring. Changes in DNA damage repair proteins, on the other hand, may be indicative of the effects of obesity on the genetic stability of sperm. Table 2 breaks down how paternal BMI affects offspring outcomes.
Diet, nutrition, and health
Before delving into the discussion of the epigenetic effects of a father’s preconception dietary habits on his offspring, it is essential to clarify that these effects may arise directly from the changes induced by the father’s dietary practices prior to conception, or they may be indirectly related to the impact of those dietary habits on the father’s BMI, which in turn affects the offspring. In this section, our focus is solely on the studies that consider the father’s pre-pregnancy diet as the primary variable of interest, to distinctly differentiate the direct influence of dietary factors on the health of the offspring.
It has been suggested that the paternal effect cannot be fully explained by the excess or deficiency of a single nutrient; rather, it is the result of the interaction of multiple nutrient balances and total energy intake [69]. As a key epigenetic enzyme, DNMT plays a central role in DNA methylation, a process critical for regulating gene expression. Variations in DNMT expression levels, particularly in response to dietary factors, may shape the epigenetic profiles of offspring, thereby affecting their development and gene expression. In rats, the impact of a paternal high-fat, high-sugar diet on DNMT expression in the adipose tissue of adult offspring appears to be sex-specific, with male offspring showing upregulated expression of DNMT1 and DNMT3a and downregulated expression of DNMT3b compared to offspring on a high-protein diet, and female offspring having a general decrease in the expression of DNMT1, DNMT3a, and DNMT3b [70]. This may indicate a different genetic response to the father’s pre-pregnancy diet in offspring of different sexes and may explain the observed correlation of the proportion of fat in the pre-pregnancy diet of mouse fathers only in the risk of obesity in female offspring [69]. Meanwhile, a high-fat diet in rat/mice paternities resulted in dyslipidemia, glucose intolerance, and abnormal insulin secretion in the offspring [71,72,73,74,75]. Notably, male offspring show significantly elevated inflammation-related gene expression, increased sperm ROS levels, and reduced circulating testosterone and its biosynthetic enzyme gene expression in the liver [76, 77]. Another study found that a pre-pregnancy high-fat, high-sucrose, high-salt diet in rats caused significant elevations in serum aspartate aminotransferase activity and changes in intestinal flora in offspring, but only male offspring had elevated lipids [78]. This suggests that unhealthy dietary habits before fathers’ pre-pregnancy may increase the risk of metabolic syndrome and cardiovascular disease in offspring, highlighting the potential for different dietary habits to induce distinct epigenetic changes.
In contrast, paternal high-protein diets have been shown in rats/mice to positively affect metabolic outcomes in offspring, improving offspring body composition, insulin sensitivity, circulating satiety hormones, and cecum short-chain fatty acids, with higher basal glucagon-like peptide-1 (GLP-1) concentrations and lower ghrelin levels in male offspring [69, 70]. Higher concentrations of GLP-1 have been associated with enhanced satiety, which contributes to reduced food intake. Lower ghrelin levels generally imply reduced appetite and better glycemic control; changes in these hormone levels may have a positive impact on weight management and metabolic health in male offspring.
Female offspring of mice whose fathers consumed a low-protein, high-carbohydrate diet prior to conception were at increased risk of obesity, and researchers have suggested that their behavioral traits may be affected by interactions between paternal dietary macronutrients [69]. In addition to contributing to offspring obesity, the mice paternal low-protein pre-pregnancy diet affects a variety of offspring characteristics related to metabolic function and vascular health, such as abnormal glucose tolerance, nonalcoholic fatty liver disease, abnormal vascular function, and altered intestinal bacterial profiles [79], with sex-specific changes in lipid abundance in the cerebellum of the offspring [80] and manifesting themselves in adulthood as abnormal alterations in heart weights and cardiorespiratory lipid profiles [80, 81]. Large-scale studies in humans have also found that offspring of fathers who experienced food shortages or famine during periods of slow growth had significantly lower mortality from cardiovascular disease [82].
Maintaining physiological functions and promoting health requires nutrients, notably essential fatty acids. Since the body cannot manufacture these essential fatty acids, they must be obtained through diet or supplementation. Fish oil, extracted from fish, is particularly valued for its richness in omega-3 polyunsaturated fatty acids (PUFA). Pre-pregnancy fish oil supplementation in mice restores offspring glucose tolerance, increases mTOR expression, and improves fatty acid oxidation in offspring females, thereby reducing offspring metabolism and cardiovascular disease risk [83, 84]. N-3 PUFA are considered essential fatty acids. Pre-pregnancy n-3 PUFA supplementation in mouse fathers attenuated anxiety and depression-like behaviors in offspring, decreased leptin expression in adipose tissue and plasma concentration, improved social, learning and memory abilities, and increased the number of synapses and neuromarkers in the hippocampus, cerebral cortex, and hypothalamus [85, 86], suggesting that pre-pregnancy supplementation with n-3 PUFA may have a positive effect on the offspring’s socialization, energy balance, and weight management. The small nuclear ribonucleoprotein polypeptide N (Snrpn) gene on chromosome 15 is imprinted and expressed by a paternal allele. In the paternity and offspring of mice supplemented with n-3 PUFA, the Snrpn gene was downregulated in testicular tissues and up-regulated in the cerebral cortex and hippocampus, with corresponding DNA methylation changes [86]. This suggests that increased paternal pre-pregnancy N-3 PUFA intake may improve brain development and function in offspring through Snrpn imprinting and that this effect is sex-specific [86]. This may explain why male offspring showed significant alterations in imprinted gene expression in adipose tissue, whereas females did not [85].
It was observed in mice that mitochondrial tRNAs (mt-tRNAs) in spermatozoa are affected by diet, and in particular, a high-fat diet induces changes in these RNAs, which are passed on to the offspring through the spermatozoa [87]. During fertilization, sperm also pass on mt-tRNAs as they deliver a genetic material to the oocyte, and although there is no direct evidence that mt-tRNAs are sufficient on their own to transfer metabolic phenotypes, their robustness, strength, dynamics, and reversibility suggest that these RNA molecules may be useful indicators for monitoring and preventing the transmission of metabolic disorders through patrilineal inheritance. We distinguish the effects of fathers pre-pregnancy diet and nutritional status on offspring outcomes in Table 3.
Exercise
Epigenetic studies of the effects of pre-pregnancy exercise on paternal have focused on rodents. Numerous studies have illustrated the potential metabolic benefits of preconceptionally exercising mouse fathers on their offspring. These benefits include improved in offspring glucose metabolism [88], elimination of abnormalities in offspring glucose tolerance, insulin sensitivity, and body weight due to high-fat diets [89, 90], and reduced risk of type 2 diabetes in offspring due to poor diets of their fathers [91, 92]. These beneficial metabolic effects may be mediated by a markedly different metabolomic phenotype in the offspring liver [88], as well as altered insulin receptor sensitivity and responsiveness to insulin signaling, and reduced methylation levels of the paternally imprinted gene insulin-like growth factor 2 (IGF2) at the DMR-2 locus of the offspring skeletal muscle cells, with these changes observed specifically in male offspring [91, 92]. However, some studies have provided different insights, stating that prolonged pre-pregnancy exercise in paternal mice may lead to reprogramming of energy expenditure patterns and insulin resistance genes in the offspring, as manifested by a significant increase in body weight, obesity, decreased glucose tolerance, and abnormally elevated insulin levels, with altered metabolic phenotypes, and altered expression of key metabolic genes in their skeletal muscle, including the epigenome, such as Pdk4, H19, Ogt, and Oga [93]. The two conflicting views above may be due to differences in the intensity of exercise prior to conception in fathers, as the effects of exercise on the male reproductive system vary by mode and intensity [94]. This also reminds the importance of further follow-up studies lies in determining whether differences in exercise intensity lead to distinct epigenetic changes affecting the health of offspring。
Animal studies have shown that exercise by fathers before conception can enhance mental health and learning abilities in their offspring. For instance, pre-pregnancy running in male rats improves anxiety levels in offspring males during adulthood and reduces conditioned fear memories in offspring males during puberty. However, the effects on female offspring were not significant [95]. While exercise by male rats or mice does not affect the development or physical performance of male offspring, it can improve the spatial learning ability of male offspring to some degree and reduce the overall DNA methylation level in the hippocampus of offspring [96,97,98]. Exercised rats exhibit changes in sperm miRNA and tRNA content [95], and it remains unclear if these changes correlate with benefits observed in their offspring. Future studies are needed to confirm this relationship. Table 4 summarizes the effects of paternal pre-conception exercise on offspring outcomes.
