Abstract
Purpose
To assess the developmental competence of oocytes matured following rescue in vitro maturation (IVM).
Methods
PubMed, EmBASE, and SCOPUS were systematically searched for peer-reviewed original papers using relevant keywords and Medical Subject Heading terms. Study quality was assessed using the Newcastle–Ottawa Scale. Odds ratios with a 95% confidence interval were calculated by applying a random effects model. The primary outcomes were fertilization and blastulation rates. Secondary outcomes included abnormal fertilization, cleavage, euploidy, clinical pregnancy, and live-birth rates.
Result
Twenty-four studies were included in the meta-analysis. The oocytes matured following rescue IVM showed significantly reduced fertilization, cleavage, blastulation, and clinical pregnancy rates compared to sibling in vivo–matured oocytes. No significant differences were found for the euploidy and live-birth rates in euploid blastocyst transfer. In poor responders, a reduced fertilization rate was observed using in vitro–matured GV but not with in vitro–matured MI. A reduced cleavage rate in MI matured overnight compared to < 6 incubation hours was found.
Conclusion
Our results showed compromised developmental competence in oocytes matured following rescue IVM. However, in poor responders, rescue IVM could maximize the efficiency of the treatment. Notably, our data suggests using in vitro MI matured within 6 incubation hours.
Clinical trial registration number: CRD42023467232.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, there has been a remarkable and swift evolution in the field of in vitro fertilization (IVF). However, one persisting and yet to be solved challenge in IVF treatments lies in the retrieval of immature oocytes. Following controlled ovarian hyperstimulation (COH), it is observed that about 15–25% of the retrieved oocytes are immature [1, 2]. Immature oocytes can be categorized into different stages of nuclear maturation: prophase I (PI), metaphase I (MI), and telophase I (TI). PI oocytes have an intracytoplasmic nucleus, commonly known as the “germinal vesicle” (GV). These oocytes have not yet resumed meiosis and are in the earliest maturation stage. MI oocytes have progressed further in meiosis, characterized by the alignment of chromosomes on the metaphase plate, and are closer to achieving full maturation. TI oocytes represent a transitional phase where the oocyte completes the first meiotic division and proceeds to metaphase II (MII), essential for successful fertilization [3]. Reaching the MII stage in oocyte development depends on the intricate interplay of two steps: nuclear and cytoplasmic maturation [1, 3, 4]. Nuclear maturation refers to the progression of the oocyte through these meiotic stages, culminating in the extrusion of the first polar body and arrest at MII. Cytoplasmic maturation, on the other hand, involves the development of the oocyte’s cytoplasm, which includes the accumulation and redistribution of organelles, RNAs, and proteins [3]. These processes are essential prerequisites for successful fertilization and embryo competence. Rescue in vitro maturation (IVM) is a technique in which immature oocytes, collected from IVF cycles with human chorionic gonadotropin (hCG) triggering, are allowed to mature within 2 to 24 h [5]. Once they reach the MII stage, intracytoplasmic sperm injection (ICSI) is performed. Several studies have explored the potential of the rescue IVM technique, reaching divergent points of view [6,7,8,9,10]. In vitro maturation process, with extended incubation periods, has been associated with potential adverse effects on fertilization, blastulation rates, and clinical outcomes [11, 12]. Hence, incorporating this approach into daily practice raises some doubts. Nevertheless, live births have been reported using embryos derived from oocytes matured following rescue IVM [13,14,15,16]. Moreover, data on embryo ploidy obtained from immature oocytes at retrieval are controversial [8, 13, 17,18,19,20]. On the other hand, as some studies emphasize [6, 9, 17], this might be useful for poor ovarian responders (PORs), in order to increase the number of available oocytes/embryos [6, 9, 17]. However, many IVF centers may not use immature oocytes even in this type of patient, assuming that the clinical benefit is negligible.
In order to provide a comprehensive overview of the existing data, we conducted a systematic review and meta-analysis of published studies focused on the developmental competence of oocytes matured following rescue IVM.
Materials and methods
Study design and protocol registration
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [21]. The protocol was recorded a priori on the international prospective register of systematic reviews PROSPERO (Registration Number: CRD42023467232). The search strategy and outcomes of interest were established before the literature search.
Search strategies and outcomes
PubMed, EmBASE, and SCOPUS were systematically searched for peer-reviewed original papers using relevant keywords and Medical Subject Heading (MeSH) terms. Keywords and MeSH terms were employed in various and overlapping combinations to strictly identify publications relevant to rescue IVM with or without hormones: (“rescue IVM” OR “immature oocytes”) AND (“fertilization” OR “embryo development” OR “euploidy” OR “blastulation” OR “IVF outcome” OR “implantation” OR “pregnancy” OR “live birth”). The primary outcomes of interest were fertilization and blastulation rates. Secondary outcomes included cleavage, euploidy, clinical pregnancy, live birth, and miscarriage rates.
