Abstract
Rationale
Identifying the long-term neurocognitive implications of opioid addiction may further our understanding of the compulsive nature of this brain disorder. The aim of this study was to examine the effects of paternal adolescent opiate exposure on cognitive performance (visual attention, impulsivity, and compulsivity) in the next generation.
Methods
Male Wistar rats received escalating doses of morphine (2.5–25 mg/kg, s.c.) or saline for 10 days during adolescence (P30–39). In adulthood (P70–80), these rats were allowed to mate with drug-naive females. Male offspring from morphine- and saline-exposed sires, once in adulthood, were trained and tested in the 5-choice serial reaction time test (5-CSRTT) to evaluate their cognitive abilities under baseline, drug-free conditions as well as following acute (1, 3, 5 mg/kg morphine) and subchronic morphine (5 mg/kg morphine for 5 days) treatment. Behavioral effects of the opioid receptor antagonist naloxone were also assessed.
Results
Morphine-sired offspring exhibited delayed learning when the shortest stimulus duration (1 s) was introduced, i.e., when cognitive load was highest. These subjects also exhibited a reduced ability to exert inhibitory control, as reflected by increased premature and perseverative responding under drug-free baseline conditions in comparison to saline-sired rats. These impairments could not be reversed by administration of naloxone. Moreover, impulsive behavior was further enhanced in morphine-sired rats following acute and subchronic morphine treatment.
Conclusion
Paternal opiate exposure during adolescence was found to primarily impair inhibitory control in male progeny. These results further our understanding of the long-term costs and risk of opioid abuse, extending across generations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ongoing opioid epidemic is resulting in widespread exposure to opioid drugs. The likelihood of initiating addictive behaviors is higher during adolescence (Gladwin et al. 2011). The combination of an underdeveloped prefrontal cortex, along with more developed limbic structures, has been hypothesized to cause a loss of top-down control that can contribute to the development of addictive behaviors during adolescence (Mills et al. 2014). As brain development continues past adolescence into young adulthood, exposure to high levels of opioid drugs can alter the normal neurodevelopmental trajectory, which in turn could lead to long-lasting neurobehavioral deficits (Azadi et al. 2019, 2020, 2021; Masrouri et al. 2020; 2021; Moazen et al. 2018; Salmanzadeh et al. 2021, 2020). We and others have shown that the effects of opioid exposure even have the potential to transfer to subsequent generations through epigenetic marks within gonadal tissue (Azadi et al. 2019, 2020, 2021; Bird 2007; Pachenari et al. 2018, 2019; Vassoler et al. 2020, 2013). This may increase the risk of psychiatric disorders for offspring of opioid-exposed individuals.
Clinical experience indicates that drug use disorders tend to run in families. It has also been reported in literature that children of addicts are more likely to have other behavioral problems, including conduct disorder, psychiatric disorders, attention-deficit/hyperactivity disorder (ADHD), and major depression (Brent et al. 2019; Edward 2000; Johnson and Leff 1999; Nunes et al. 1998; Parvaresh et al. 2015; Vidal et al. 2012). However, distinguishing between confounding variables in humans is extraordinarily difficult. Moreover, no longitudinal human studies have to our knowledge been published that examined the consequences of preconception drug use on outcomes in children. Genetic and environmental factors can be more readily controlled in rodent models compared to humans. Such models are thus well-suited to study the specific impact of opioid exposure on neuropsychological function in offspring.
Accumulating evidence points towards the development of a complex phenotype in individuals following adolescent maternal opioid exposure (Vassoler et al. 2014; Vassoler and Byrnes 2021). Conversely, much less is known about the inter- and transgenerational neurobehavioral consequences of adolescent paternal opioid exposure. Our laboratory has recently reported that adolescent morphine exposure in male rats causes several consequences in male offspring: (1) a reduction in the rewarding effects of low doses of morphine along with a reduction in spontaneous burst firing of ventral tegmental area dopamine neurons (Azadi et al. 2019); (2) augmentation of somatic and aversive aspects of opiate withdrawal, which was found to be mediated, at least in part, by epigenetic changes in lateral paragigantocellularis-related brain circuitry (Azadi et al. 2020); 3) altered pain perception (Pachenari et al. 2018); (4) increased expression of Ca2+-activated K+ channels within locus coeruleus neurons (Pachenari et al. 2019); and (5) impaired short-term memory performance (Azadi et al. 2021). Here, we wanted to extend these findings by assessing intergenerational effects of morphine exposure on cognitive abilities in the offspring.
Cognitive functioning encompasses several behavioral domains, including attention and executive control. One of the more commonly reported cognitive consequences of opiate exposure is a loss of impulse control (Alaee et al. 2021; Baldacchino et al. 2012; Clark et al. 2006; Kirby et al. 1999; Mintzer et al. 2005; Moazen et al. 2018; Pattij et al. 2009; Schippers et al. 2012; Wiskerke et al. 2011). As a trait, impulsivity—the tendency to act prematurely without sufficient forethought and/or with a bias towards short-term rewards (Dalley and Robbins 2017; Evenden 1999; Pattij and Vanderschuren 2020)—has been linked to increased probability of substance use disorders and increased likelihood to relapse during abstinence (Dalley and Ersche 2019; Pattij and De Vries 2013; Skóra et al. 2020; Sliedrecht et al. 2020; Verdejo-Garcia and Albein-Urios 2020). With adolescence being a critical time period in the development of executive functioning, it is perhaps not surprising that exposure to drugs of abuse particularly during this period of life has been found to have lasting detrimental effects on cognitive functions such as impulse control (Casey and Jones 2010; Hamidullah et al. 2020; Salmanzadeh et al. 2020; Spear 2018). Indeed, previous studies in humans and particularly rodents have revealed that adolescent exposure to alcohol, nicotine, cannabis, psychostimulants, or opiates can impair inhibitory control and/or attentional functioning later in adulthood (Brent et al. 2019; Counotte et al. 2009; Infante et al. 2020; Johnson et al. 2019; Moazen et al. 2018; Parvaresh et al. 2015; Reynolds et al. 2019; Sanchez‐Roige et al. 2014; Vidal et al. 2012). Although still limited, data from some recent rodent studies suggest that cognitive deficits related to drug use may not be restricted to the exposed individuals themselves. Specifically, it has been reported that preconception parental exposure to drugs such as nicotine, Δ9-THC, cocaine, or morphine can induce impairments in attention and memory function in offspring (Brent et al. 2019; Ellis et al. 2020; Holloway et al. 2020; McCarthy et al. 2018; Parvaresh et al. 2015; Renaud and Fountain 2016; Sadat-Shirazi et al. 2020; Vidal et al. 2012; Wimmer et al. 2017). Transgenerational effects due to paternal drug exposure are thought to involve drug-induced epigenetic modifications in gonadal tissue (Goldberg and Gould 2019; Salmanzadeh et al. 2021, 2020; Vassoler and Wimmer 2020; Yohn et al. 2015). Opiate exposure may be particularly harmful due to the critical modulatory role the endogenous opioid system plays not only in the brain but also throughout the hypothalamic-pituitary–gonadal axis as well as locally in the testes (Vassoler et al. 2020; Vassoler and Wimmer 2020). Given that adolescence is thought to be a particularly sensitive time period during which environmental factors can induce epigenetic marks (Mychasiuk and Metz 2016), we hypothesized that exposing males to opioid drugs during adolescence may result in long-lasting cognitive deficits, such as increased impulsivity, in their future offspring.
