Abstract
Purpose
Spermatogonial stem cells (SSCs) are the source for the mature male gamete. SSC technology in humans is mainly focusing on preserving fertility in cancer patients. Whereas in livestock, it is used for mining the factors associated with male fertility. The review discusses the present status of SSC biology, methodologies developed for in vitro culture, and challenges ahead in establishing SSC technology for the propagation of superior germplasm with special reference to livestock.
Method
Published literatures from PubMed and Google Scholar on topics of SSCs isolation, purification, characterization, short and long-term culture of SSCs, stemness maintenance, epigenetic modifications of SSCs, growth factors, and SSC cryopreservation and transplantation were used for the study.
Result
The fine-tuning of SSC isolation and culture conditions with special reference to feeder cells, growth factors, and additives need to be refined for livestock. An insight into the molecular mechanisms involved in maintaining stemness and proliferation of SSCs could facilitate the dissemination of superior germplasm through transplantation and transgenesis. The epigenetic influence on the composition and expression of the biomolecules during in vitro differentiation of cultured cells is essential for sustaining fertility. The development of surrogate males through gene-editing will be historic achievement for the foothold of the SSCs technology.
Conclusion
Detailed studies on the species-specific factors regulating the stemness and differentiation of the SSCs are required for the development of a long-term culture system and in vitro spermatogenesis in livestock. Epigenetic changes in the SSCs during in vitro culture have to be elucidated for the successful application of SSCs for improving the productivity of the animals.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
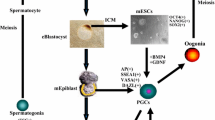
The spermatogonial stem cells (SSCs) are the adult stem cell pool capable of self-renewal and differentiation and regulates the sustained production of sperm. They form a niche in the seminiferous tubules, and the fate of the cells for self-renewal, differentiation into sperm, or apoptosis is determined by the signals of the testicular microenvironment [1]. SSCs in the testicular microenvironment are regulated by juxtaposed cells, autocrine, and paracrine factors essential for maintaining the stem cell reservoir [2]. In mice, A-single(As) spermatogonia are single cells that divide and produce inter-connected A-paired (Apr) cells, which further divide in turn to produce four A-aligned (Aln) cells. A-alinged cells again divide to produce chains of eight cells and 16 cells [3]. These are collectively grouped as undifferentiated A-type spermatogonia, expressing most of SSCs markers and possessing stem cell properties.
Improving productivity through augmenting reproduction is the prime focus of the livestock sector. Production potential has been accelerated in livestock with the upcoming assisted reproductive technology (ART) over the period of time. Artificial insemination (AI) is the most successful biotechnological tool that facilitated faster dissemination of the male germplasm using streamlined management of breeding bull [4]. SSC technology is a promising area for faster dissemination of superior male germplasm and also facilitates the production of transgenic animals with high productive and reproductive traits [5,6,7]. SSC-mediated transgenic animal production has the advantage of permanent modification of the germline. SSC transplantation and cryopreservation methods for the restoration of fertility and in vitro sperm production attained varying degrees of success. To establish germ cell transplantation in agriculturally important animals, identification of factors produced in testicular SSCs niche is essential. The establishment of a reliable and robust culture system for SSCs maintenance is of utmost important [8]. The factors regulating SSC self-renewal and exponential growth with inherent stemness remain elusive. A long-term culture system that supports, expands and maintains SSCs from livestock is yet to be developed [9].