Eligibility criteria and study selection
In this meta-analysis, we included observational prospective or retrospective studies focusing on at least one of the outcomes of interests related to the rescue IVM. Full-length articles were considered eligible if written in English, excluding studies involving animal models. No time restrictions were applied. No attempt was made to identify unpublished studies. Data extraction was performed in 24 papers. A summary of the extraction results is shown in Table 1.
Data extraction
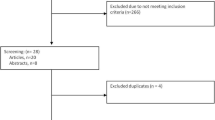
Two reviewers (A.B. and A.Bu.) assessed independently all studies for inclusion or exclusion. Disagreements were solved in discussion with two other investigators (S.d.G. and L.P.). During the first screening, titles and abstracts were investigated and studies with lack of any relevance were excluded (Fig. 1). The remaining articles were retrieved full-length and assessed according to the eligibility criteria. The following information on included studies was collected: first author’s last name, year of publication, study design, number of patients, number of analyzed oocytes (mature and immature oocytes), and outcomes investigated. If the full-text papers lacked adequate information, we reached out to the authors via email to request supplementary data.
Quality assessment
The risk of bias and quality assessment of the included studies were conducted using the Newcastle–Ottawa Scale (NOS) [22]. Three authors (A.B., S.d.G., and L.P.) independently assessed the risk of bias for each included study. The most experienced author (A.Bu.) resolved conflicts. The NOS is a validated scale of 8 items in three domains: selection, comparability, and outcome or exposure depending on the study type (cohort or case–control series). Studies are graded one point each for all items except comparability which has the potential to score up to two points, with the maximum possible score being nine. We categorized studies as high quality (scoring 9 points), medium quality (scoring 8–7 points), or low quality (scoring ≤ 6 points) as determined by this scale.
Data analysis
Statistical analysis was conducted utilizing the RevMan software (Version 5.4) developed by The Nordic Cochrane Centre as part of the Cochrane Collaboration. Data from mature oocytes versus oocytes matured following rescue IVM were pooled to derive a combined odds ratio, adopting a more conservative approach with the random effects model. To address between-study heterogeneity, we employed I2, representing the percentage of total variation in the estimated effect across studies. An I2 value exceeding 50% indicated substantial heterogeneity. Statistical significance was considered for p-value less than 0.05. We chose the odd ratio (OR) as the metric, using the Mantel–Haenszel approach.
For laboratory outcomes, our measures included:
-
1.
Fertilization rate, obtained by dividing the number of fertilized oocytes by all injected oocytes.
-
2.
Abnormal fertilization rate, deduced as the fraction of the number of abnormal fertilized oocytes (1PN and or 3PN) against the total number of injected oocytes.
-
3.
Cleavage rate, obtained by dividing the number of cleaved zygotes (in day 2 with 2–4 blastomeres) by all fertilized oocytes.
-
4.
Blastulation rate, reflecting the percentage of blastocyst obtained (day 5 to day 7) from all fertilized oocytes.
-
5.
Euploidy rate, pinpointed by dividing the number of euploid blastocyst against all biopsied blastocyst.
For clinical outcomes, were included:
-
1.
Clinical pregnancy rate, deduced as the fraction of gestational sacs against the number of embryo transfers.
-
2.
Live birth rate, obtained as the fraction of live birth achieved against the entirety of embryo transfers.
-
3.
Miscarriage rate, ascertained by dividing miscarriage occurrences against the full count of clinical pregnancies.
Subgroup analysis
Sub-analyses were performed for (i) PORs, (ii) in vitro–matured oocytes < 6 incubation hours Vs in vivo–matured sibling oocytes, (iii) in vitro–matured oocytes after overnight incubation Vs in vivo–matured sibling oocytes, (iv) MI matured < 6 h Vs MI matured overnight, (v) in vitro–matured MI Vs in vitro–matured GV, and (vi) euploid blastocyst transfer (clinical outcomes).