The 5-choice serial reaction time task (5-CSRTT) is a well-established translational task used for measuring inhibitory control, compulsivity, and attention (Bari et al. 2008; Robbins 2002). In the current study, we used the 5-CSRTT to examine the effects of adolescent opioid exposure in male rats on cognitive performance in their male offspring under drug-free conditions and in response to acute and subchronic morphine challenges. Our results indicated that morphine-sired rats show a lasting deficit in inhibitory control along with manifestations of delayed task learning. Furthermore, morphine-sired rats showed a significantly larger increase in premature responding following a morphine challenge compared to saline-sired controls. Together, these data indicate that paternal opioid exposure can lead to transgenerational changes in the neural circuitry that mediates inhibitory control.
Materials and methods
All experiments were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Ethical Committee of Faculty of Medical Sciences, Tarbiat Modares University (IR.TMU.REC.1395.321). Employed animals in this study were housed in cages measuring 43 × 29 × 16 cm in groups of four under a 12-h light/dark cycle (light off at 7:00 pm) in a thermo-regulated room. Food and water were provided ad libitum, unless stated otherwise. 5-CSRTT was performed during the dark phase. In order to ameliorate stress, animals were habituated to the environment and handled daily for 3–5 min, beginning 5 days prior to the start of the 5-CSRTT training.
Subjects
F0 generation
Male Wistar rats were purchased from Razi Institute, Iran. Animals were group housed and allowed to acclimate to the vivarium for 10 days. At PND30, rats were randomly assigned to the F0 morphine-treated (n = 16) or F0 saline-treated (n = 18) groups and were administered morphine and saline, respectively. Following the last drug injection, rats remained undisturbed until PND70 at which time each of the morphine- and saline-treated male rats were allowed to copulate with a naive female counterpart from our colony.
F1 generation
Once a female was visibly pregnant (approximately E16-E20), it was separated and single housed during the remainder of the gestation and nursing period. Parturition usually occurred 21 days after initial pairing with a male. Litters were examined for obvious morphological anomalies. Litters were weaned on PND21 and housed with same-sex littermates and remained undisturbed until adulthood (PND70). As we reported previously, F0 generation morphine administration in adolescent male rats did not affect litter size, gender composition, or the pups’ body weight at either time point (PND1 and PND 30) (Azadi et al. 2019, 2020).
Behavioral procedures were performed on the F1 male offspring, starting when these rats were 70 days old (bodyweights 300–350 g). Rats were divided into two treatment groups, based on whether their male parent had been exposed to saline (saline-sired group, n = 18) or morphine (morphine-sired group, n = 16). Importantly, only one, randomly chosen, rat was used from each litter. From this moment onwards, rats were kept single housed with ad libitum access to water. Food access was restricted to maintain rats’ weight at 85–90% of free-feeding values. Food was given at the end of each day. During training, nine rats did not reach the final performance criterion. These rats were excluded from the experiment, leaving n = 14 and n = 11 for the saline-sired and morphine-sired group, respectively.
Morphine exposure F0 generation
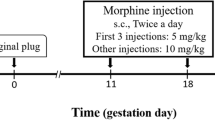
As depicted in Fig. 1, a 10-day morphine dosing regimen (9:00am and 4:00 pm) was employed during postnatal days (PND) 30–39, beginning at 2.5 mg/kg subcutaneously (s.c.) and increasing by 2.5 mg/kg daily until day 10, for a final dose of 25 mg/kg. Age-matched control animals (F0 saline-treated) instead received repeated saline injections. This increasing protocol is used frequently to avoid mortality (Azadi et al. 2019, 2020; Byrnes et al. 2011). In addition, as we aimed to generalize this study to human consumption, we employed this increasing protocol since both opioid prescriptions and drug abuse typically begin with low doses and gradually escalate to higher doses.
Behavioral experiment F1 generation
Apparatus
Operant chambers (44 × 35 × 36 cm) (Panlab, Spain) on one side contained an array of nine nose poke holes, positioned 2 cm above the grid floor. Each hole contained a 2.5-W cue light. In the current study, only five of the nine response holes were used, and the others were masked by a cover. The opposite wall contained a magazine that was connected to a food dispenser. All of the response holes as well as the food magazine were fitted with infrared beams crossing their entrance to detect nose poke responses. All chambers also contained a white house light that can provide ambient lighting.