Present scenario of SSC technology in farm animals
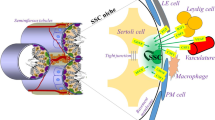
SSC research has various applications in livestock such as mining factors regulating male fertility, SSC transplantation and faster dissemination of superior germplasm as an alternative to AI, understanding the process and the pathways associated with SSC self-renewal and differentiation and preservation of the genetic material of valuable male [5, 10] and endangered species [11] (Fig. 1). Production of good-quality sperm from infertile animals is possible through transplantation of SSCs from superior fertile bulls [6]. In vitro spermatogenesis though successful in rodents [12] and fishes [13], still has not gained momentum in livestock and humans. However, with the help of advanced bioengineering technology, 3D scaffolds are developed that support the complete differentiation of SSCs into sperm [14]. Germ cells transplanted from donor to a recipient testis resulted in donor-derived spermatogenesis and fertile sperm production in rodents, monkeys, and livestock [15, 16], and offspring with donor germplasm was produced through natural breeding in rodents and livestock [15, 17]. Hence, SSC technology has great potential for the production of superior beef and dairy animals through natural breeding. In humans, SSC technology is advocated for prepubertal cancer patients that enable to restore the fertility at a later phase once the treatments are over. Research is being undertaken for testicular tissue grafting and xenografting, testicular tissue organ culture, and de novo testicular morphogenesis in rodents and humans [18,19,20] for facilitating the production of donor-derived spermatogenesis and restoration of fertility, but to a less extent in livestock. Gene editing in mouse SSCs using CRISPR-Cas9 technology corrected male infertility due to genetic defects and sex chromosome-linked dominant genetic diseases [21]. Such applications indicate the importance of SSC-based fertility-related research in the future, and attempts are carried out in livestock recently [15]. The researches on the isolation, purification, characterization, and culture of SSCs in livestock are advancing in many livestock species such as cattle [17], buffalo [22, 23], goat [24], sheep [25], and pig [26]. However, identification of specific SSC markers, long-term SSC culture and cryopreservation, and SSC transplantation procedures need refinement for successful application in livestock [15, 27, 28].
The various applications of SSCs culture are, a propagation of superior germplasm through transplantation of SSCs from the high fertile to the low fertile animals, b transgenic animal production, c in vitro differentiation of SSCs into the functional sperm, d characterization of SSCs in vitro to understand the physiology of SSCs, unveiling the possibility to predict fertility and to study the genetic and epigenetic changes associated with long-term culture, e understanding the spermatogenesis process by unraveling the mechanism of SSCs self-renewal and differentiation, f cryopreservation of valuable male germplasm
SSC isolation
The testis consists of various types of cells such as Sertoli cells, Leydig cells, different stages of differentiating germ cells, and the SSCs. Undifferentiated SSC pool and cell kinetics are identified using prevailing models such as As, Apr and Aal spermatogonia, based on SSC clones in rodents [3]; and Adark and Apale spermatogonia in humans, based on nuclear morphology and hematoxylin staining [29]. In livestock, only limited studies on SSC pool and self-renewal dynamics were conducted as compared to rodents and humans. The subset of SSCs is rare in germ cells population in the testis, as 0.03% in mice [30], 4% in monkey [31], 22% in humans [32], and 0.2 to 0.3% in bovine [33]. The population of SSCs in the testis of other livestock species such as sheep, goats, pigs, and buffaloes is not documented. However, we could isolate 7.33% promyelocytic leukemia zinc finger (PLZF +)cells from prepubertal sheep testis using enzymatic method [34].
SSCs were isolated from the testis using mechanical and enzymatic digestion methods with high viability in bovine, caprine, porcine, and bubaline [28]. An enzymatic method using collagenase IV, trypsin, DNase I, and hyaluronidase is commonly adopted for the dissociation of livestock testicular cells. Since a single enzyme is not sufficient to isolate effectively SSCs, two-step, three-step, or sequential enzymatic digestion with different enzymes have been carried out [24].
In the mechanical isolation method, tunica albuginea and visible connective tissues are removed, and seminiferous tubules are mechanically dissociated using scissors and forceps. Seminiferous tubular cells are dissociated by repeated vigorous pipetting and passing through a 10-ml hypodermic syringe fitted with a 22G needle. The cells are then filtered through 70- and 40-micron nylon meshes [35]. Enzymatic digestion of testicular tissues yields a higher number of SSCs than mechanical dissociation [36]. The STA-PUT method of SSC isolation combines the enzymatic method with velocity gradient separation [37]. This method yields a higher number of SSCs from the testis as compared to fluorescence-activated cell sorting (FACS) and is also less expensive than a cell sorter or an elutriator methods of isolation [37]. STA-PUT is an assembly of specialized glass apparatus, wherein SSCs are separated by a linear BSA gradient and sedimentation velocity at unit gravity. SSCs get separated based on the size and mass of the cells [38]. The advantages of STA-PUT method are low cost as it only involves the assembly of specialized glassware but yields SSCs with increased purity and doesn’t require specific biomarker [39]. SSCs from mice [40, 41], humans [39], bovine [42], and porcine [43] are isolated using STA-PUT method. A combination of the STA-PUT and magnetic-activated cell sorting (MACS) can be used for isolating the subpopulation of SSCs expressing a particular marker [44].