Results
Study selection and characteristics
The literature search initially yielded n = 3630 records, excluding duplicates. Following a thorough screening of titles and abstracts, n = 3565 records were excluded and n = 65 manuscripts were considered potentially eligible. After carefully reviewing the full content of these manuscripts, we excluded n = 41 of them. Among these exclusions, n = 34 were off-topic, n = 4 were case reports, n = 4 were non-extractable data, and n = 1 was a review article. Overall, we included n = 24 studies in our meta-analysis. The characteristics and the risk of bias within studies are reported in Table 1. The overall oocyte number included in this meta-analysis was 74,136 (59,144 MII, 11,326 MI, and 3666 GV). Ten out of 24 included studies allowed us to calculate the maturation rate (MR) of immature oocytes (MI and GV) at different timings (< 6 h and overnight incubation). The overall MR of oocytes matured following rescue IVM was 38.8% (2706/6971) and 58.2% (609/1047) for MI and GV, respectively. All GVs matured only after an overnight incubation. The MR for MI mature within 6 incubation hours and after overnight incubation was 36.0% (2359/6545) and 81.5% (347/426), respectively. The sub-analyses in the (i) PORs, (ii) in vitro–matured oocytes < 6 incubation hours Vs in vivo–matured sibling oocytes included 10 studies, and (iii) in vitro–matured oocytes after overnight incubation Vs in vivo–matured sibling oocytes, (iv) MI matured < 6 h Vs MI matured overnight, (v) in vitro–matured MI Vs in vitro–matured GV, and (vi) euploid blastocyst transfer included 2, 10, 9, 2, 5, and 2 studies, respectively. Two studies assessed rescue IVM in PORs; one study considered PORs when fewer than 5 mature oocytes and at least one immature oocyte were retrieved, while the second study considered PORs when fewer than 3 mature oocytes were retrieved. All studies followed the protocol for IVM rescue described below: after removing the cumulus cells, the nuclear maturation status of the denuded oocytes was assessed and graded as GV, MI, and MII stage. The MII oocytes were injected into ICSI, and immature oocytes (GV and MI) were separately cultured in incubators at 37 °C with 5% O2 and 6% CO2, without the addition of hormones. The maturation status of the oocytes was assessed at 2 h, 4 h, 6 h, and after overnight incubation.
Quality assessment
All studies had an NOS assessment performed where a maximum score of 9 could be achieved. Three studies had a top score of 9 so considered high quality [8, 10, 17]; eight studies obtained a medium score [7, 8] so considered medium quality [1, 7, 12, 13, 23,24,25,26]; the remaining studies obtained a low quality score (≤ 6) so considered of low quality [3, 11, 18, 19, 27,28,29,30,31,32,33,34,35]. The quality assessment of included studies showed a low or moderate risk of bias. In fact, among the nine applicable stars assessing the three main categories of selection, comparability, and outcomes, the vast majority of included studies received between 6 and 9 stars (Table 1).
Meta-analyses: biological outcomes
Fertilization rate
Overall assessment
The fertilization rate (FR) was assessed in 23 studies. The oocytes that matured following rescue IVM showed a significantly reduced fertilization rate [MI to MII OR = 0.49 95% CI (0.41–0.59), p < 0.00001; GV to MII OR = 0.53 95% CI (0.38–0.74), p = 0.0002] compared to in vivo–matured sibling oocytes (Fig. 2). There was significant statistical heterogeneity among the studies (MI to MII I2 = 93%, and GV to MII I2 = 88%).
Subgroup analysis
In PORs, the use of in vitro–matured GV resulted in a significantly reduced fertilization rate [GV to MII OR = 0.18, 95% CI (0.10–0.29), p < 0.00001, I2 = not applicable]. However, there was no significant difference in fertilization rate with the use of in vitro–matured MI [MI to MII OR = 0.92, 95% CI (0.33–2.63), p = 0.89, I2 = 67%]. Oocytes matured in vitro within 6 h and overnight showed lower fertilization rates compared to in vivo–matured sibling oocytes (Table 2). There was no difference in fertilization rate between in vitro–matured GV and in vitro–matured MI, as well as between MI matured overnight and MI matured within 6 h (Table 2).
Abnormal fertilization
The abnormal fertilization rate (AFR) was assessed in 9 studies. The results showed a significantly increased AFR in in vitro–matured MI [MI to MII OR = 1.35 95% CI (1.07–1.69), p = 0.01] but not in in vitro–matured GV [GV to MII OR = 0.82 95% CI (0.30–2.22), p = 0.70] compared to in vivo sibling oocytes (Fig. 2). There was significant statistical heterogeneity among the studies related to in vitro–matured GV (GV to MII I2 = 65%) but not in in vitro–matured MI (MI to MII I2 = 46%).
Subgroup analysis
A reduced AFR was observed in in vitro–matured oocytes overnight [MI to MII OR = 0.53, 95% CI (0.42–0.67), p < 0.00001, I2 = 96%], but not in in vitro–matured oocytes within 6 h of incubation, when compared to in vivo sibling oocytes (Table 2).