5-choice serial reaction time task
5-CSRTT experiments were performed largely according to methods applied previously in our lab (Moazen et al. 2020). The first trial was started when rats made a nose poke response in the food magazine. After a fixed 5-s inter-trial interval (ITI), a light stimulus was turned on randomly in one of the five holes. If rats detected the light cue and made a nose poke into the illuminated hole during the stimulus presentation or the following limited hold period, it was deemed a correct response and they received a chocolate pellet as a reward. A nose poke in a non-illuminated hole or failure to respond before the end of the limited hold period was considered as incorrect response and omission, respectively. If rats made nose poke responses in any hole before the onset of the light cue, no pellets were delivered as a reward and a premature response was recorded. Incorrect responses, errors of omission, and premature responses were followed by a 5-s timeout period during which the house light was turned off and no reward was delivered. Additionally, nose poke responses after a correct response, but before reward collection, were recorded as perseverative responses (but had no behavioral consequence). Each training testing session lasted 30 min, and training sessions were conducted once daily for 7 days/week. Acquisition of baseline performance was conducted over several stages. At the first stage of training, light stimulus duration and limited hold were both set at 30 s. Stimulus duration was then gradually decreased to 20, 10, 5, 2.5, 1.25, and 1 s over sessions until animals reached stable baseline performance (accuracy > 80% correct choice and < 20% errors of omission). Limited hold times were decreased in parallel to 20, 10, and 5 s. Once performance at the final training stage (stimulus duration 1 s, limited hold 5 s) was stable for at least six sessions, the data from the last 4 days of training were used to provide a baseline index of performance in the standard 5-CSRTT (fixed ITI: 5 s) (Bari et al. 2008). Several performance measures were recorded (Robbins 2002): (1) accuracy, an attention index, calculated as [number correct trials / (correct + incorrect trials)] × 100; (2) omission errors, i.e., the number of omitted trials during a session, as a measure of motivation and sustained attention; (3) inhibitory control, evaluated by the number of premature responses; (4) compulsivity, counted by the number of perseverative responses; (5) motor activity, as measured by the average latency to respond correctly; and (6) food motivation as measured by the average latency to collect rewards.
Behavioral pharmacology
Rats were tested in a high demand version of the 5-CSRTT with variable ITI (2 and 5 s). ITI durations occurred pseudorandomly so that a rat was exposed to equal numbers of trials at each ITI during a session. As depicted in Fig. 2, drug testing sessions were interleaved with two baseline training sessions (with fixed 5-s ITI) to prevent carry-over effects of the drugs. Adding these baseline sessions also helped assure that the observed drug effects were not due to order effects. Statistical analyses indicated no significant effects on baseline behavior over the test period (data not shown). For clarity, baseline data have therefore not been included in any of the figures.
On consecutive test days, saline 1 mL/kg, naloxone 1 mg/kg, morphine 1 mg/kg, morphine 3 mg/kg, morphine 5 mg/kg, and subchronic morphine (5 days of 5 mg/kg) were administered to each rat. This approach was chosen since we aimed to generalize this study to human consumption, and, normally, both opioid prescriptions and drug abuse are started with low doses and gradually develop to higher doses. Morphine and naloxone (Sigma Aldrich, USA) were dissolved in physiological saline and were administrated subcutaneously (s.c.) in a volume of 1.0 mL/kg of body weight. Drug doses were based on previous studies (Pattij et al. 2009; Wiskerke et al. 2011). All drugs were administered at 9:00am and 5-CSRTT sessions commenced 30 min following injection.
Data analysis
Data are presented as mean ± standard error of the mean (SEM), and statistical analyses were performed using GraphPad Prism Software version 9.0. One-sample Kolmogorov–Smirnov tests were used to ascertain that all data were normally distributed. 5-CSRTT learning rates were analyzed in two ways: using an unpaired Student’s t test to analyze total number of training sessions required to reach the final performance criteria, and a mixed-model ANOVA to analyze the total number of sessions per training stage. For the latter, treatment group was used as between subject factor (levels: saline-sired vs. morphine-sired) and training stage as within subject factor (levels: stages 1–7). To analyze 5-CSRTT baseline performance, we employed unpaired Student’s t tests. In the second part of the study, repeated measures ANOVA with drugs as within subject factor (levels: saline; naloxone; morphine 1, 3, and 5 mg/kg; and subchronic morphine) and treatment group as a between subject factor (levels: saline-sired vs. morphine-sired) were employed to analyze the behavioral pharmacology data. Moreover, baseline training data in between drug tests were analyzed by repeated measures ANOVA with day as within subject factor (levels: b1, b2, and b3) and treatment group as a between subject factor (levels: saline-sired vs. morphine-sired). For all ANOVAs, in case of violation of the sphericity assumption, Greenhouse–Geisser correction was applied. In such cases, we reported the Greenhouse–Geisser epsilon, the adjusted p values, but—for simplicity—the non-adjusted degrees of freedom. Statistically significant main effects were further analyzed using Bonferroni multiple comparison post hoc tests. In all analyses, significance was set at P < 0.05.
Results
The objective of this study was to investigate the transgenerational cognitive consequences of adolescent opiate exposure. To this end, F1 male offspring of saline- and morphine-treated male rats were trained in the 5-CSRTT, after which the effects of opiate receptor antagonism and agonism on executive functioning were measured.
5-CSRTT learning rate
The number of days required for each rat to reach training criteria is shown in Fig. 3. Overall, the morphine-sired group required more days to be trained than the saline-sired group (Fig. 3A; t23 = 2.31, P = 0.02). Subsequently, splitting data over the 7 stages of training (Fig. 3B) revealed a significant interaction effect between training stage and treatment group (stage, [F (6, 138) = 151.7, P < 0.001. ε = 0.73]; group, [F (1, 23) = 5.19, P = 0.03]; stage × group, [F (6, 138) = 2.27, P = 0.04]. Specifically, the data showed that there was no difference between saline-sired and morphine-sired animals during acquisition of the task when the duration of the visual stimuli was 20, 10, 5, 2.5, and 1.25 s. However, at the final stage when a 1-s stimulus duration was used—and the attentional demands were highest—the performance of morphine-sired rats was compromised (Bonferroni’s post hoc test at stage 7, P = 0.002). Together, these data suggest that offspring of morphine-treated male rats show learning deficits in a task requiring inhibitory control and divided attention.