SSC characterization
Characterization of isolated, cultured, or transplanted SSCs is performed using the cell morphology, biochemical assays, or molecular markers. The morphological characterization of SSCs based on cell shape, size, nuclear to cytoplasm ratio, and chromatin condensation status has been carried out in mice [45], humans [46], bovine, porcine, caprine, and ovine [28, 47,48,49]. Undifferentiated spermatogonia contain euchromatin while differentiated spermatogonia have heterochromatin in mice [45]. Alkaline phosphatase activity is markedly high in undifferentiated spermatogonia as compared to differentiated cells and other testicular cells in humans [50], rodents [51], porcine [52], bovine [53], caprine [54], ovine [34], and bubaline [55]. Though morphological and biochemical methods are used for the initial characterization of SSCs, it cannot be confirmatory in nature. Hence, various biomolecules have been established as markers, which are expressed on the cell surface, cytoplasm, and nucleus (Fig. 2, Supplementary table 1). SSC markers have been identified in cattle [PLZF, Dolichos biflorus agglutinin (DBA), protein gene product 9.5 (PGP 9.5), Thy-1 cell surface antigen (THY1) and GDNF family receptor alpha 1(GFRα1)] [56,57,58]), buffalo (PLZF, PGP 9.5 and THY1) [22], goat [(α6 integrin (ITGA6), PLZF, β1 integrin (ITGB1), Octamer binding transcription factor 4 (OCT-4) and THY1)] [24, 59], sheep [(PLZF, PGP 9.5 and Cadherin 1(CDH1)], and pig (PLZF, DBA, PGP 9.5 and GFRα1) [26, 60]. Even though independent studies have reported these SSC markers in livestock, species-specific markers to differentiate between SSCs and other cell types are still lacking [28]. ITGA6 was reported to be a nonreliable SSC marker when compared to GFRα1 in bovine [58, 61]. PLZF and GFRα1 showed a similar pattern of expression in domestic cat SSCs [62]. Inhibitor of DNA binding 4 (ID4) is an established SSC marker in rodents [63] and humans [64], but not well characterized in livestock. Undifferentiated embryonic cell transcription factor 1 (UTF1), an SSC marker reported in rodents, humans, and monkeys [65, 66], is also conserved as a marker in stallion [67] and pig [68] but has not been documented in other species. It is also to be noted that SSC lineage-wise markers have been established in rodents and humans [69], but not in livestock. The identification of species-specific SSC markers is critical for enrichment and in vitro culture [70]. In addition, identification of SSC surface marker is necessary for isolation of viable cells through FACS or MACS method. Apart from molecular markers, retrospective studies such as tracing the SSC efficiency by teratoma formation and transplantation capability are performed as functional assays [71].
Spermatogonial stem cell markers are identified on the cell surface, cytoplasm, and nucleus. Cell surface markers such as THY1, ITGA6, ITGB1, GFRα1, CD9, PLD6, IGFBP3, SSEA1, CD14, CDH1, CD209, and GFR125; cytoplasmic markers such as UCHL1, OCT4, KLF4, UTF1, FOXO1 and PLZF; and nuclear markers such as FOXO1, OCT4, KLF4, UTF1, and PLZF are pictorially represented on the SSC
SSC enrichment/purification
Since the SSC population is very less in the testicular cells isolate, several methods of enrichment procedures have been tried to obtain sufficient cell numbers for culture work. Some of the techniques utilized are MACS, FACS, differential plating, selection with extracellular matrix (ECM), and velocity sedimentation or density gradient centrifugation [58, 72, 73]. The combination of enrichment techniques also significantly augment the purity of spermatogonia [74]. The selection of enrichment method is crucial for successful culture and downstream applications of SSCs.