Cleavage rate
Overall assessment
The cleavage rate (CR) was assessed in 16 studies. The oocytes that matured following rescue IVM showed a significantly reduced cleavage rate [MI to MII OR = 0.38 95% CI (0.23–0.63), p = 0.0002; GV to MII OR = 0.29 95% CI (0.18–0.49), p < 0.00001] compared to in vivo sibling oocytes (Fig. 3). There was significant statistical heterogeneity among the studies (MI to MII I2 = 96%, and GV to MII I2 = 80%).
Subgroup analysis
In PORs, only one study provided data on CR [MI to MII OR = 2.17, 95% CI (0.33–14.31), p = 0.42, I2 = not applicable]. A reduced CR was found in in vitro–matured oocytes overnight compared to in vivo–matured oocytes (Table 2), but not in in vitro–matured oocytes within 6 incubation hours compared to in vivo–matured oocytes (Table 2). A reduced CR was also observed in MI matured overnight compared to MI matured within 6 h (Table 2). No difference in CR between in vitro–matured GV and MI was found (Table 2).
Blastulation rate
Overall assessment
The blastulation rate (BR) was evaluated in 5 studies. The oocytes matured following rescue IVM showed a significantly reduced blastulation rate [MI to MII OR = 0.27 95% CI (0.21–0.34), p < 0.0001; GV to MII OR = 0.23 95% CI (0.12–0.48), p = 0.0001] compared to in vivo sibling oocytes (Fig. 3). There was significant statistical heterogeneity among the studies related to in vitro–matured GV (I2 = 67%) but not in in vitro–matured MI (I2 = 29%).
Subgroup analysis
A reduced BR was observed in in vitro–matured oocytes within 6 h and overnight compared to in vivo–matured sibling oocytes (Table 2) and in vitro–matured GV compared to in vitro–matured MI (Table 2).
Euploidy rate
Overall assessment
The proportion of euploid embryos (ER) was evaluated in 2 studies. There was no difference in euploidy rate [MI to MII OR = 0.91 95% CI (0.30–2.73), p = 0 0.35] (Fig. 4) between in vitro–matured oocytes and in vivo–matured sibling oocytes. There was significant statistical heterogeneity among the studies (I2 = 87%).
Meta-analyses (not sibling oocytes): clinical outcomes
Clinical pregnancy
Overall assessment
The clinical pregnancy (CP) rate was evaluated in 3 studies. All 3 studies performed day-3 embryo transfer. The oocytes matured following rescue IVM showed a significantly reduced CP [MI to MII OR = 0.40 95% CI (0.18–0.90), p = 0.03] compared to in vivo–matured oocytes (Fig. 4). There was no significant statistical heterogeneity among the studies (I2 = 0%).
Subgroup analysis
No difference in CP in euploid blastocyst transfer was observed between in vitro–matured oocytes and in vivo–matured oocytes [MI to MII OR = 1.44 95% CI (0.63–3.28), p = 0 0.39, I2 = 0%] (Table 2).
Miscarriage
Overall assessment
Only one study, with a small sample size, reported data on miscarriage rate (MR) in in vitro–matured GV [GV to MII OR = 7.08 95% CI (0.63–79.05), p = 0.11, I2 = not applicable] compared to in vivo–matured oocytes (Fig. 4).
Subgroup analysis
No difference in MR in euploid blastocyst transfer was observed [MI to MII OR = 2.80 95% CI (0.20–38.51), p = 0.44, I2 = 69%] between in vitro–matured oocytes and in vivo–matured oocytes (Table 2).
Live birth
Overall assessment
No studies reported data on live birth (LB) in the overall assessment.
Subgroup analysis
No difference in LB in euploid blastocyst transfer was observed [MI to MII OR = 0.95 95% CI (0.28–3.25), p = 0.94, I2 = 46%] between in vitro–matured oocytes and in vivo–matured oocytes (Table 2).