Training performance in the 5-CSRTT in morphine-sired and saline-sired rats. A Total sessions to reach the criteria; unpaired Student’s t test, B number of sessions required to meet criteria at each training stage; mixed-model ANOVA. Data (n = 11 for morphine-sired group and n = 14 for saline-sired group) are presented as mean ± SEM. *P < 0.05, **P < 0.01 morphine-sired vs saline-sired
Baseline 5-CSRTT performance
Next, data from the last 4 days of training (1-s stimulus duration) were used to provide an index of baseline performance in a 5-CSRTT with fixed inter-trial interval (Fig. 4). Here, unpaired Student t test analyses revealed significant differences between saline-sired and morphine-sired groups for both premature (t23 = 3.11, P = 0.004) and perseverative (t23 = 3.18; P = 0.004) responding. In contrast, no significant group differences were observed for any of the other behavioral parameters (accuracy, t23 = 0.27; errors of omission, t23 = 0.36; correct response latency, t23 = 0.35; reward collection latency, t23 = 0.42; all N.S.). Thus, it appears that morphine-sired rats had a lasting deficit in behavioral control as measured in the 5-CSRTT.
Comparison of baseline 5-CSRTT behavior in morphine-sired and saline-sired rats. Data depict the average baseline 5-CSRTT performance across 4 days prior to the onset of pharmacological testing in the morphine-sired (n = 11) and saline-sired (n = 14) groups. Data are expressed as mean ± SEM. Unpaired Student’s t test; **P < 0.01 morphine-sired vs saline-sired
Pharmacological challenges
In the final part of this study, we wanted to see whether the opioid system differentially modulates executive functioning in morphine-sired rats as compared to their saline-sired counterparts. Following the establishment of stable 5-CSRTT performance, we therefore assessed the behavioral changes in response to a series of pharmacological challenges (see Fig. 2): 1 mg/kg naloxone, increasing doses of acute morphine (1–5 mg/kg), and a subchronic regiment of morphine (5 days of 5 mg/kg). Naloxone, an opioid receptor antagonist with preference for the mu opioid receptor, was used to assess the contribution of the endogenous opioid system in executive functioning. Administering the mu opioid receptor agonist morphine on the other hand allowed us to investigate opioid receptor modulation of 5-CSRTT behavior. Subchronic exposure to morphine was also included to be able to detect possible differential adaptation to the cognitive effects of morphine in morphine-sired vs. saline-sired rats (e.g., development of tolerance or sensitization). To make the timing of the task less predictable, hence increase the cognitive load, we introduced a variable inter-trial interval at this stage of the study.
Premature responses
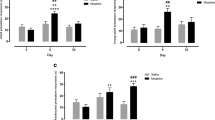
As depicted in Fig. 5A, a two-way ANOVA indicated a significant main effect of group [F (1, 23) = 121.5, P < 0.0001], drug [F (5, 115) = 8.08, P < 0.001, ε = 0.62, and group × drug interaction [F (5, 115) = 2.39, P = 0.04] on premature responding. Post hoc analyses revealed that although naloxone did not affect premature responding, the number of premature responses in morphine-sired rats was significantly increased after acute treatment with morphine at doses of 1 mg/kg (P = 0.01), 3 mg/kg (P = 0.04), and 5 mg/kg (P = 0.03) as well as following subchronic morphine treatment (P = 0.006), compared to saline administration. In contrast, none of the treatments significantly affected the number of premature responses made by saline-sired rats, leading to a significant difference between the morphine-sired and saline-sired groups following acute treatment with morphine 1 mg/kg (P < 0.001), 3 mg/kg (P = 0.009), and 5 mg/kg (P = 0.02) and a strong trend towards a between-group difference after subchronic morphine (P = 0.06).
Assessment of opioid modulation of 5-CSRTT performance in morphine-sired and saline-sired rats. Graphs show the effects of 1 mg/kg naloxone, 1–5 mg/kg acute morphine and 5 days of 5 mg/kg morphine administration on different behavioral parameters measured in the 5-CSRTT for morphine-sired (n = 11) and saline-sired (n = 14) rats. Data are expressed as mean ± SEM. Mixed-model ANOVA; + indicate within-group differences compared to acute saline administration, * indicate between-group differences for morphine-sired vs saline-sired groups, whereas # indicate between-drug differences compared to acute saline administration (i.e., independent of treatment group). For all types of symbols, one, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001, respectively
Perseverative responses
Despite significant main effects of group [F (1, 23) = 19.04, P < 0.001], drug [F (5, 115) = 8.12, P < 0.001, ε = 0.63], and group × drug interaction [F (5, 115) = 2.30, P = 0.05] on perseverative responding in the 5-CSRTT (Fig. 5B), post hoc testing revealed no significant between-group differences for any of the treatments. These follow-up comparisons did reveal that, relative to saline administration, acute administration of 3 mg/kg morphine caused a significant decrease in perseverative responding specifically in the morphine-sired group (P = 0.03), while subchronic treatment with 5 mg/kg morphine reduced the number of perseverative responses made in both groups (P = 0.01 and P = 0.003 for the morphine-sired and saline-sired group, respectively). Again, naloxone did not affect behavior in saline-sired or morphine-sired rats.
Accuracy
Statistical analysis here revealed a significant main effect of drug [F (5, 115) = 4.35, P = 0.006, ε = 0.64] but not group [F (1, 23) = 0.84, P = 0.36] or group × drug interaction [F (5, 115) = 0.31, P = 0.90] on response accuracy, an indicator for attention (Fig. 5C). Post hoc analyses showed that the drug effect was caused by acute administration of 3 and 5 mg/kg of morphine reducing accuracy in both groups (P = 0.02 and 0.003 for, respectively, 3 and 5 mg/kg morphine).
Errors of omission
As illustrated in Fig. 5D, there were no significant main effects of group [F (1, 23) = 1.44, P = 0.24] or group × drug interaction [F (5, 115) = 0.22, P = 0.95] on the number of trials in which rats omitted a response. There was, however, a significant main effect of drug [F (5, 115) = 7.48, P < 0.001, ε = 0.72], due to a similar increase in omissions in both groups following acute (P < 0.001 for 1, 3, and 5 mg/kg) as well as subchronic (P < 0.001) administration of morphine.