Addictive substance use
Addictive substances refer to a class of chemical substances that can trigger intense cravings and dependence, such as amphetamines and cannabis. These substances are capable of altering a person’s state of mind, producing feelings of pleasure or relaxation, and their long-term use alters neural pathways and signaling systems in the brain, leading to increased tolerance and a reduced response to normally rewarding stimuli. Among these addictive substances, cocaine (COC) stands out as a particularly potent CNS stimulant that can have profound effects on brain function and behavior. Chronic COC exposure causes promoter hypermethylation of key genes in rat sperm [99]. The genetic effects of COC exposure of animal fathers prior to conception on offspring are multifaceted, mainly at the neurodevelopmental and behavioral levels, such as mild cognitive deficits in male offspring of rats and mice, reduction in anxiety-like behaviors, impaired hippocampus-dependent memory for object location, deficits in synaptic plasticity in the basolateral amygdala (BLA), and elevated hippocampal D-serine levels and Dao1 mRNA expression levels [100] and altered oxytocin receptor binding in rat offspring in brain regions that regulate social behavior [101]. Female offspring of COC-exposed male rats show higher conditioned freezing responses when re-exposed to cues associated with aversive stimuli [102]. The BLA is an important region of the brain for processing emotions, and the hippocampus is involved in memory processes, spatial cognition, and orienting functions, implying that paternal exposure to COCs may have contributed to the range of behavioral changes described above by interfering with the functioning of both the BLA and the hippocampus in the brains of the offspring. At the same time, pre-pregnancy COC-exposed rat offspring have reduced levels of dopamine and dopamine metabolites in the orbitofrontal cortex, the nucleus ambiguus (NAcc), and the dorsal striatum and male offspring exhibit higher levels of cocaine-seeking compulsion and motivation, with significant downregulated of Gabrg3 expression in the ventral tegmental area (VTA) of the brain [103, 104], and attenuated behavioral plasticity accompanied by a remodeling of histone modifications in the NAcc and selective increase in Cdkn1a expression [99, 105]. Gabrg3 is a subunit of the GABA(A) receptor, and GABAergic neurons in the VTA mainly regulate peripheral dopaminergic neurons, which in turn affects the release of dopamine from downstream brain regions. Reduced levels of dopamine may affect offspring’s sensitivity and response to reward, which may suggest that the relationship with the dopamine-related neural pathways play a central role in behavioral alterations in offspring. NAcc is thought to be the reward center of the brain that responds to stimuli such as food, sex, and drugs. Remodeling of NAcc histone modifications in offspring may increase or decrease specific types of histone modifications, altering expression of addiction-related genes and affecting drug response and addictive behaviors, and selective increases in Cdkn1a expression may be driven by a range of epigenetic changes.
Morphine, a powerful opioid, has CNS excitatory and depressive effects, mostly depressing. It stimulates CNS opioid receptors and is used for analgesia, but it can cause addiction and abuse. Pre-pregnancy exposure to morphine in rats leads to delayed learning and reduced inhibitory control in offspring, effects that are not mitigated by naloxone, and impulsive behavior which is further enhanced in these rats after acute and subchronic morphine treatment of the offspring [106]. Meanwhile, pre-pregnancy morphine exposure in rats/mice affects the expression of neurobiochemical markers in their offspring, such as downregulation of BDNF mRNA expression in the NAcc, elevated levels of phosphorylated cAMP, D2 dopamine receptors, and BDNF in the medial prefrontal cortex [107, 108], and the male offspring show reduced levels of acetylated histone H3 in the prefrontal cortex and hippocampus and new object recognition index [109,110,111], elevated cerebrospinal fluid and plasma corticotropin-releasing hormone (CRH) levels, and increased CRH receptor 1 expression in the brain [109]. The elevated CRH in male rat offspring is associated with increased anxiety behavior, which researchers suggested may be a stress response due to hypothalamic–pituitary–adrenal (HPA) axis dysregulation [109]. However, another study found no change in anxiety-like behavior or stress-triggered HPA axis activation in offspring following pre-pregnancy morphine exposure in rat fathers [112]. The reason for the discrepancy in results may be related to the dose and length of drug exposure in different preconception paternal rats, and further studies are needed in follow-up to determine the effects of morphine exposure on anxiety behaviors in offspring.
Morphine exposure in rats/mouse fathers does not alter heroin or cocaine self-administration in minor offspring [113], but adult offspring have reduced levels of COC self-administration and reinforcing effects of COC [107]. In addition, female offspring animals show greater sensitivity to the rewarding effects and self-administration levels of morphine [107, 111], which may imply that organisms have evolved adaptive responses to drug exposure to help offspring better survive and reproduce in potentially drug-induced environments. Complex and specific abnormalities in neurotransmitters, receptors, or signal transduction molecules in neural networks in the brain that process and regulate reward-related behaviors may cause offspring with different or even opposite responses to different drug classes.
Methamphetamine (METH) is a potent amphetamine-class CNS stimulant with harmful and addictive properties. Paternal pre-pregnancy exposure to METH significantly alters the DNA methylation profile in spermatozoa and the mPFC transcriptome profile in male offspring, with elevated expression of sortilin1 and Shank2, observed in both spermatozoa and the mPFC of male offspring [114]. These concurrent alterations suggest a need to investigate the association between paternal METH exposure and autism in offspring [115, 116]. Offspring of METH-exposed fathers exhibit depressive-like behaviors and diminished activity in the prelimbic cortex, sublimbic cortex, and nucleus accumbens in response to mild stimulation, with sex-specific differences in brain activity patterns [117]. Male offspring showed preferential behavior towards METH and increased amygdala activity within the brain, while female offspring showed predominantly increased anxiety-like behavior and spatial memory deficits and diminished neuronal activity in orbitofrontal cortex, cingulate cortex, NAcc shell, medial habenula, dorsal hippocampal CA1, and ventral hippocampal CA1 [117, 118]. β1-Adrenergic receptors (ADRB1) on CaMKII-positive neurons in the mPFC may mediate the greater susceptibility of male mouse offspring to drug addiction resulting from paternal METH exposure[118].
The main psychotropic component in cannabis is tetrahydrocannabinol (THC). Cannabis is extensively abused but used medically to treat chronic pain, nausea, and other symptoms. Cannabis can cause euphoria, relaxation, altered time perception, heightened senses, mood swings, anxiety, panic, hallucinations, poor memory and cognitive functions, and dependence. Pre-pregnancy exposure of rat fathers to cannabis causes male offspring to exhibit dopamine utilization deficits throughout maturation, which persist even after the father’s pre-pregnancy cannabis use has ceased and after a long drug-free period, exceeding one full spermatogenesis cycle [119]. However, pre-pregnancy exposure of rat fathers to THC does not result in defective dopamine utilization in offspring [119]. Interestingly, when the dose of THC was reduced to a certain level, male offspring showed an increase in dopamine utilization that differed from cannabis exposure and significant hyperkinetic activity [119]. The authors hypothesized that this may be related to the fact that cannabis and THC target different genes are related to the Y chromosome or hormonal status. In male rats exposed to THC, the discs-large associated protein 2 (DLGAP2) gene showed significant hypomethylation in sperm. This gene is uniquely expressed in the testis from the paternal allele, which is linked to a variety of neuropsychiatric disorders, such as autism spectrum disorders. Interestingly, this hypomethylated state was also observed in their offspring NAcc [120]. This finding suggests that changes in sperm DNA methylation induced by cannabis use could potentially be passed down through generations, influencing the neurodevelopment of offspring. Paternal pre-pregnancy exposure to THC in rats significantly affects offspring brain development and produces long-term behavioral deficits, with dose-dependent deficits in presynaptic activity of the acetylcholine system in the offspring [121, 122]. At low doses, acetylcholine receptors upregulate to compensate for this activity deficit but, at greater doses, receptor malfunction, impairing presynaptic inputs and causing cognitive impairment in offspring [122, 123]. Table 5 shows how the father’s addictive substance use affects kids.
Environmental and occupational exposures
Polycyclic aromatic hydrocarbons (PAHs), persistent organic pollutants, are extensively found in the environment due to incomplete combustion or thermal cracking of fossil fuels including coal and oil. Studies in humans have found that pre-pregnancy exposure of fathers to PAHs increases the risk of acute lymphoblastic leukemia (ALL) in offspring[124]. Benzo[a]pyrene (BaP), a structurally complex and carcinogenic PAH compound consisting of five benzene rings, can lead to alterations in mitochondrial protein abundance and enzyme activity in the liver of male mouse offspring and reduction in mitochondrial DNA copy number and induce alterations in sperm DNA methylation of imprinted genes in both fathers and their offspring when mouse fathers are exposed pre-conception [125, 126]. Both BaP and benzene contain benzene rings and exposure to the father’s benzene can raise the chance of offspring ALL before childbirth. However, there appears to be no correlation between benzene exposure in human fathers and childhood cancer in their offspring [127, 128]. Phthalates are a widely used class of plasticizers and are commonly used in toys, food packaging materials, medical blood bags, and hoses. Pre-pregnancy exposure to phthalates in human fathers is associated with lower levels of internalizing problems in offspring [129]. Internalizing problems include anxiety, withdrawal, fear, obsessive–compulsive behaviors, and other emotions or behaviors typically exhibited by children, such as “over-control, inhibition, or shyness anxiety.” Another study concluded that the effects were gender-specific, with male offspring having increased internalizing behaviors and female offspring having decreased internalizing behaviors [130]; differences in results may stem from inter-individual variability among humans and different versions of questionnaire forms for scoring. Paternal pre-pregnancy MBzP concentrations in human fathers have been linked to offspring’s emotional overeating, food response, and water craving [131]. Preservatives like propylparaben (PP) are para-hydroxybenzoates (PHBs) derived from benzoic acid. The offspring of human fathers with higher paternal pre-pregnancy urine methyl and PP concentrations had more internalizing and behavioral issues[132]. Another study revealed no correlation between paternal pre-pregnancy PHB concentrations in human fathers and offspring negative behaviors [133]. Different sample numbers, evaluation parameters, and scale selections may cause this disparity. Bisphenol A (BPA), an industrial chemical used in epoxy resins and polycarbonate plastics, functions as an endocrine disruptor due to its estrogen-like structure. BPA affects spermatogenesis by reducing the size and number of spermatogenic epithelial cells in mice’s testes, leading to sustained hypermethylation of sperm DNA [134], resulting in a decrease in the total number of spermatozoa from parents to grandchildren, suggesting that exposure to BPA in males may lead to reproductive problems for up to three generations. Pre-pregnancy BPA exposure in human fathers does not affect child internalization or externalization behaviors [133] but does affect food craving, seeking, and consumption [131].