Discussion
Principal findings
This study represents the first review and meta-analysis assessing the developmental competence of oocytes matured following rescue IVM. The integration of rescue IVM into daily practice raises some concerns due to the frequent presence of meiotic abnormalities [36]. Additionally, considering the time and cost-effectiveness, it would be optimistic to consider this approach as a routine practice for all patients. Nevertheless, developmentally competent and genetically normal embryos [17] and live births have been reported using embryos derived from oocytes matured following rescue IVM [13,14,15,16]. This constitutes a pivotal aspect with significant ethical implications, particularly due to numerous IVF laboratories not using immature oocytes. The latest good practice recommendation on add-ons in reproductive medicine [37] suggested that rescue IVM is currently not recommended for clinical practice, pinpointing a lack of established effectiveness, and long-term safety data. Accordingly, the overall assessment of this meta-analysis indicates that the use of oocytes matured following rescue IVM is associated with a significant reduction of developmental competence in terms of fertilization, abnormal fertilization, cleavage, blastulation, and clinical pregnancy rates when compared to sibling in vivo–matured oocytes. On the other hand, no statistically significant difference in the euploidy rate was found. A reduced clinical pregnancy rate for in vitro–matured oocytes when compared to in vivo sibling oocytes was observed. It should be noted these transfers are derived from embryos untested with preimplantation genetic testing. When assessing euploid blastocyst transfer (sub-analysis), no significant differences in clinical pregnancy and live birth were found. Collectively, our data suggest that in vitro–matured oocytes produced lower blastocyst but with the same developmental competence. Assessing the results of the blastulation rate in the overall analysis reveals that the odds ratios related to in vitro–matured MI are higher than those of in vitro–matured GV. These findings are further supported by a sub-analysis comparing in vitro–matured MI and GV derived from the same patients. Specifically, a reduced blastulation rate was observed in in vitro–matured GV compared to in vitro–matured MI [OR = 0.67 95% CI (0.45–0.99), p = 0.005, I2 = 0%].
In the sub-analysis of PORs, a significantly reduced fertilization rate with the use of in vitro–matured GV but not with the use of in vitro–matured MI was observed. We could speculate that the use of in vitro–matured MI could increase the number of oocytes/embryos available in PORs, improving the overall efficiency of treatments. This sub-analysis confirmed the previous hypothesis that the developmental competence of in vitro–matured GV might be more severely compromised compared to in vitro–matured MI. Only one study reported data on cleavage rate in PORs showing no difference between in vitro–matured oocytes and in vivo sibling oocytes [MI to MII OR = 2.17 95% CI (0.33–14.31), p = 0.42, I2 = not applicable]. We might speculate that in PORs the poor intrinsic oocyte quality may balance the developmental competence between those matured in vivo and in vitro. However, it is important to consider that these findings are based on a small sample size in terms of absolute numbers and included studies.
In the sub-analysis of different maturation timing, a compromised developmental competence in terms of fertilization, cleavage, and blastulation rates was found in both MI matured within 6 incubation hours and in in vitro–matured oocytes after overnight incubation compared to in vivo–matured sibling oocytes. Upon closer examination of the results, it becomes evident that oocytes matured within 6 incubation hours exhibit higher odds ratios compared to those matured overnight. This observation is further substantiated by our sub-analysis that showed a reduced cleavage rate in MI matured overnight [OR = 0.41 95% CI (0.29–0.56), p < 0.00001, I2 = 0%] compared to MI matured within 6 incubation hours. Notably, our data suggest that in vitro MI matured within 6 h exhibit superior developmental competence compared to those matured after overnight incubation. Consistent with our results, a recent study demonstrated that ICSI of in vitro–matured oocytes within 4–6 h may enhance the production of usable embryos [23]. Additionally, another study by Strassburger and colleagues reported severely compromised results in terms of embryo development and chromosomal constitution for oocytes matured after 24 h of in vitro culture [38]. These findings collectively suggest that oocytes matured overnight should not be considered for insemination. Finally, only one study reported data on perinatal outcomes of embryos derived from in vitro–matured oocytes showing no difference in gestational age, birth weight, delivery mode, and gender [17]. In the longer term, large studies based on national birth registries are needed to clarify possible adverse effects for the newborn achieved from rescue IVM.
Strengths and limitations
To the best of our knowledge, the present meta-analysis is the first to give a comprehensive view on the developmental competence of oocytes matured following rescue IVM. The methodology applied is another strength of this meta-analysis. Indeed, the protocol was registered a priori in the International Prospective Register of Systematic Reviews (PROSPERO) and published [21]. The systematic review was conducted according to international PRISMA guidelines and the quality of the included studies was evaluated systematically by the Newcastle–Ottawa Scale [22]. The major limitation of this analysis is represented by the observational nature of the studies (prospective or retrospective) included, but they are the only source of evidence available in the literature. Another weakness of this review is that several of the results are based on statistically heterogeneous studies. This may be due to the underlying differences in study design, varied from small cohorts selected to large sample size. To compensate for these limitations, we adopted a conservative approach using random effects models and performing subgroup analyses. Additionally, due to the lack of detail, the inability to separately analyze the two different abnormal fertilization conditions (1PN and 3PN) is another limitation.