Correct response latency
As demonstrated in Fig. 5E, for response latencies during correct trials, there were no significant main effects of group [F (1, 23) = 0.93, P = 0.35] or group × drug interaction [F (5, 115) = 0.38, P = 0.86]. Similar to the results for errors of omission, however, there was significant main effects of drug [F (5, 115) = 3.42, P = 0.02, ε = 0.62], which post hoc comparisons showed was caused by a significant increase in response times specifically following acute administration of 5 mg/kg of morphine (P = 0.02).
Latency to collect rewards
As demonstrated in Fig. 5F, when looking at in the reward latency as a reflection of task motivation, neither naloxone nor morphine (acute or subchronic) administration significantly affected performance in saline-sired or morphine-sired rats [group, F (1, 23) = 0.46, P = 0.50; drug, F (5, 115) = 0.85, P = 0.51, ε = 0.69; group × drug, F (5, 115) = 0.22, P = 0.95].
Overall, the results of the pharmacological challenges indicate that activation of opioid receptors had a differential effect in saline-sired vs. morphine-sired rats only when looking at premature and to a lesser extent perseverative responding, which are thought to reflect respectively impulsive and compulsive actions (Robbins 2002).
Discussion
The world is currently facing an opioid epidemic, resulting in widespread exposure to exogenous opioids. One of the known consequences of opioid abuse, both acute and chronic, is increased impulsivity (Baldacchino et al. 2012; Clark et al. 2006; Kirby et al. 1999; Maguire et al. 2016; Mintzer et al. 2005; Moazen et al. 2018; Pattij et al. 2009; Pattij and Vanderschuren 2020; Schippers et al. 2012; Wiskerke et al. 2011). Recent studies have highlighted that neuropsychological effects of exposure to opioid drugs are not necessarily restricted to the user but can also be passed on to the next generation via epigenetic marks in gonadal tissue (Azadi et al. 2019, 2020; Vassoler et al. 2020, 2013). In this paper, we present the first experimental evidence of a link between paternal morphine exposure and decreased executive functioning in offspring. Specifically, we found that paternal morphine exposure during adolescence affected executive function in the offspring. More specifically, morphine-sired offspring displayed impaired task learning and decreased inhibitory control in the 5-CSRTT. These results extend our knowledge on the long-term costs and risks of opioid abuse.
In the first part of this study, we employed a standard version of the 5-CSRTT with fixed ITI to compare task learning and executive functioning under drug-free conditions between rats paternally exposed to either morphine or saline. Results showed that offspring from morphine-exposed sires displayed significantly delayed learning in the last training phase of the 5-CSRTT, when stimulus duration was reduced to 1 s and the cognitive load was highest. Additionally, these rats showed impaired inhibitory control as manifested by a persistent increase in premature and perseverative responding. In contrast, there were no significant differences between the morphine- and saline-sired rats on other task parameters such as response accuracy, latency to respond, omission errors, and latency to collect food reward. The latter negative findings suggest that processes such as attention, motivation, and motor activity were not grossly affected in offspring from morphine-exposed sires. To our knowledge, these data provide some of the first evidence of persistent transgenerational cognitive effects of exposure to opioids. The results extend recent studies showing that paternal exposure to various drugs of abuse, including morphine, can disrupt novel object recognition memory in progeny (Ellis et al. 2020; McCarthy et al. 2018; Sadat-Shirazi et al. 2020; Schiele et al. 2020; Wimmer et al. 2017). The selectivity of the cognitive deficit observed in the current study to the domain of inhibitory control is interesting as we observed a similarly restricted impairment when we exposed adolescent male rats to repeated morphine injections and tested them in the 5-CSRTT 1 month after the last morphine exposure (Moazen et al. 2018). In the latter study, no lasting impairments were seen in rats that received morphine during adulthood. Together with the current study, this raises the possibility that adolescence might be a uniquely sensitive period to induce persistent cognitive deficits, not only in exposed individuals but also in their future offspring. Opioid medications are routinely given to adolescent and young adult males in clinical settings. Thus, it may be important to investigate whether such treatment can lead to cognitive impairments in their children, even when those children are conceived long after discontinuation of the opioid treatment.
In the second part of our study, we administered morphine, either acutely or subchronically, to the rats immediately prior testing them in a higher demand version of the 5-CSRTT with a variable ITI. The main reason for using a variable ITI was to reduce the predictability of the stimulus presentation. This way we aimed to assure that any observed morphine effects were not related to alterations in time perception (Befort et al. 2011; Mahoney et al. 2013). Within the morphine-sired group, we observed increased impulsivity manifested by more premature responses following acute (1, 3, and 5 mg/kg) as well as subchronic (5 * 5 mg/kg) morphine treatment compared to when these rats were injected with saline. This morphine effect was not seen in the saline-sired rats. The difference in the behavioral response to morphine in the two groups of rats is in line with studies showing that opioid exposure in males may alter sensitivity to opioids in offspring (see for review Goldberg and Gould (2018)). The lack of effect of morphine on premature responding in the saline-sired rats contrasts with two previous 5-CSRTT studies that did report such effects (Pattij et al. 2009; Wiskerke et al. 2011). Several reasons may account for this discrepancy. First, it could be related to the use of a variable ITI in the current study as compared to the fixed ITI that was used in the studies by Pattij and co-workers. Indeed, it was previously found that morphine-induced impulsive behavior could not be observed when rats were prevented from being able to time their response by making the stimulus onset temporally unpredictable (Befort et al. 2011; Mahoney et al. 2013). Secondly, we here used relatively short ITIs (2–5 s) and baseline impulsivity was low. Thus, our task presumably placed a relatively low demand on inhibitory control. This may have prevented the morphine challenges from significantly disrupting the brain circuitry mediating impulse control. Finally, it is conceivable that effects on impulsivity in the saline-sired group were masked by the well-known sedative effects of morphine. Akin to previous studies (Maguire et al. 2016; Mahoney et al. 2013; Pattij et al. 2009), we did observe behavioral effects of morphine that likely reflected sedation: an increase in errors of omission and response latencies combined with a reduction in accuracy and preservative responding. Importantly, these effects were not different between the two groups of rats. In contrast to morphine, acute administration of the opioid receptor antagonist naloxone did not affect impulsivity in morphine- or saline-sired rats, nor did this drug affect any other behavioral parameter. Although it cannot be ruled out that the low levels of impulsive responding in our rats caused a floor effect, a lack of effect of naloxone on trait-like inhibitory control is in line with several previous studies (Mahoney et al. 2013; Pattij et al. 2009; Wiskerke et al. 2011, 2012).