Fathers working in different occupations may be exposed to different sources of exposure, resulting in an elevated risk of a variety diseases of offspring, and studies in this area have focused on the risk of cancer in offspring. For example, fathers working in manufacturing, animal slaughtering, steel and metal processing, and land transportation industries, especially the food and beverage industry, increase the risk of retinoblastoma in their offspring [135]. Those exposed to emissions from gasoline or diesel fuel at work may have a slightly elevated risk of Hodgkin’s lymphoma and soft tissue sarcoma in their offspring [136]. Organic solvent exposure among fathers has been linked to an increase risk of childhood ALL [124], while paternal exposure to zoonotic viruses, organic dust, and other microorganisms from animal-related activities increases the risk of Acute Myeloid Leukemia (AML) in offspring [138]. Offspring of fathers in the painting and printing industry had a threefold increased risk of AML, with no such effect in offspring of mothers in the same industry [139]. In addition, parental employment in the painting and printing industry was associated with a higher risk of CNS cancers in offspring [139], possibly due to the high exposure to organic compounds that these jobs require. However, other studies have concluded that pre-pregnancy occupational dust exposure of fathers is not associated with childhood cancers, leukemias, or CNS tumors [140]. Differences in study outcomes may arise due to several factors. First, the selection of populations included in the studies can influence results. Second, the timing of the fathers’ occupational exposure is another critical factor. Additionally, the ventilation performance of the premises where the exposure occurs can affect outcomes. Lastly, the variability in the specific items to which workers are exposed at their jobs contributes to the differences observed. Heavy metals like mercury, lead, and cadmium can cause health issues due to their persistence and accumulation in the body. While direct exposure to heavy metals may increase cancer risk, paternal occupational exposure does not appear to raise the risk of cancer in children [141]. Occupational exposure of fathers to microorganisms, pesticides, allergens, or reactive chemicals prior to pregnancy does not appear to have an effect on asthma risk in offspring [142]. Table 6 shows how father pre-pregnancy environmental and occupational exposures affect kids.
Aging
Sperm DNA methylation changes with age [143], showing the opposite pattern to that of somatic cells, with age-associated methylation alterations in spermatozoa showing a global tendency to be hypermethylated and a tendency to be hypomethylated in specific regions [144, 145]. The distribution of these age-related methylation changes in spermatozoa is not random; hypermethylated sites are often found in distal gene regions, while hypomethylated sites are near transcriptional start sites. Genes most affected by age are associated with development, neuronal projection, differentiation and recognition, and behavior, suggesting a potential link to an increased risk of neurodevelopmental disorders in offspring born to older fathers [145, 146]. Embryos from older human fathers exhibit transcriptome dysregulation affecting multiple neural signaling pathways in the inner cell mass (ICM) at the preimplantation. Both the ICM and trophoblast ectodermal cell lineages show methylation group changes associated with neurotransmission and neural signaling, which have been linked to genes associated with neurodevelopmental disorders such as autism and schizophrenia, as well as to the regulation of the expression of imprinted genes. Epigenetic variation in these genes may arise in sperm and persist during embryonic development and after birth [147]. No differences in small RNA expression were observed between sperm samples from young and old human fathers, although significant DNA methylation changes were found, suggesting that miRNA regulation is not affected by paternal aging and that sperm DNA methylation is progressively impaired with age, which may be related to the inheritance of neurodevelopmental disorders [148]. Advanced paternal age is a risk factor for autism, schizophrenia, birth defects (especially orofacial clefts and down syndrome), and musculoskeletal congenital anomalies in offspring [149,150,151]. Furthermore, a large cohort study from China showed that older fathers had a 28–31% increased risk of having macrosomia offspring compared to fathers under 30 years of age, after adjusting for multiple factors including occupation, smoking and alcohol consumption [152]. There is an elevated risk of death in offspring of older fathers, with offspring of fathers aged 40 years or older having an increased risk of death mainly due to congenital anomalies, malignant neoplasms, and external causes [153]. From Taiwan, China, three population-based studies reported that older fathers may be associated with an increased risk of mental health and physical health in their offspring [154,155,156]. The age of the father showed a linear increasing trend with the risk of a range of mental disorders such as schizophrenia [154], autism, and ADHD in the offspring [155]. Fathers’ age of 40 years or older was correlated with offspring lung function and remained statistically significant even after the inclusion of multiple confounders into the multivariate model, suggesting that advanced paternal age may have an impact on offspring’s large and small airway obstruction, increasing the risk of respiratory disorders in offspring [156]. In aged mice, genes near hypomethylated differentially methylated regions (hypo-DMRs) in sperm DNA are associated with cellular projections and processes such as learning or memory. Motif analysis has revealed a significant enrichment of specific REST/NRSF binding motifs within these hypo-DMRs in the sperm of aged mice. Some hypo-DMRs detected near REST/NRSF binding sites in the sperm of aged mice are found in proximity to genes related to neurodevelopmental disorders such as autism spectrum disorder and schizophrenia, including genes like Shank2 and Htr6. Transcriptome analysis of embryonic brains indicates that genes related to the REST/NRSF target gene set are upregulated in offspring sired by aged mouse fathers, and these offspring exhibit reduced diversity in vocal communication [143]. The male offspring of aged mice exhibit poor glucose tolerance, hepatic lipid accumulation, increased adipogenesis, and impaired energy homeostasis. Concurrently, there are significant alterations in the expression of genes in pathways related to lipid metabolism and thermogenesis and activation of peroxisome proliferator-activated receptor signaling pathway [157], which may be the result of the aged fathers’ carrying specific epigenetic markers in sperm during fertilization that are passed on to the offspring, affecting the energy balance and metabolic efficiency of the offspring. Aging in mice alters the profile of transfer RNA-derived small RNAs (tsRNAs) in sperm, and their male offspring show anxiety-like behavior and reprogramming of cerebral cortex and hippocampal tissues for gene expression involved in dopamine synapses and neurotrophic signaling pathways [158], suggesting that paternal aging may affect the offspring’s neurological system by altering tsRNAs in spermatozoa development and behavioral traits.
Increasing age in humans leads to an increase in sex chromosome aneuploidy in sperm [66], which may lead to certain genetic diseases or developmental disorders if aneuploid spermatozoa are successfully fertilized and develop into embryos [159]. Sperm condensed chromatin increases linearly with age [160], and condensed chromatin may be influential in causing defective embryos after fertilization, affecting the health of the offspring. Table 7 displays offspring outcomes by a father’s age. These data suggest that the father’s reproductive age may affect offspring health and survival, but it is insufficient to fully explain how paternal age affects offspring epigenetics. Health professionals should consider paternal age when providing pre-pregnancy counseling to better assess and communicate potential risks and provide couples with more comprehensive health guidance.
Gut microbial
Environmental variables that disrupt the gut microbial population may affect cellular tissue physiological responses and pathology [161]. A recent study found that paternal gut bacteria can affect offspring [162]. Offspring born to mouse fathers with dysbiosis had significantly reduced growth and increased perinatal mortality, and their brain and brown adipose tissue showed differentially expressed metabolic genes. However, their gut flora was unaffected, and in vitro fertilization experiments showed that spermatozoa mediated this effect. Although few studies have examined the epigenetic effects of paternal gut bacteria on offspring, our findings suggest future investigation.
Interactions between exposures and cumulative effects
It is essential to consider the interactions and cumulative effects of various paternal exposures, as many studies have only examined their separate impacts. For instance, cigarette smoke generates PAHs even in indoor settings. Unhealthy eating habits and lack of physical activity may contribute to obesity. Smoking, obesity, and exposure to environmental toxins may impact men’s sperm quality, leading to a passive delay in childbearing. Use of addictive substances can result in neglecting one’s own nutritional and physical well-being. Obesity may contribute to epigenetic aging of spermatozoa [163], and some of these exposures may subject men to potential harm. Men’s motility may be enhanced by occupations that include exposure to harmful substances. When these factors are combined, they may act on the male reproductive system, leading to an overall decline in sperm quality, affecting the success of conception while also affecting the health of the offspring through epigenetic mechanisms, increasing the risk of various diseases in the offspring. Conducting large-scale, long-term cumulative effect studies in humans is challenging due to the significant resources, time, and participant cooperation required, as well as the need to eliminate numerous complex confounding factors. Animal model studies that examine multifactorial exposures can provide follow-up insights, helping to better understand how these factors interact and their potential impact on reproductive health and offspring well-being.