Implications for clinical practice
The results of this meta-analysis show that oocytes matured through rescue IVM have lower developmental competence compared to oocytes matured in vivo. Therefore, rescue IVM is not recommended for patients with a good prognosis. However, it is well-established that a higher number of oocytes/embryos significantly improves the cumulative birth rate [39, 40]. Given this premise, the application of rescue IVM could be considered for patients with a poor prognosis. In such cases, even obtaining just a few extra MII oocytes could help maximize the efficiency of the treatment. Moreover, rescue IVM could be an alternative approach that might be considered for polycystic ovarian syndrome patients undergoing IVF treatment and who cannot safely use hCG triggering. This is particularly relevant for patients who have ovaries that are resistant to high doses of stimulation and show poor unexpected ovarian response [41, 42]. Another important point to note is the maturation timing. Our results suggest the use of in vitro MI matured within 6 incubation hours. Furthermore, a reduced blastulation rate but a similar euploidy rate between in vitro and in vivo–matured oocytes was found. These findings may have significant relevance in clinical practice. A blastocyst transfer policy should be recommended with the use of oocytes matured following rescue IVM.
Our data showed that an average MI oocyte has a 36% (2359/6545) maturation rate within 6 h, a 55% (3344/6006) fertilization rate, and a 35% (136/400) blastulation rate. Based on these data, we can calculate the probability that a blastocyst will be obtained from an MI matured within 6 incubation hours through the following calculation: 0.36 × 0.55 × 0.35 = 0.069. This means each MI oocyte has a 7% chance of developing into a blastocyst. Applying this to a POR population, with a reduced number of oocytes and at least one MI retrieved, not applying rescue IVM would have caused a relative reduction in the chance of obtaining an additional blastocyst available for transfer in at least 7% of these patients.
Finally, these findings could be translated into the context of fertility preservation for medical reasons. In fertility preservation patients, immature oocytes should be frozen routinely. Two reviews summarized that the cryopreservation of immature oocytes in the context of fertility preservation has the potential to enhance the availability of mature oocytes [43, 44]. Nevertheless, there is still a debate about the optimal timing for cryopreservation, specifically whether it should occur before or after in vitro rescue IVM (45).
Conclusions
This meta-analysis provides the state-of-the-art best evidence on rescue IVM. Indeed, we conducted a systematic review of the literature using stringent eligibility criteria to ensure a comparable oocyte cohort. We included all papers that reported data for at least one clinical outcome (fertilization, cleavage, blastulation, euploidy, clinical pregnancy, live birth, and miscarriage rates) for oocytes matured following rescue IVM. Our results showed compromised developmental competence in oocytes matured following rescue IVM. However, in patients with a low number of retrieved mature oocytes as PORs, rescue IVM could maximize the efficiency of the treatment. Notably, our data suggests the use of in vitro MI matured within 6 incubation hours. The available evidence supporting these findings is limited, necessitating cautious interpretation. Moreover, it is crucial to note that the long-term safety data of rescue IVM remain unestablished. Consequently, this meta-analysis emphasizes the necessity for additional large, high-quality observational studies in this area, particularly in PORs, in order to identify an optimal cut-off for the maturation timing.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14(7):1859–63.
Huang FJ, Chang SY, Tsai MY, Lin YC, Kung FT, Wu JF, et al. Relationship of the human cumulus-free oocyte maturational profile with in vitro outcome parameters after intracytoplasmic sperm injection. J Assist Reprod Genet. 1999;16(9):483–7.
Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update. 2018;24(3):245–66.
Balakier H, Sojecki A, Motamedi G, Librach C. Time-dependent capability of human oocytes for activation and pronuclear formation during metaphase II arrest. Hum Reprod. 2004;19(4):982–7.
Jie H, Zhao M, Alqawasmeh OAM, Chan CPS, Lee TL, Li T, et al. In vitro rescue immature oocytes - a literature review. Hum Fertil (Camb) 2022;25(4):640–50.
Lee H-J, Barad DH, Kushnir VA, Shohat-Tal A, Lazzaroni-Tealdi E, Wu Y-G, et al. Rescue in vitro maturation (IVM) of immature oocytes in stimulated cycles in women with low functional ovarian reserve (LFOR). Endocrine. 2016;52(1):165–71.
de Almeida Ferreira Braga DP, de CássiaSávioFigueira R, Ferreira RC, Pasqualotto FF, Iaconelli A, Borges E. Contribution of in-vitro maturation in ovarian stimulation cycles of poor-responder patients. Reprod Biomed Online. 2010;20(3):335–40.
Moon JH, Zhao Q, Zhang J, Reddy V, Han J, Cheng Y, et al. The developmental competence of human metaphase I oocytes with delayed maturation in vitro. Fertil Steril. 2023;119(4):690–6.