Accumulating evidence points toward a complex phenotype with regard to parental drug exposure prior to conception and their future offspring’s response to drugs of abuse (Vassoler et al. 2014; Yohn et al. 2015). The complexity may, however, provide insight into brain systems vulnerable to the impact of preconception exposure to drugs of abuse. Based on our findings of selective transgenerational impairments in the domain of response inhibition, brain regions modulating behavioral control appear to be among the vulnerable neurocircuitries. Two candidate brain regions are the medial prefrontal cortex and the shell subregion of the nucleus accumbens. In both regions, mu-opioid receptor activation has been shown to promote impulsive actions (Selleck et al. 2015; Wiskerke et al. 2011). These opioid effects appear to, at least in part, involve interactions with the mesolimbic dopamine system (Wiskerke et al 2011, 2012). Accordingly, there is ample evidence showing that exposing adolescent rats to morphine leads to neural plasticity in their future offspring, including significantly increased levels of mu-opioid receptors and altered dopamine signaling inside the nucleus accumbens (Ashabi et al. 2018; Byrnes et al. 2013; Vassoler et al. 2017, 2016; Vathy et al. 2003). In addition to the mesolimbic dopamine system, the locus coeruleus-noradrenaline system is known to be important for the type of inhibitory control measured in the 5-CSRTT (for review, see Dalley and Robbins 2017; Pattij and Vanderschuren 2020). We have previously found that paternal exposure to morphine in adolescence changes the electrophysiological properties of locus coeruleus neurons (Pachenari et al. 2019). Hence, it is possible that paternal morphine exposure may have altered this brain circuitry in the offspring, resulting in impaired inhibitory control. It was beyond the scope of the current study to unravel the molecular mechanisms via which morphine exposure can induce transgenerational behavioral changes. However, it is tempting to speculate that it involves transgenerational epigenetic inheritance. First, sires were last exposed to morphine 1 month before they were allowed to mate, arguing against any direct effect of the drug on the pups and/or their postnatal care (Witte 1995). Secondly, it has been shown that many drugs of abuse can directly bind to germline cells and leave epigenetic marks in these cells in exposed males (He et al. 2006; Jenab and Morris 2000; Le et al. 2017; Vassoler et al. 2020, 2013; Yohn et al. 2015). For example, a recent study showed increased levels of acetylated histone H3 seminiferous tubules in sires exposed to morphine during adolescence (Vassoler et al. 2020). Thirdly, there is a rapidly growing body of literature indicating that repeated exposure to addictive substances such as morphine can induce epigenetic changes that carry over to future generations (Le et al. 2017; Vassoler and Sadri-Vakili 2014; Wimmer et al. 2017; Yohn et al. 2015). Future studies may be able to confirm whether epigenetic changes indeed underlie impaired inhibitory control observed in offspring of morphine-exposed sires. Such studies should perhaps also look at other drugs of abuse to determine whether the current findings with morphine generalize to other substances as well.
In summary, our results indicate that exposing adolescent male rats to opioid drugs promotes the occurrence of an impulsive phenotype in the next generation. This finding may have important implications given that maladaptive levels of impulsive behavior are thought to promote risky behaviors and are commonly associated with a range of brain disorders such as ADHD and drug addiction (Edition 2013). More studies are needed to uncover the biological mechanisms underlying the transgenerational cognitive effects observed here. Multiple other questions remain unanswered as well. For example, does exposing sires to other types of addictive substances cause similar deficits in their male offspring? Are these effects sex-specific or would female offspring be similarly affected? Investigating the transgenerational effects of opiate exposure on other aspects of cognition, such as behavioral flexibility and impulsive decision-making, may also help identify the specific brain circuitries involved. Together, this line of research may further our understanding of the consequences of drug addiction beyond those experienced by the drug user, as well as provide novel insights into the etiology of this debilitating psychiatric disorder.
References
Alaee E, Moazen P, Pattij T, Semnanian S, Azizi H (2021) Prenatal exposure to morphine impairs attention and impulsivity in adult rats. Psychopharmacology. https://doi.org/10.1007/s00213-021-05888-7
Ashabi G, Sadat-Shirazi M-S, Akbarabadi A, Vousooghi N, Kheiri Z, Toolee H, Khalifeh S, Zarrindast M-R (2018) Is the nociception mechanism altered in offspring of morphine-abstinent rats? J Pain 19:529–541
Azadi M, Azizi H, Haghparast A (2019) Paternal exposure to morphine during adolescence induces reward-resistant phenotype to morphine in male offspring. Brain Res Bull 147:124–132
Azadi M, Gompf HS, Azizi H (2020) Paternal exposure to morphine during adolescence potentiates morphine withdrawal in male offspring: involvement of the lateral paragigantocellularis nucleus. J Psychopharmacol 34:1289–1299
Azadi M, Zare M, Pachenari N, Shojaei A, Semnanian S, Azizi H (2021) Sex-specific transgenerational effects of adolescent morphine exposure on short-term memory and anxiety-like behavior: male linage. Neuroscience Letters: 136111.
Baldacchino A, Balfour DJ, Passetti F, Humphris G, Matthews K (2012) Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev 36:2056–2068
Bari A, Dalley JW, Robbins TW (2008) The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3:759–767
Befort K, Mahoney MK, Chow C, Hayton SJ, Kieffer BL, Olmstead MC (2011) Effects of delta opioid receptors activation on a response inhibition task in rats. Psychopharmacology 214:967–976
Bird A (2007) Perceptions of epigenetics. Nature 447:396
Brent DA, Hur K, Gibbons RD (2019) Association between parental medical claims for opioid prescriptions and risk of suicide attempt by their children. JAMA Psychiat 76:941–947
Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM (2011) Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res 218:200–205
Byrnes JJ, Johnson NL, Carini LM, Byrnes EM (2013) Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology 227:263–272
Casey B, Jones RM (2010) Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry 49:1189–1201
Clark L, Robbins TW, Ersche KD, Sahakian BJ (2006) Reflection impulsivity in current and former substance users. Biol Psychiatry 60:515–522
Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T (2009) Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology 34:299–306
Dalley JW, Ersche KD (2019) Neural circuitry and mechanisms of waiting impulsivity: relevance to addiction. Philos Trans R Soc B 374:20180145
Dalley JW, Robbins TW (2017) Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci 18:158–171
Edition F (2013) Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc 21.