Clinical implications and preventive measures
The findings from this study have significant implications for clinical practice. Understanding the epigenetic impact of paternal exposures before conception can inform targeted interventions and preventive measures for prospective parents. Healthcare professionals should educate and counsel prospective fathers about the potential risks associated with environmental toxins, smoking, poor diet, and other harmful exposures. One preventive measure is pre-pregnancy care, including lifestyle changes such as smoking cessation, maintaining a healthy weight, sensible exercise, a balanced diet with essential nutrients such as omega-3 fatty acids, and avoidance of addictive substances, which can help mitigate adverse epigenetic modifications in sperm, thereby improving health outcomes for offspring. In addition, public health should emphasize the importance of fathers’ health and its impact on offspring, and policies aimed at reducing exposure to environmental pollutants, promoting healthy lifestyles and providing resources for smoking cessation programs can also play a key role. At the same time, men should be advised to consider the timing of having offspring to avoid developmental and genetic defects in the offspring caused by advanced age. In clinical settings, routine assessment of paternal health and environmental exposures should be incorporated into pre-pregnancy care programs. For couples with a history of adverse exposures, genetic counseling and testing are recommended to assess potential risks and guide individualized interventions. Overall, these prevention strategies can help to reduce the risk of offspring health problems and improve the well-being of future generations by addressing the critical period of pre-pregnancy paternal health.
New contributions and hypotheses
This review article contributes to the field by offering new insights into the epigenetic impact of paternal exposures before conception. First, our review article emphasizes that the process of epigenetic effects is influenced by factors such as sex and developmental stage. While epigenetic effects may provide offspring with adaptations to specific environments, not all changes result in positive adaptive benefits. For example, adverse environmental exposures such as paternal smoking, obesity, and addictive substance abuse may have gender-specific and stage-specific adverse effects on offspring health through epigenetic mechanisms. These factors may affect gene expression and physiologic functioning in the offspring and increase the risk of disease. Therefore, in-depth studies of paternal epigenetic effects must take into account the influence of gender and developmental stage and be alert to epigenetic changes that may negatively affect the health of offspring at different stages. Second, as stated previously, ROS are critical in spermatogenesis and development, and exposures such as smoking and obesity affect ROS levels within male sperm, and subsequent observation of the effects of paternal epigenetics on the levels of ROS within the offspring’s sperm is necessary. Third, paternal exposure to different factors may cause alterations in specific miRNAs, mt-tRNAs, and tsRNAs in their own sperm, and we speculate that these specific molecular changes have the potential to resist reprogramming mechanisms during embryonic development to cause genetic effects on the offspring. These new contributions emphasize the complexity and importance of paternal epigenetic inheritance and open new avenues for research on preventive and therapeutic strategies aimed at mitigating adverse health outcomes in offspring.
Conclusion
The review article examines several elements that affect paternal epigenetic impacts, such as environmental chemicals, dietary patterns, lifestyle choices, and aging on the development and well-being of offspring. Understanding these mechanisms is crucial for developing targeted interventions and preventive measures aimed at improving public health. Future research should focus on standardizing methodologies, increasing cohort diversity, and conducting long-term follow-up studies to validate and expand upon these findings. Additionally, exploring the role of non-coding RNAs and other emerging epigenetic markers will provide deeper insights into the complex mechanisms of epigenetic inheritance. The importance of pre-pregnancy paternal health cannot be overstated, as it has profound implications for the health of future generations. Public health initiatives should prioritize educating and supporting prospective fathers in adopting healthier lifestyles to mitigate potential risks. By addressing these factors, we can enhance reproductive outcomes and promote the well-being of future populations In summary, this review underscores the need for a comprehensive approach to paternal pre-pregnancy health, integrating clinical practice, public health policies, and further scientific research to fully understand and address the epigenetic influences on offspring health.
Limitations of current research
While this review provides a comprehensive overview of the impact of paternal pre-pregnancy exposures on offspring health, several limitations must be acknowledged. Firstly, the studies reviewed encompass a variety of experimental designs, populations, and exposure types, which can introduce heterogeneity and make direct comparisons challenging. Potential confounding factors, such as genetic predispositions and environmental influences, may also affect the results but were not consistently controlled for across studies. Secondly, the generalizability of these findings is limited by the diversity of the study cohorts. Many studies were conducted in specific populations or under controlled laboratory conditions, which may not fully represent the broader human population. This limits the applicability of the findings to diverse demographic and environmental contexts. Thirdly, methodological limitations in the reviewed studies, such as small sample sizes, retrospective designs, and varying exposure assessment methods, can affect the reliability and validity of the results. Additionally, the long-term follow-up required to observe transgenerational effects is often lacking, which makes it difficult to draw definitive conclusions about the persistence and impact of epigenetic changes over generations. Lastly, while animal models provide valuable insights into the mechanisms of epigenetic inheritance, their relevance to human health must be interpreted with caution. Differences in physiology, lifespan, and environmental interactions between species can influence the extent to which findings from animal studies can be extrapolated to humans. Recognizing these limitations is crucial for interpreting the findings of this review and for guiding future research. Further studies with standardized methodologies, larger and more diverse cohorts, and long-term follow-up are needed to confirm and expand upon the insights gained from the current literature.
Data availability
Not applicable.
References
Chen T, Li E. Structure and function of eukaryotic DNA methyltransferases. Curr Top Dev Biol. 2004;60:55–89. https://doi.org/10.1016/S0070-2153(04)60003-2.
Montjean D, Zini A, Ravel C, et al. Sperm global DNA methylation level: association with semen parameters and genome integrity. Andrology. 2015;3(2):235–40. https://doi.org/10.1111/andr.12001.
Zhang Y, Sun Z, Jia J, et al. Overview of histone modification. Adv Exp Med Biol. 2021;1283:1–16. https://doi.org/10.1007/978-981-15-8104-5_1.
Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15(suppl_1):R17–29. https://doi.org/10.1093/hmg/ddl046.
Perez MF, Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat Cell Biol. 2019;21(2):143–51. https://doi.org/10.1038/s41556-018-0242-9.
Xing Y, Shi S, Le L, Lee CA, Silver-Morse L, Li WX. Evidence for transgenerational transmission of epigenetic tumor susceptibility in Drosophila. PLoS Genet. 2007;3(9):e151. https://doi.org/10.1371/journal.pgen.0030151.
Blake GE, Watson ED. Unravelling the complex mechanisms of transgenerational epigenetic inheritance. Curr Opin Chem Biol. 2016;33:101–7. https://doi.org/10.1016/j.cbpa.2016.06.008.
Xavier MJ, Roman SD, Aitken RJ, Nixon B. Transgenerational inheritance: how impacts to the epigenetic and genetic information of parents affect offspring health. Hum Reprod Update. 2019;25(5):518–40. https://doi.org/10.1093/humupd/dmz017.
Seisenberger S, Andrews S, Krueger F, et al. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell. 2012;48(6):849–62. https://doi.org/10.1016/j.molcel.2012.11.001.
Hackett JA, Sengupta R, Zylicz JJ, et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339(6118):448–52. https://doi.org/10.1126/science.1229277.
Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13(3):153–62. https://doi.org/10.1038/nrg3188.
Hajkova P, Erhardt S, Lane N, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117(1–2):15–23. https://doi.org/10.1016/s0925-4773(02)00181-8.
Guibert S, Forné T, Weber M. Global profiling of DNA methylation erasure in mouse primordial germ cells. Genome Res. 2012;22(4):633–41. https://doi.org/10.1101/gr.130997.111.
Rathke C, Baarends WM, Awe S, Renkawitz-Pohl R. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta. 2014;1839(3):155–68. https://doi.org/10.1016/j.bbagrm.2013.08.004.
Moritz L, Schon SB, Rabbani M, et al. Sperm chromatin structure and reproductive fitness are altered by substitution of a single amino acid in mouse protamine 1. Nat Struct Mol Biol. 2023;30(8):1077–91. https://doi.org/10.1038/s41594-023-01033-4.
Guo F, Li X, Liang D, et al. Active and passive demethylation of male and female pronuclear DNA in the mammalian zygote. Cell Stem Cell. 2014;15(4):447–59. https://doi.org/10.1016/j.stem.2014.08.003.
Wang L, Zhang J, Duan J, et al. Programming and inheritance of parental DNA methylomes in mammals [published correction appears in Cell. Cell. 2014;157(4):979–91. https://doi.org/10.1016/j.cell.2014.04.017.
Burton A, Torres-Padilla ME. Epigenetic reprogramming and development: a unique heterochromatin organization in the preimplantation mouse embryo. Brief Funct Genomics. 2010;9(5–6):444–54. https://doi.org/10.1093/bfgp/elq027.