Shani AK, Haham LM, Balakier H, Kuznyetsova I, Bashar S, Day EN, et al. The developmental potential of mature oocytes derived from rescue in vitro maturation. Fertil Steril. 2023;120(4):860–9.
Elkhatib I, Nogueira D, Bayram A, Abdala A, Del Gallego R, Melado L, et al. How to identify patients who would benefit from delayed-matured oocytes insemination: a sibling oocyte and ploidy outcome study. Hum Reprod. 2023;38(8):1473–83.
Álvarez C, García-Garrido C, Taronger R, González de Merlo G. In vitro maturation, fertilization, embryo development & clinical outcome of human metaphase-I oocytes retrieved from stimulated intracytoplasmic sperm injection cycles. Indian J Med Res. 2013;137(2):331–8.
Li M, Li Y, Ma S-Y, Feng H-L, Yang H-J, Wu K-L, et al. Evaluation of the developmental potential of metaphase I oocytes from stimulated intracytoplasmic sperm injection cycles. Reprod Fertil Dev. 2011;23(3):433–7.
Escrich L, Galiana Y, Grau N, Insua F, Soler N, Pellicer A, et al. Do immature and mature sibling oocytes recovered from stimulated cycles have the same reproductive potential? Reprod Biomed Online. 2018;37(6):667–76.
Ko DS, Lee S-H, Park D-W, Yang KM, Lim CK. Pregnancy and fertilization potential of immature oocytes retrieved in intracytoplasmic sperm injection cycles. Clin Exp Reprod Med. 2015;42(3):118–25.
Edirisinghe WR, Junk SM, Matson PL, Yovich JL. Birth from cryopreserved embryos following in-vitro maturation of oocytes and intracytoplasmic sperm injection. Hum Reprod. 1997;12(5):1056–8.
Liu J, Lu G, Qian Y, Mao Y, Ding W. Pregnancies and births achieved from in vitro matured oocytes retrieved from poor responders undergoing stimulation in in vitro fertilization cycles. Fertil Steril. 2003;80(2):447–9.
Li Y, Jin L, Tian W, Yan E, Li Y, Ren X, et al. The ploidy of blastocysts from in-vitro-matured metaphase I oocytes. Reprod Biomed Online. 2023;48:103571.
Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74(2):295–8.
Emery BR, Wilcox AL, Aoki VW, Peterson CM, Carrell DT. In vitro oocyte maturation and subsequent delayed fertilization is associated with increased embryo aneuploidy. Fertil Steril. 2005;84(4):1027–9.
Zhang XY, Ata B, Son W-Y, Buckett WM, Tan S-L, Ao A. Chromosome abnormality rates in human embryos obtained from in-vitro maturation and IVF treatment cycles. Reprod Biomed Online. 2010;21(4):552–9.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45.
Mandelbaum RS, Awadalla MS, Smith MB, Violette CJ, Klooster BL, Danis RB, et al. Developmental potential of immature human oocytes aspirated after controlled ovarian stimulation. J Assist Reprod Genet. 2021;38(9):2291–9.
De Vincentiis S, De Martino E, Buffone MG, Brugo-Olmedo S. Use of metaphase I oocytes matured in vitro is associated with embryo multinucleation. Fertil Steril. 2013;99(2):414–21.
Margalit T, Ben-Haroush A, Garor R, Kotler N, Shefer D, Krasilnikov N, et al. Morphokinetic characteristics of embryos derived from in-vitro-matured oocytes and their in-vivo-matured siblings after ovarian stimulation. Reprod Biomed Online. 2019;38(1):7–11.
Vanhoutte L, De Sutter P, Van der Elst J, Dhont M. Clinical benefit of metaphase I oocytes. Reprod Biol Endocrinol. 2005;3:71.
Avci B, Kasapoglu I, Cakir C, Ozbay A, Ata B, Uncu G. Fertilisation and early embryonic development of immature and rescue in vitro-matured sibling oocytes. Hum Fertil (Camb). 2022;25(1):107–16.
Alcoba DD, Pimentel AM, Brum IS, Corleta HE. Developmental potential of in vitro or in vivo matured oocytes. Zygote. 2015;23(1):93–8.
Bilibio JP, Lorenzzoni PL, Meireles AJC, Maciel Y, Sales P, de Nascimento FC. The usefulness of metaphase I oocytes in women who undergo controlled ovarian hyperstimulation for intracytoplasmic sperm injection. JBRA Assist Reprod. 2021;25(1):115–21.
Strassburger D, Friedler S, Raziel A, Kasterstein E, Schachter M, Ron-El R. The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19(7):1587–90.