Edward (2000) Psychiatric disorders and impairment in the children of opiate addicts: prevalances and distribution by ethnicity. American Journal on Addictions 9:232–241
Ellis AS, Toussaint AB, Knouse MC, Thomas AS, Bongiovanni AR, Mayberry HL, Bhakta S, Peer K, Bangasser DA, Wimmer ME (2020) Paternal morphine self-administration produces object recognition memory deficits in female, but not male offspring. Psychopharmacology: 1–13.
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146:348–361
Gladwin TE, Figner B, Crone EA, Wiers RW (2011) Addiction, adolescence, and the integration of control and motivation. Dev Cogn Neurosci 1:364–376
Goldberg LR, Gould TJ (2018) Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. European Journal of Neuroscience.
Goldberg LR, Gould TJ (2019) Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur J Neurosci 50:2453–2466
Hamidullah S, Thorpe HH, Frie JA, Mccurdy RD, Khokhar JY (2020) Adolescent Substance Use and the Brain: Behavioral, Cognitive and Neuroimaging Correlates. Front Hum Neurosci 14:298
He F, Lidow IA, Lidow MS (2006) Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol 28:198–209
Holloway ZR, Hawkey AB, Pippin E, White H, Wells C, Kenou B, Rezvani AH, Murphy SK, Levin ED (2020) Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology 78:57–63
Infante MA, Nguyen-Louie TT, Worley M, Courtney KE, Coronado C, Jacobus J (2020) Neuropsychological trajectories associated with adolescent alcohol and cannabis use: a prospective 14-year study. Journal of the International Neuropsychological Society: JINS 26:480
Jenab S, Morris PL (2000) Interleukin-6 regulation of kappa opioid receptor gene expression in primary sertoli cells. Endocrine 13:11–15
Johnson JL, Leff M (1999) Children of substance abusers: Overview of research findings. Pediatrics 103:1085–1099
Johnson KR, Boomhower SR, Newland MC (2019) Behavioral effects of chronic WIN 55,212–2 administration during adolescence and adulthood in mice. Exp Clin Psychopharmacol 27:348
Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 128:78–87
Le Q, Yan B, Yu X, Li Y, Song H, Zhu H, Hou W, Ma D, Wu F, Zhou Y (2017) Drug-seeking motivation level in male rats determines offspring susceptibility or resistance to cocaine-seeking behaviour. Nat Commun 8:1–13
Maguire D, Henson C, France C (2016) Daily morphine administration increases impulsivity in rats responding under a 5-choice serial reaction time task. Br J Pharmacol 173:1350–1362
Mahoney MK, Silveira MM, Olmstead MC (2013) Increased impulsive action in rats: effects of morphine in a short and long fixed-delay response inhibition task. Psychopharmacology 230:569–577
Masrouri H, Azadi M, Semnanian S, Azizi H (2020) Maternal deprivation induces persistent adaptations in putative dopamine neurons in rat ventral tegmental area: in vivo electrophysiological study. Exp Brain Res.
Masrouri H, Azadi M, Semnanian S, Azizi H (2021) Early life maternal deprivation attenuates morphine induced inhibition in lateral paragigantocellularis neurons in adult rats. Brain Res Bull 169:128–135
McCarthy DM, Morgan Jr TJ, Lowe SE, Williamson MJ, Spencer TJ, Biederman J, Bhide PG (2018) Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS biology 16: e2006497.
Mills KL, Goddings A-L, Clasen LS, Giedd JN, Blakemore S-J (2014) The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci 36:147–160
Mintzer MZ, Copersino ML, Stitzer ML (2005) Opioid abuse and cognitive performance. Drug Alcohol Depend 78:225–230
Moazen P, Azizi H, Salmanzadeh H, Semnanian S (2018) Adolescent morphine exposure induces immediate and long-term increases in impulsive behavior. Psychopharmacology 235:3423–3434
Moazen P, Torabi M, Azizi H, Fathollahi Y, Mirnajafi-Zadeh J, Semnanian S (2020) The locus coeruleus noradrenergic system gates deficits in visual attention induced by chronic pain. Behavioural Brain Research: 112600.
Mychasiuk R, Metz GA (2016) Epigenetic and gene expression changes in the adolescent brain: What have we learned from animal models? Neurosci Biobehav Rev 70:189–197
Nunes EV, Weissman MM, Goldstein RB, McAVAY G, Seracini AM, Verdeli H, Wickramaratne PJ (1998) Psychopathology in children of parents with opiate dependence and/or major depression. J Am Acad Child Adolesc Psychiatry 37:1142–1151
Pachenari N, Azizi H, Ghasemi E, Azadi M, Semnanian S (2018) Exposure to opiates in male adolescent rats alters pain perception in the male offspring. Behav Pharmacol 29:255–260
Pachenari N, Azizi H, Semnaniann S (2019) Adolescent morphine exposure in male rats alters the electrophysiological properties of locus coeruleus neurons of the male offspring. Neuroscience 410:108–117
Parvaresh N, Mazhari S, Nazari-Noghabi M (2015) Frequency of psychiatric disorders in children of opioid or methamphetamine-dependent patients. Addict Health 7:140
Pattij T, De Vries TJ (2013) The role of impulsivity in relapse vulnerability. Curr Opin Neurobiol 23:700–705
Pattij T, Schetters D, Janssen MC, Wiskerke J, Schoffelmeer AN (2009) Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology 205:489–502
Pattij T, Vanderschuren LJ (2020) The neuropharmacology of impulsive behaviour, an update. Recent Advances in Research on Impulsivity and Impulsive Behaviors: 3–22.