Zhu P, Guo H, Ren Y, et al. Single-cell DNA methylome sequencing of human preimplantation embryos. Nat Genet. 2018;50(1):12–9. https://doi.org/10.1038/s41588-017-0007-6.
Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146(6):866–72. https://doi.org/10.1016/j.cell.2011.08.042.
Zeng Y, Chen T. DNA Methylation reprogramming during mammalian development. Genes. 2019;10(4):257. https://doi.org/10.3390/genes10040257.
Jung YH, Kremsky I, Gold HB, et al. Maintenance of CTCF- and transcription factor-mediated interactions from the gametes to the early mouse embryo. Mol Cell. 2019;75(1):154-171.e5. https://doi.org/10.1016/j.molcel.2019.04.014.
Eskenazi B, Castorina R. Association of prenatal maternal or postnatal child environmental tobacco smoke exposure and neurodevelopmental and behavioral problems in children. Environ Health Perspect. 1999;107(12):991–1000. https://doi.org/10.1289/ehp.99107991.
Liang J, Fu Z, Liu Q, et al. Interactions among maternal smoking, breastfeeding, and offspring genetic factors on the risk of adult-onset hypertension. BMC Med. 2022;20(1):454. https://doi.org/10.1186/s12916-022-02648-y.
Wang R, Sun T, Yang Q, et al. Low birthweight of children is positively associated with mother’s prenatal tobacco smoke exposure in Shanghai: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):603. https://doi.org/10.1186/s12884-020-03307-x.
Liu Y, Chen S, Pang D, et al. Effects of paternal exposure to cigarette smoke on sperm DNA methylation and long-term metabolic syndrome in offspring. Epigenetics Chromatin. 2022;15(1):3. https://doi.org/10.1186/s13072-022-00437-8.
You Y, Liu R, Zhou H, et al. Effect of exposure to paternal smoking on overweight and obesity in children: findings from the children lifeway cohort in Shenzhen. Southern China Obes Facts. 2022;15(4):609–20. https://doi.org/10.1159/000525544.
Mørkve Knudsen GT, Rezwan FI, Johannessen A, et al. Epigenome-wide association of father’s smoking with offspring DNA methylation: a hypothesis-generating study. Environ Epigenetics. 2019;5(4):dvz023. https://doi.org/10.1093/eep/dvz023.
Mexal S, Berger R, Pearce L, et al. Regulation of a novel αN-catenin splice variant in schizophrenic smokers. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):759–68. https://doi.org/10.1002/ajmg.b.30679.
Havdahl A, Wootton RE, Leppert B, et al. Associations between pregnancy-related predisposing factors for offspring neurodevelopmental conditions and parental genetic liability to attention-deficit/hyperactivity disorder, autism, and schizophrenia. JAMA Psychiat. 2022;79(8):799. https://doi.org/10.1001/jamapsychiatry.2022.1728.
Kitaba NT, Knudsen GTM, Johannessen A, et al. Fathers’ pre-pregnancy smoking and offspring DNA methylation. Clin Epigenetics. 2023;15(1):131. https://doi.org/10.1186/s13148-023-01540-7.
Northstone K, Golding J, Davey Smith G, Miller LL, Pembrey M. Prepubertal start of father’s smoking and increased body fat in his sons: further characterisation of paternal transgenerational responses. Eur J Hum Genet. 2014;22(12):1382–6. https://doi.org/10.1038/ejhg.2014.31.
Knudsen GTM, Dharmage S, Janson C, et al. Parents’ smoking onset before conception as related to body mass index and fat mass in adult offspring: findings from the RHINESSA generation study. PLoS ONE. 2020;15(7):e0235632. https://doi.org/10.1371/journal.pone.0235632.
Accordini S, Calciano L, Johannessen A, et al. Prenatal and prepubertal exposures to tobacco smoke in men may cause lower lung function in future offspring: a three-generation study using a causal modelling approach. Eur Respir J. 2021;58(4):2002791. https://doi.org/10.1183/13993003.02791-2020.
Accordini S, Calciano L, Johannessen A, et al. A three-generation study on the association of tobacco smoking with asthma. Int J Epidemiol. 2018;47(4):1106–17. https://doi.org/10.1093/ije/dyy031.
Liu J, Bowatte G, Pham J, et al. Pre-pubertal smoke exposure of fathers and increased risk of offspring asthma: a possible transgenerational effect. Eur Respir J. 2022;60(4):2200257. https://doi.org/10.1183/13993003.00257-2022.
Wu CC, Hsu TY, Chang JC, et al. Paternal tobacco smoke correlated to offspring asthma and prenatal epigenetic programming. Front Genet. 2019;10:471. https://doi.org/10.3389/fgene.2019.00471.
Mejia-Lancheros C, Mehegan J, Murrin CM, et al. Smoking habit from the paternal line and grand-child’s overweight or obesity status in early childhood: prospective findings from the lifeways cross-generation cohort study. Int J Obes. 2018;42:1853–70. https://doi.org/10.1038/s41366-018-0039-8.
The ALSPAC Study Team, Pembrey ME, Bygren LO, et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14(2):159–66. https://doi.org/10.1038/sj.ejhg.5201538.
Bailey HD, Lacour B, Guerrini-Rousseau L, et al. Parental smoking, maternal alcohol, coffee and tea consumption and the risk of childhood brain tumours: the ESTELLE and ESCALE studies (SFCE, France). Cancer Causes Control. 2017;28(7):719–32. https://doi.org/10.1007/s10552-017-0900-4.
Milne E, Greenop KR, Scott RJ, et al. Parental smoking and risk of childhood brain tumors. Int J Cancer. 2013;133(1):253–9. https://doi.org/10.1002/ijc.28004.
Filippini G, Maisonneuve P, McCredie M, et al. Relation of childhood brain tumors to exposure of parents and children to tobacco smoke: The Search International Case-Control Study. Int J Cancer. 2002;100(2):206–13. https://doi.org/10.1002/ijc.10465.
Hamad MF, Dayyih WAA, Laqqan M, AlKhaled Y, Montenarh M, Hammadeh ME. The status of global DNA methylation in the spermatozoa of smokers and non-smokers. Reprod Biomed Online. 2018;37(5):581–9. https://doi.org/10.1016/j.rbmo.2018.08.016.
Murphy PJ, Guo J, Jenkins TG, et al. NRF2 loss recapitulates heritable impacts of paternal cigarette smoke exposure. PLoS Genet. 2020;16(6):e1008756. https://doi.org/10.1371/journal.pgen.1008756.
Hammer B, Kadalayil L, Boateng E, et al. Preconceptional smoking alters spermatozoal miRNAs of murine fathers and affects offspring’s body weight. Int J Obes. 2021;45(7):1623–7.
Glantz SA, Bareham DW. E-Cigarettes: use, effects on smoking, risks, and policy implications. Annu Rev Public Health. 2018;39:215–35. https://doi.org/10.1146/annurev-publhealth-040617-013757.
Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol. 2017;14(8):447–56. https://doi.org/10.1038/nrcardio.2017.36.
Maurer JJ, Wimmer ME, Turner CA, et al. Paternal nicotine taking elicits heritable sex-specific phenotypes that are mediated by hippocampal Satb2. Mol Psychiatry. 2022;27(9):3864–74. https://doi.org/10.1038/s41380-022-01622-7.
Hawkey AB, White H, Pippen E, et al. Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicol Teratol. 2019;74:106808. https://doi.org/10.1016/j.ntt.2019.05.001.
McCarthy DM, Morgan TJ, Lowe SE, et al. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biol. 2018;16(10):e2006497. https://doi.org/10.1371/journal.pbio.2006497.
Zhang M, Zhang D, Dai J, et al. Paternal nicotine exposure induces hyperactivity in next-generation via down-regulating the expression of DAT. Toxicology. 2020;431:152367. https://doi.org/10.1016/j.tox.2020.152367.
Vallaster MP, Kukreja S, Bing XY, et al. Paternal nicotine exposure alters hepatic xenobiotic metabolism in offspring. eLife. 2017;6:e24771. https://doi.org/10.7554/eLife.24771.
Keyhan S, Burke E, Schrott R, et al. Male obesity impacts DNA methylation reprogramming in sperm. Clin Epigenetics. 2021;13(1):17. https://doi.org/10.1186/s13148-020-00997-0.
Noor N, Cardenas A, Rifas-Shiman SL, et al. Association of periconception paternal body mass index with persistent changes in DNA methylation of offspring in childhood. JAMA Netw Open. 2019;2(12):e1916777. https://doi.org/10.1001/jamanetworkopen.2019.16777.
Sharp GC, Alfano R, Ghantous A, et al. Paternal body mass index and offspring DNA methylation: findings from the PACE consortium. Int J Epidemiol. 2021;50(4):1297–315. https://doi.org/10.1093/ije/dyaa267.
Potabattula R, Dittrich M, Schorsch M, Hahn T, Haaf T, El Hajj N. Male obesity effects on sperm and next-generation cord blood DNA methylation. PLoS ONE. 2019;14(6):e0218615. https://doi.org/10.1371/journal.pone.0218615.