Kim BK, Lee SC, Kim KJ, Han CH, Kim JH. In vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil Steril. 2000;74(6):1153–8.
Shin SB, Cho JW, Lee S-H, Yang KM, Lim CK, Lee H-S. Fertilization and pregnancy potential of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Clin Exp Reprod Med. 2013;40(1):7–11.
Shu Y, Gebhardt J, Watt J, Lyon J, Dasig D, Behr B. Fertilization, embryo development, and clinical outcome of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Fertil Steril. 2007;87(5):1022–7.
Fesahat F, Kalantar SM, Sheikhha MH, Saeedi H, Montazeri F, Firouzabadi RD, et al. Developmental and cytogenetic assessments of preimplantation embryos derived from in-vivo or in-vitro matured human oocytes. Eur J Med Genet. 2018;61(4):235–41.
Yılmaz N, Özyer Ş, Taş D, Özer MC, Türkkanı A, Yılmaz EŞ, et al. Fertilization and early embryonic development of in vitro matured metaphase I oocytes in patients with unexpected low oocyte maturity rate. Zygote. 2022;30(3):319–23.
De Vos M, Grynberg M, Ho TM, Yuan Y, Albertini DF, Gilchrist RB. Perspectives on the development and future of oocyte IVM in clinical practice. J Assist Reprod Genet. 2021;38(6):1265–80.
ESHRE Add-ons working group, Lundin K, Bentzen JG, Bozdag G, Ebner T, Harper J, et al. Good practice recommendations on add-ons in reproductive medicine†. Hum Reprod. 2023;38(11):2062–104.
Strassburger D, Goldstein A, Friedler S, Raziel A, Kasterstein E, Mashevich M, et al. The cytogenetic constitution of embryos derived from immature (metaphase I) oocytes obtained after ovarian hyperstimulation. Fertil Steril. 2010;94(3):971–8.
Fanton M, Cho JH, Baker VL, Loewke K. A higher number of oocytes retrieved is associated with an increase in fertilized oocytes, blastocysts, and cumulative live birth rates. Fertil Steril. 2023;119(5):762–9.
Scaravelli G, Zacà C, Levi Setti PE, Livi C, Ubaldi FM, Villani MT, et al. Fertilization rate as a novel indicator for cumulative live birth rate: a multicenter retrospective cohort study of 9,394 complete in vitro fertilization cycles. Fertil Steril. 2021;116(3):766–73.
Guo W, Zheng X, Zheng D, Yang Z, Yang S, Yang R, et al. Effectiveness, flexibility and safety of switching IVF to IVM as a rescue strategy in unexpected poor ovarian response for PCOS infertility patients. J Clin Med. 2023;12(5):1978.
Fatum M, Bergeron ME, Ross C, Ding A, Bhevan A, Turner K, et al. Rescue in vitro maturation in polycystic ovarian syndrome patients undergoing in vitro fertilization treatment who overrespond or underrespond to ovarian stimulation: is it A viable option? A case series study. Int J Fertil Steril. 2020;14(2):137–42.
Son W-Y, Chung J-T, Das M, Buckett W, Demirtas E, Holzer H. Fertilization, embryo development, and clinical outcome of immature oocytes obtained from natural cycle in vitro fertilization. J Assist Reprod Genet. 2013;30(1):43–7.
Sirait B, Jusuf AA, Wiweko B, Handayani N, Aubry DA, Muharam R. Potential use of immature oocyte to improve fertility preservation outcome: a narrative review. J Hum Reprod Sci. 2022;15(1):3–11.
Wang H, Racowsky C, Combelles CMH. Is it best to cryopreserve human cumulus-free immature oocytes before or after in vitro maturation? Cryobiology. 2012;65(2):79–87.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
A.B., L.P., S.d.G., and A.Bu. undertook the systematic literature review, extracted the data, conducted the bias analysis and grading, performed the meta-analysis, and collated the results. All authors reviewed the data, discussed the conclusions, and reviewed and approved the drafts.
Corresponding author
Ethics declarations
Conflict of interest
E.P. reported grants and personal fees from MSD, grants from Ferring, IBSA, TEVA, and Gedeon Richter, and grants and personal fees from Merck. The other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Attestation
Data regarding any of the subjects in the study has not been previously published unless specified.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bartolacci, A., Busnelli, A., Pagliardini, L. et al. Assessing the developmental competence of oocytes matured following rescue in vitro maturation: a systematic review and meta-analysis. J Assist Reprod Genet 41, 1939–1950 (2024). https://doi.org/10.1007/s10815-024-03211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-024-03211-9