Renaud SM, Fountain SB (2016) Transgenerational effects of adolescent nicotine exposure in rats: Evidence for cognitive deficits in adult female offspring. Neurotoxicol Teratol 56:47–54
Reynolds LM, Yetnikoff L, Pokinko M, Wodzinski M, Epelbaum JG, Lambert LC, Cossette M-P, Arvanitogiannis A, Flores C (2019) Early adolescence is a critical period for the maturation of inhibitory behavior. Cereb Cortex 29:3676–3686
Robbins T (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163:362–380
Sadat-Shirazi M-S, Asgari P, Mahboubi S, Zadeh-Tehrani SN, Ashabi G, Rohbani K, Sabzevari S, Soltani H, Khalifeh S, Zarrindast M-R (2020) Effect of morphine exposure on novel object memory of the offspring: The role of histone H3 and ΔFosB. Brain Res Bull 156:141–149
Salmanzadeh H, Ahmadi-Soleimani M, Azadi M, Halliwell RF, Azizi H (2021) Adolescent Substance abuse, transgenerational consequences and epigenetics. Current neuropharmacology.
Salmanzadeh H, Ahmadi-Soleimani SM, Pachenari N, Azadi M, Halliwell RF, Rubino T, Azizi H (2020) Adolescent drug exposure: a review of evidence for the development of persistent changes in brain function. Brain Res Bull 156:105–117
Sanchez‐Roige S, Peña‐Oliver Y, Ripley TL, Stephens DN (2014) Repeated ethanol exposure during early and late adolescence: double dissociation of effects on waiting and choice impulsivity. Alcoholism: Clinical and Experimental Research 38: 2579–2589.
Schiele MA, Bandelow B, Baldwin DS, Pini S, Domschke K (2020) A neurobiological framework of separation anxiety and related phenotypes. Eur Neuropsychopharmacol 33:45–57
Schippers MC, Binnekade R, Schoffelmeer AN, Pattij T, De Vries TJ (2012) Unidirectional relationship between heroin self-administration and impulsive decision-making in rats. Psychopharmacology 219:443–452
Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, Baldo BA (2015) Endogenous opioid signaling in the medial prefrontal cortex is required for the expression of hunger-induced impulsive action. Neuropsychopharmacology 40:2464–2474
Skóra MN, Pattij T, Beroun A, Kogias G, Mielenz D, de Vries T, Radwanska K, Müller CP (2020) Personality driven alcohol and drug abuse: New mechanisms revealed. Neuroscience & Biobehavioral Reviews.
Sliedrecht W, Roozen HG, Witkiewitz K, de Waart R, Dom G (2020) The association between impulsivity and relapse in patients with alcohol use disorder: a literature review. Alcohol and Alcoholism.
Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19:197
Vassoler F, Byrnes E, Pierce R (2014) The impact of exposure to addictive drugs on future generations: physiological and behavioral effects. Neuropharmacology 76:269–275
Vassoler FM, Byrnes EM (2021) Transgenerational effects on anxiety-like behavior following adolescent morphine exposure in female rats. Behavioural Brain Research 406: 113239.
Vassoler FM, Oliver DJ, Wyse C, Blau A, Shtutman M, Turner JR, Byrnes EM (2017) Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacology 113:271–280
Vassoler FM, Sadri-Vakili G (2014) Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience 264:198–206
Vassoler FM, Toorie AM, Teceno DN, Walia P, Moore DJ, Patton TD, Byrnes EM (2020) Paternal morphine exposure induces bidirectional effects on cocaine versus opioid self-administration. Neuropharmacology 162: 107852.
Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC (2013) Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16:42–47
Vassoler FM, Wimmer ME (2020) Consequences of parental opioid exposure on neurophysiology, behavior, and health in the next generations. Cold Spring Harbor Perspectives in Medicine: a040436.
Vassoler FM, Wright SJ, Byrnes EM (2016) Exposure to opiates in female adolescents alters mu opiate receptor expression and increases the rewarding effects of morphine in future offspring. Neuropharmacology 103:112–121
Vathy I, Šlamberová R, Rimanóczy Á, Riley MA, Bar N (2003) Autoradiographic evidence that prenatal morphine exposure sex-dependently alters μ-opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Prog Neuropsychopharmacol Biol Psychiatry 27:381–393
Verdejo-Garcia A, Albein-Urios N (2020) Special issue on vulnerabilities to substance abuse impulsivity traits and neurocognitive mechanisms conferring vulnerability to substance use disorders. Neuropharmacology: 108402.
Vidal SI, Vandeleur C, Rothen S, Gholam-Rezaee M, Castelao E, Halfon O, Aubry J-M, Ferrero F, Preisig M (2012) Risk of mental disorders in children of parents with alcohol or heroin dependence: a controlled high-risk study. Eur Addict Res 18:253–264
Wimmer M, Briand L, Fant B, Guercio L, Arreola A, Schmidt H, Sidoli S, Han Y, Garcia B, Pierce R (2017) Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol Psychiatry 22:1641–1650
Wiskerke J, Schetters D, van Es IE, van Mourik Y, den Hollander BR, Schoffelmeer AN, Pattij T (2011) mu-Opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. J Neurosci 31:262–272
Wiskerke J, Van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T (2012) On the role of cannabinoid CB1-and μ-opioid receptors in motor impulsivity. Front Pharmacol 3:108
Witte K (1995) The differential-allocation hypothesis: does the evidence support it? Evolution 49:1289–1290
Yohn NL, Bartolomei MS, Blendy JA (2015) Multigenerational and transgenerational inheritance of drug exposure: the effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol 118:21–33
Acknowledgements
We are immensely grateful to Professor Trevor Robbins (Department of Psychiatry, University of Cambridge) for helpful comments on the manuscript. In addition, we would like to thank the financial support of the Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no potential conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azadi, M., Moazen, P., Wiskerke, J. et al. Preconception paternal morphine exposure leads to an impulsive phenotype in male rat progeny. Psychopharmacology 238, 3435–3446 (2021). https://doi.org/10.1007/s00213-021-05962-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05962-0