Fontelles CC, Carney E, Clarke J, et al. Paternal overweight is associated with increased breast cancer risk in daughters in a mouse model. Sci Rep. 2016;6(1):28602. https://doi.org/10.1038/srep28602.
Johannessen A, Lønnebotn M, Calciano L, et al. Being overweight in childhood, puberty, or early adulthood: changing asthma risk in the next generation? J Allergy Clin Immunol. 2020;145(3):791-799.e4. https://doi.org/10.1016/j.jaci.2019.08.030.
Bowatte G, Bui DS, Priyankara S, et al. Parental pre-pregnancy BMI trajectories from childhood to adolescence and asthma in the future offspring. J Allergy Clin Immunol. 2022;150(1):67-74.e30. https://doi.org/10.1016/j.jaci.2021.11.028.
Lønnebotn M, Calciano L, Johannessen A, et al. Parental prepuberty overweight and offspring lung function. Nutrients. 2022;14(7):1506. https://doi.org/10.3390/nu14071506.
Deveci AC, Keown-Stoneman CDG, Maguire JL, et al. Paternal BMI in the pre-pregnancy period, and the association with child zBMI. Int J Obes. 2023;47(4):280–7. https://doi.org/10.1038/s41366-023-01261-0.
Jääskeläinen A, Pussinen J, Nuutinen O, et al. Intergenerational transmission of overweight among Finnish adolescents and their parents: a 16-year follow-up study. Int J Obes. 2011;35(10):1289–94. https://doi.org/10.1038/ijo.2011.150.
Loomba R, Hwang S, O’Donnell CJ, et al. Parental obesity and offspring serum alanine and aspartate aminotransferase levels: the Framingham Heart Study. Gastroenterology. 2008;134(4):953-959.e1. https://doi.org/10.1053/j.gastro.2008.01.037.
Retnakaran R, Wen SW, Tan H, et al. Paternal weight prior to conception and infant birthweight: a prospective cohort study. Nutr Diabetes. 2021;11(1):28. https://doi.org/10.1038/s41387-021-00172-1.
Lin Y, Chen Z, Qian Q, et al. Effects of paternal obesity on maternal-neonatal outcomes and long-term prognosis in adolescents. Front Endocrinol. 2023;14:1114250. https://doi.org/10.3389/fendo.2023.1114250.
Punjabi U, Goovaerts I, Peeters K, Van Mulders H, De Neubourg D. Sperm as a carrier of genome instability in relation to paternal lifestyle and nutritional conditions. Nutrients. 2022;14(15):3155. https://doi.org/10.3390/nu14153155.
Raee P, Shams Mofarahe Z, Nazarian H, et al. Male obesity is associated with sperm telomere shortening and aberrant mRNA expression of autophagy-related genes. Basic Clin Androl. 2023;33(1):13. https://doi.org/10.1186/s12610-023-00188-w.
Pini T, Parks J, Russ J, et al. Obesity significantly alters the human sperm proteome, with potential implications for fertility. J Assist Reprod Genet. 2020;37(4):777–87. https://doi.org/10.1007/s10815-020-01707-8.
Crean AJ, Senior AM, Freire T, et al. Paternal dietary macronutrient balance and energy intake drive metabolic and behavioral differences among offspring. Nat Commun. 2024;15(1):2982. https://doi.org/10.1038/s41467-024-46782-y.
Chleilat F, Schick A, Deleemans JM, et al. Paternal high protein diet modulates body composition, insulin sensitivity, epigenetics, and gut microbiota intergenerationally in rats. FASEB J. 2021;35(9):e21847. https://doi.org/10.1096/fj.202100198RR.
Ng SF, Lin RCY, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–6. https://doi.org/10.1038/nature09491.
Fullston T, McPherson NO, Owens JA, Kang WX, Sandeman LY, Lane M. Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet. Physiol Rep. 2015;3(3):e12336. https://doi.org/10.14814/phy2.12336.
Fullston T, Teague EMCO, Palmer NO, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013;27(10):4226–43. https://doi.org/10.1096/fj.12-224048.
Wei S, Luo S, Zhang H, Li Y, Zhao J. Paternal high-fat diet altered SETD2 gene methylation in sperm of F0 and F1 mice. Genes Nutr. 2023;18(1):12. https://doi.org/10.1186/s12263-023-00731-4.
Gong P, Bailbé D, Bianchi L, et al. Paternal high-protein diet programs offspring insulin sensitivity in a sex-specific manner. Biomolecules. 2021;11(5):751. https://doi.org/10.3390/biom11050751.
Shrestha A, Dellett SK, Yang J, Sharma U, Ramalingam L. Effects of fish oil supplementation on reducing the effects of paternal obesity and preventing fatty liver in offspring. Nutrients. 2023;15(24):5038. https://doi.org/10.3390/nu15245038.
Sanchez-Garrido MA, Ruiz-Pino F, Velasco I, et al. Intergenerational influence of paternal obesity on metabolic and reproductive health parameters of the offspring: male-preferential impact and involvement of kiss1-mediated pathways. Endocrinology. 2018;159(2):1005–18. https://doi.org/10.1210/en.2017-00705.
Zhang X, Dong Y, Sun G, et al. Paternal programming of liver function and lipid profile induced by a paternal pre-conceptional unhealthy diet: potential association with altered gut microbiome composition. Kidney Blood Press Res. 2019;44(1):133–48. https://doi.org/10.1159/000497487.
Watkins AJ, Dias I, Tsuro H, et al. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc Natl Acad Sci. 2018;115(40):10064–9. https://doi.org/10.1073/pnas.1806333115.
Furse S, Morgan HL, Koulman A, Watkins AJ. Characterisation of the paternal influence on intergenerational offspring cardiac and brain lipid homeostasis in mice. Int J Mol Sci. 2023;24(3):1814. https://doi.org/10.3390/ijms24031814.
Morgan HL, Furse S, Dias IHK, et al. Paternal low protein diet perturbs inter-generational metabolic homeostasis in a tissue-specific manner in mice. Commun Biol. 2022;5(1):929. https://doi.org/10.1038/s42003-022-03914-8.
Kaati G, Bygren L, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10(11):682–8. https://doi.org/10.1038/sj.ejhg.5200859.
Pérez Lugo MI, Salas ML, Shrestha A, Ramalingam L. Fish oil improves offspring metabolic health of paternal obese mice by targeting adipose tissue. Biomolecules. 2024;14(4):418. https://doi.org/10.3390/biom14040418.
Xiong L, Dorus S, Ramalingam L. Role of fish oil in preventing paternal obesity and improving offspring skeletal muscle health. Biomedicines. 2023;11(12):3120. https://doi.org/10.3390/biomedicines11123120.
Shi Q, Liu X, Fan X, Wang R, Qi K. Paternal dietary ratio of n-6: n-3 polyunsaturated fatty acids programs offspring leptin expression and gene imprinting in mice. Front Nutr. 2022;9:1043876. https://doi.org/10.3389/fnut.2022.1043876.
Li M, Shi Q, Jiang X, et al. Paternal pre-pregnancyal diet enriched with n-3 polyunsaturated fatty acids affects offspring brain function in mice. Front Nutr. 2022;9:969848. https://doi.org/10.3389/fnut.2022.969848.
Tomar A, Gomez-Velazquez M, Gerlini R, et al. Epigenetic inheritance of diet-induced and sperm-borne mitochondrial RNAs. Nature. 2024;630(8017):720–7. https://doi.org/10.1038/s41586-024-07472-3.
Hernández-Saavedra D, Markunas C, Takahashi H, et al. Maternal exercise and paternal exercise induce distinct metabolite signatures in offspring tissues. Diabetes. 2022;71(10):2094–105. https://doi.org/10.2337/db22-0341.
Stanford KI, Rasmussen M, Baer LA, et al. Paternal exercise improves glucose metabolism in adult offspring. Diabetes. 2018;67(12):2530–40. https://doi.org/10.2337/db18-0667.
Zheng J, Alves-Wagner AB, Stanford KI, et al. Maternal and paternal exercise regulate offspring metabolic health and beta cell phenotype. BMJ Open Diabetes Res Care. 2020;8(1):e000890. https://doi.org/10.1136/bmjdrc-2019-000890.
Krout D, Roemmich JN, Bundy A, Garcia RA, Yan L, Claycombe-Larson KJ. Paternal exercise protects mouse offspring from high-fat-diet-induced type 2 diabetes risk by increasing skeletal muscle insulin signaling. J Nutr Biochem. 2018;57:35–44. https://doi.org/10.1016/j.jnutbio.2018.03.013.
Costa-Júnior JM, Ferreira SM, Kurauti MA, et al. Paternal exercise improves the metabolic health of offspring via epigenetic modulation of the germline. Int J Mol Sci. 2021;23(1):1. https://doi.org/10.3390/ijms23010001.
Murashov AK, Pak ES, Koury M, et al. Paternal long-term exercise programs offspring for low energy expenditure and increased risk for obesity in mice. FASEB J. 2016;30(2):775–84. https://doi.org/10.1096/fj.15-274274.
Belladelli F, Basran S, Eisenberg ML. Male fertility and physical exercise. World J Mens Health. 2023;41(3):482. https://doi.org/10.5534/wjmh.220199.
Short AK, Yeshurun S, Powell R, et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl Psychiatry. 2017;7(5):e1114–e1114. https://doi.org/10.1038/tp.2017.82.
Spindler C, Segabinazi E, Meireles AFD, et al. Paternal physical exercise modulates global DNA methylation status in the hippocampus of male rat offspring. Neural Regen Res. 2019;14(3):491. https://doi.org/10.4103/1673-5374.245473.
Mega F, De Meireles ALF, Piazza FV, et al. Paternal physical exercise demethylates the hippocampal DNA of male pups without modifying the cognitive and physical development. Behav Brain Res. 2018;348:1–8. https://doi.org/10.1016/j.bbr.2018.03.040.
Yin MM, Wang W, Sun J, et al. Paternal treadmill exercise enhances spatial learning and memory related to hippocampus among male offspring. Behav Brain Res. 2013;253:297–304. https://doi.org/10.1016/j.bbr.2013.07.040.
Swinford-Jackson SE, Fant B, Wimmer ME, et al. Cocaine-induced changes in sperm Cdkn1a methylation are associated with cocaine resistance in male offspring. J Neurosci. 2022;42(14):2905–16. https://doi.org/10.1523/JNEUROSCI.3172-20.2022.
Wimmer ME, Briand LA, Fant B, et al. Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol Psychiatry. 2017;22(11):1641–50. https://doi.org/10.1038/mp.2017.8.
Yaw AM, Glass JD, Prosser RA, Caldwell HK. Paternal cocaine in mice alters social behavior and brain oxytocin receptor density in first generation offspring. Neuroscience. 2022;485:65–77. https://doi.org/10.1016/j.neuroscience.2022.01.010.
Rich MT, Worobey SJ, Mankame S, Pang ZP, Swinford-Jackson SE, Pierce RC. Sex-dependent fear memory impairment in cocaine-sired rat offspring. Sci Adv. 2023;9(42):eadf6039. https://doi.org/10.1126/sciadv.adf6039.
Cui J, Huang N, Fan G, et al. Paternal cocaine-seeking motivation defines offspring’s vulnerability to addiction by down-regulating GABAergic GABRG3 in the ventral tegmental area. Transl Psychiatry. 2024;14(1):107. https://doi.org/10.1038/s41398-024-02835-w.
Huang N, Cui J, Fan G, et al. Transcriptomic effects of paternal cocaine-seeking on the reward circuitry of male offspring. Transl Psychiatry. 2024;14(1):120. https://doi.org/10.1038/s41398-024-02839-6.
Wimmer ME, Vassoler FM, White SL, et al. Impaired cocaine-induced behavioral plasticity in the male offspring of cocaine-experienced sires. Eur J Neurosci. 2019;49(9):1115–26. https://doi.org/10.1111/ejn.14310.
Azadi M, Moazen P, Wiskerke J, Semnanian S, Azizi H. pre-pregnancy paternal morphine exposure leads to an impulsive phenotype in male rat progeny. Psychopharmacology. 2021;238(12):3435–46. https://doi.org/10.1007/s00213-021-05962-0.
Vassoler FM, Toorie AM, Teceno DN, et al. Paternal morphine exposure induces bidirectional effects on cocaine versus opioid self-administration. Neuropharmacology. 2020;162:107852. https://doi.org/10.1016/j.neuropharm.2019.107852.
Rohbani K, Sabzevari S, Sadat-Shirazi MS, et al. Parental morphine exposure affects repetitive grooming actions and marble burying behavior in the offspring: potential relevance for obsessive-compulsive like behavior. Eur J Pharmacol. 2019;865:172757. https://doi.org/10.1016/j.ejphar.2019.172757.
Sabzevari S, Rohbani K, Sadat-Shirazi MS, et al. Morphine exposure before conception affects anxiety-like behavior and CRF level (in the CSF and plasma) in the adult male offspring. Brain Res Bull. 2019;144:122–31. https://doi.org/10.1016/j.brainresbull.2018.11.022.
Sadat-Shirazi MS, Asgari P, Mahboubi S, et al. Effect of morphine exposure on novel object memory of the offspring: the role of histone H3 and ΔFosB. Brain Res Bull. 2020;156:141–9. https://doi.org/10.1016/j.brainresbull.2020.01.011.
Brynildsen JK, Sanchez V, Yohn NL, Carpenter MD, Blendy JA. Sex-specific transgenerational effects of morphine exposure on reward and affective behaviors. Behav Brain Res. 2020;395:112842. https://doi.org/10.1016/j.bbr.2020.112842.
Ellis AS, Toussaint AB, Knouse MC, et al. Paternal morphine self-administration produces object recognition memory deficits in female, but not male offspring. Psychopharmacology. 2020;237(4):1209–21. https://doi.org/10.1007/s00213-019-05450-6.
Zeid D, Toussaint AB, Dressler CC, et al. Paternal morphine exposure in rats reduces social play in adolescent male progeny without affecting drug-taking behavior in juvenile males or female offspring. Mol Cell Neurosci. 2023;126:103877. https://doi.org/10.1016/j.mcn.2023.103877.
Li Z, Liu D, Wang G, et al. METH exposure alters sperm DNA methylation in F0 mice and mPFC transcriptome in male F1 mice. Psychopharmacology. 2024;241(5):897–911. https://doi.org/10.1007/s00213-023-06516-2.
Monteiro P, Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18(3):147–57. https://doi.org/10.1038/nrn.2016.183.
Febbraro F, Andersen HHB, Kitt MM, Willnow TE. Spatially and temporally distinct patterns of expression for VPS10P domain receptors in human cerebral organoids. Front Cell Dev Biol. 2023;11:1229584. https://doi.org/10.3389/fcell.2023.1229584.
Fan Y, Li Z, Zheng Y, et al. Sex-specific neurobehavioural outcomes and brain stimulation pattern in adult offspring paternally exposed to methamphetamine. Addict Biol. 2022;27(3):e13175. https://doi.org/10.1111/adb.13175.
Zheng Y, Liu D, Guo H, et al. Paternal methamphetamine exposure induces higher sensitivity to methamphetamine in male offspring through driving ADRB1 on CaMKII-positive neurons in mPFC. Transl Psychiatry. 2023;13(1):324. https://doi.org/10.1038/s41398-023-02624-x.
Slotkin TA, Levin ED, Seidler FJ. Paternal cannabis exposure prior to mating, but not δ9-tetrahydrocannabinol, elicits deficits in dopaminergic synaptic activity in the offspring. Toxicol Sci. 2021;184(2):252–64. https://doi.org/10.1093/toxsci/kfab117.
Schrott R, Acharya K, Itchon-Ramos N, et al. Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics. 2020;15(1–2):161–73. https://doi.org/10.1080/15592294.2019.1656158.
Holloway ZR, Hawkey AB, Torres AK, et al. Paternal cannabis extract exposure in rats: pre-pregnancy timing effects on neurodevelopmental behavior in offspring. Neurotoxicology. 2020;81:180–8. https://doi.org/10.1016/j.neuro.2020.10.007.
Slotkin TA, Skavicus S, Levin ED, Seidler FJ. Paternal Δ9-tetrahydrocannabinol exposure prior to mating elicits deficits in cholinergic synaptic function in the offspring. Toxicol Sci. 2020;174(2):210–7. https://doi.org/10.1093/toxsci/kfaa004.
Holloway ZR, Hawkey AB, Pippin E, et al. Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology. 2020;78:57–63. https://doi.org/10.1016/j.neuro.2020.01.009.
Metayer C, Scelo G, Kang AY, et al. A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ Res. 2016;151:174–83. https://doi.org/10.1016/j.envres.2016.06.047.
Godschalk R, Remels A, Hoogendoorn C, et al. Paternal exposure to environmental chemical stress affects male offspring’s hepatic mitochondria. Toxicol Sci. 2018;162(1):241–50. https://doi.org/10.1093/toxsci/kfx246.
Zhang W, Yang J, Lv Y, Li S, Qiang M. Paternal benzo[a]pyrene exposure alters the sperm DNA methylation levels of imprinting genes in F0 generation mice and their unexposed F1–2 male offspring. Chemosphere. 2019;228:586–94. https://doi.org/10.1016/j.chemosphere.2019.04.092.
Spycher BD, Lupatsch JE, Huss A, et al. Parental occupational exposure to benzene and the risk of childhood cancer: a census-based cohort study. Environ Int. 2017;108:84–91. https://doi.org/10.1016/j.envint.2017.07.022.
Heck JE, He D, Contreras ZA, Ritz B, Olsen J, Hansen J. Parental occupational exposure to benzene and the risk of childhood and adolescent acute lymphoblastic leukaemia: a population-based study. Occup Environ Med. 2019;76(8):527–9. https://doi.org/10.1136/oemed-2019-105738.
Leader J, Mínguez-Alarcón L, Williams PL, et al. Paternal and maternal pre-pregnancy and maternal pregnancy urinary phthalate metabolite and BPA concentrations in relation to child behavior. Environ Int. 2024;183: 108337. https://doi.org/10.1016/j.envint.2023.108337.
Messerlian C, Bellinger D, Mínguez-Alarcón L, et al. Paternal and maternal pre-pregnancy urinary phthalate metabolite concentrations and child behavior. Environ Res. 2017;158:720–8. https://doi.org/10.1016/j.envres.2017.07.032.
Leader J, Mínguez-Alarcón L, Williams PL, et al. Associations of parental pre-pregnancy and maternal pregnancy urinary phthalate biomarker and bisphenol-a concentrations with child eating behaviors. Int J Hyg Environ Health. 2024;257: 114334. https://doi.org/10.1016/j.ijheh.2024.114334.
Leader J, Mínguez‐Alarcón L, Williams PL, et al. Paternal and maternal pre-pregnancy and maternal pregnancy urinary concentrations of parabens in relation to child behavior. Andrology. 2023;n/a(n/a):andr.13576. https://doi.org/10.1111/andr.13576
Skarha J, Messerlian C, Bellinger D, et al. Parental pre-pregnancy and prenatal urinary bisphenol A and paraben concentrations and child behavior. Environ Epidemiol. 2020;4(1): e082. https://doi.org/10.1097/EE9.0000000000000082.
Rahman MS, Pang WK, Ryu DY, Park YJ, Pang MG. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum Reprod. 2020;35(8):1740–52. https://doi.org/10.1093/humrep/deaa139.
Omidakhsh N, Hansen J, Ritz B, et al. Parental occupation and risk of childhood retinoblastoma in Denmark. J Occup Environ Med. 2021;63(3):256–61. https://doi.org/10.1097/JOM.0000000000002120.
Rossides M, Kampitsi CE, Talbäck M, et al. Risk of cancer in children of parents occupationally exposed to hydrocarbon solvents and engine exhaust fumes: a register-based nested case–control study from Sweden (1960–2015). Environ Health Perspect. 2022;130(7): 077002. https://doi.org/10.1289/EHP11035.
Onyije FM, Olsson A, Erdmann F, et al. Parental occupational exposure to combustion products, metals, silica and asbestos and risk of childhood leukaemia: findings from the Childhood Cancer and Leukaemia International Consortium (CLIC). Environ Int. 2022;167: 107409. https://doi.org/10.1016/j.envint.2022.107409.
Patel DM, Jones RR, Booth BJ, et al. parental occupational exposure to pesticides, animals, and organic dust and risk of childhood leukemia and central nervous system tumors: findings from the International Childhood Cancer Cohort Consortium (I4C). Int J Cancer. 2020;146(4):943–52. https://doi.org/10.1002/ijc.32388.
Volk J, Heck JE, Schmiegelow K, Hansen J. Risk of selected childhood cancers and parental employment in painting and printing industries: a register-based case-control study in Denmark 1968–2015. Scand J Work Environ Health. 2019;45(5):475–82. https://doi.org/10.5271/sjweh.3811.
Rossides M, Kampitsi C, Talbäck M, Wiebert P, Feychting M, Tettamanti G. Childhood cancer risk in offspring of parents occupationally exposed to dusts: a register-based nested case-control study from Sweden of 5 decades. Cancer. 2022;128(8):1637–48. https://doi.org/10.1002/cncr.34116.
Rossides M, Mogensen H, Kampitsi CE, et al. Parental occupational exposure to metals and risk of cancer in the offspring: a register-based case-control study from Sweden. Eur J Cancer. 2023;191:113243. https://doi.org/10.1016/j.ejca.2023.113243.
Pape K, Svanes C, Sejbæk CS, et al. Parental occupational exposure pre- and post-conception and development of asthma in offspring. Int J Epidemiol. 2021;49(6):1856–69. https://doi.org/10.1093/ije/dyaa085.
Yoshizaki K, Kimura R, Kobayashi H, et al. Paternal age affects offspring via an epigenetic mechanism involving REST/NRSF. EMBO Rep. 2021;22(2): e51524. https://doi.org/10.15252/embr.202051524.
Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014;10(7): e1004458. https://doi.org/10.1371/journal.pgen.1004458.
Cao M, Shao X, Chan P, et al. High-resolution analyses of human sperm dynamic methylome reveal thousands of novel age-related epigenetic alterations. Clin Epigenetics. 2020;12(1):192. https://doi.org/10.1186/s13148-020-00988-1.
Bernhardt L, Dittrich M, Prell A, et al. Age-related methylation changes in the human sperm epigenome. Aging. 2023;15(5):1257–78. https://doi.org/10.18632/aging.204546.
Denomme MM, McCallie BR, Haywood ME, Parks JC, Schoolcraft WB, Katz-Jaffe MG. Paternal aging impacts expression and epigenetic markers as early as the first embryonic tissue lineage differentiation. Hum Genomics. 2024;18(1):32. https://doi.org/10.1186/s40246-024-00599-4.
Denomme MM, Haywood ME, Parks JC, Schoolcraft WB, Katz-Jaffe MG. The inherited methylome landscape is directly altered with paternal aging and associated with offspring neurodevelopmental disorders. Aging Cell. 2020;19(8): e13178. https://doi.org/10.1111/acel.13178.
Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol Psychiatry. 2011;16(12):1203–12. https://doi.org/10.1038/mp.2010.121.
Oldereid NB, Wennerholm UB, Pinborg A, et al. The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(3):320–89. https://doi.org/10.1093/humupd/dmy005.
Urhoj SK, Mortensen LH, Nybo AA. Advanced paternal age and risk of musculoskeletal congenital anomalies in offspring. Birth Defects Res B Dev Reprod Toxicol. 2015;104(6):273–80. https://doi.org/10.1002/bdrb.21167.
Yin S, Zhou Y, Zhao C, et al. Association of paternal age alone and combined with maternal age with perinatal outcomes: a prospective multicenter cohort study in China. J Epidemiol Glob Health. 2024;14(1):120–30. https://doi.org/10.1007/s44197-023-00175-4.
Urhoj SK, Jespersen LN, Nissen M, Mortensen LH, Nybo Andersen AM. Advanced paternal age and mortality of offspring under 5 years of age: a register-based cohort study. Hum Reprod. 2014;29(2):343–50. https://doi.org/10.1093/humrep/det399.
Lan KC, Chiang HJ, Huang TL, et al. Association between paternal age and risk of schizophrenia: a nationwide population-based study. J Assist Reprod Genet. 2021;38(1):85–93. https://doi.org/10.1007/s10815-020-01936-x.
Wang SH, Wu CS, Hsu LY, et al. Paternal age and 13 psychiatric disorders in the offspring: a population-based cohort study of 7 million children in Taiwan. Mol Psychiatry. 2022;27(12):5244–54. https://doi.org/10.1038/s41380-022-01753-x.
Gau CC, Lee HJ, Lu HY, et al. Association of advanced paternal age with lung function at school age. Respir Res. 2022;23(1):259. https://doi.org/10.1186/s12931-022-02178-4.
Mao Y, Zhao Y, Luo S, et al. Advanced paternal age increased metabolic risks in mice offspring. Biochim Biophys Acta Mol Basis Dis. 2022;1868(5): 166355. https://doi.org/10.1016/j.bbadis.2022.166355.
Guo Y, Bai D, Liu W, et al. Altered sperm tsRNAs in aged male contribute to anxiety-like behavior in offspring. Aging Cell. 2021;20(9): e13466. https://doi.org/10.1111/acel.13466.
Rodrigo L. Sperm genetic abnormalities and their contribution to embryo aneuploidy & miscarriage. Best Pract Res Clin Endocrinol Metab. 2020;34(6): 101477. https://doi.org/10.1016/j.beem.2020.101477.
Evenson DP, Djira G, Kasperson K, Christianson J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil Steril. 2020;114(2):311–20. https://doi.org/10.1016/j.fertnstert.2020.03.028.
De Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–32. https://doi.org/10.1136/gutjnl-2021-326789.
Argaw-Denboba A, Schmidt TSB, Di Giacomo M, et al. Paternal microbiome perturbations impact offspring fitness. Nature. 2024;629(8012):652–9. https://doi.org/10.1038/s41586-024-07336-w.
Salas-Huetos A, James ER, Broberg DS, Aston KI, Carrell DT, Jenkins TG. The combined effect of obesity and aging on human sperm DNA methylation signatures: inclusion of BMI in the paternal germ line age prediction model. Sci Rep. 2020;10(1):15409. https://doi.org/10.1038/s41598-020-71979-8.
Author information
Authors and Affiliations
Contributions
Conceptualization, H.C. and J.W.; writing—original draft preparation, X.W. and W.Z.; writing—review and editing, X.W. and J.W. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study did not require ethical approval and was based on publicly available studies.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Zhang, W., Chen, H. et al. Multifaceted paternal exposures before conception and their epigenetic impact on offspring. J Assist Reprod Genet (2024). https://doi.org/10.1007/s10815-024-03243-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-024-03243-1