Magnetic-activated cell sorting
MACS uses the immune magnetic method for the separation of the interested cells population based on their surface markers. The knowledge of the surface marker and its expression in the cells are needed to obtain a sufficient specific cell population for culture and other down-stream applications. In humans, MACS has been efficiently used for isolation of SSCs utilizing markers such as G protein-coupled receptor 125, (GPR 125), CD49f, and THY1 [19]. THY1 (CD90) is used as a marker to isolate SSCs from cattle, pigs, and goats [75,76,77]. THY1 is a reliable surface marker for goat [77, 78], pig [75], and cattle [76]. However, THY1 is not specific to humans SSCs, and the sorting efficiency using FACS also varied between humans and porcine [79]. GFRα1 is used for the isolation of pig SSCs [26]. Surface marker, ITGA6 in humans [73], yielded 3-fold, while DBA-FITC in bovine yielded 4.6-fold [74] and c-kit in rodents yielded 4–7-fold [80] enrichment of SSCs through MACS. This approach is a high-throughput method involving less operational cost and time when compared to FACS. MACS microbeads do not alter the structure, function, and viability and will not interfere with the culture characteristics of sorted cells. However, the purity of cells for certain cell type is not adequate in MACS when compared to FACS due to the sorting of cells based only on cell affinity [81].
Fluorescent activated cell sorting
FACS is a more specific and efficient method employed for the purification of SSCs based on multiple parameters such as cell size, density, viability, and numerous SSC markers. Hence, SSCs can be sorted with high accuracy from the mixture of cells isolate by employing FACS technique [81]. Further, sorting using various surface markers enables to enrich the subset of stem cell population from the whole testicular cell isolates. The surface markers, ITGA6, THY1, GFRα1, and DBA are targeted for SSC separation in rodents and livestock. The enrichment efficiency is high using FACS (12-fold) when compared to MACS (3.3-fold) [73]. In mice, selection of cells based on FACS employing light-scattering properties and expression of the cell surface markers ITGA6, ITGAV, and the c-kit receptor resulted in 166-fold enrichment of SSCs [82]. In bovine, sorting by FACS increases in DBA-positive SSCs from 12.3% in the input sample to 50.5% in the sorted fraction [74]. When coupled with molecular marker screening and the stem cell transplant assay to validate sorted fractions, FACS becomes a powerful tool for dissecting the molecular phenotype of SSCs [73]. Identification of a greater number of specific surface markers in livestock enables to enrich SSCs more efficiently using FACS.
Differential plating
Different extracellular matrix substrates are used for the enrichment of SSCs. Laminin is used for the positive selection [70], while lectin and gelatin are used for the negative selection of SSCs [34]. Laminin is secreted by Sertoli cells as an adhesion molecule and contributes to the basement membrane. Laminin binds to ITGα6/β1 receptors on the spermatogonial cell surface, and this property is used for purifying the SSCs by differential plating. SSCs are plated on such matrixes for improving self-renewal and proliferation for a long time [34, 83, 84]. The enrichment using laminin yielded a higher percentage of SSCs when compared to gelatin. Ficoll gradient centrifugation in combination with laminin differential plating further improved the purity to 64% in the testis of cats [85]. Datura stramonium agglutinin (DSA), a lectin that especially binds to β(1-4) linked oligomers of N-acetyl-D-glucosamine in Sertoli cells is used for the isolation and purification of Sertoli cells [86]. The testicular cells isolated are enriched over DSA lectin (5 μg/mL for 1 h), yielded 2.8-fold (VASA+) enriched SSCs [87]. The overnight incubation in flasks coated with 20 μg/ml DSA results in a 3.6-fold increase in bovine typeA spermatogonia in the nonadherent fraction [74]. Where as in goat DSA- lectin enriches the SSC population up to 11.23% in goat [88]. In addition, combining Percoll density gradient centrifugation with DSA enrichment, the purity of SSC increased up to 60% in buffalo [22]. Gelatin is obtained from the matrix component and favors the attachment of somatic cells, whereas the nonadherent cells are enriched with SSCs [26, 72, 83, 89]. The floating cells from a gelatin-coated plate after 1 day of incubation relatively enriched mouse male germline stem cells for long-term proliferation in mice. In most of the studies, the concentration of gelatin used for differential plating is 0.2% [26, 72, 83], and enrichment efficiency improved on increasing the incubation duration of differential plating.
Combined enrichment methods
A multiparameter selection was adopted to improve the purification efficiency. Isolation of SSCs using multiple parameters such as intracellular complexity along with marker selection α6-integrin+, c-kit -, and α v-integrin -(CD51-) enriched SSCs up to 152- to 166-fold in FACS [82]. A combined enrichment method employing extracellular matrix, laminin, and gelatin effectively purified undifferentiated cells to 2.7-fold from monkey testis [90]. The purity of the SSCs after the combination of Percoll, laminin and gelatin was the highest with 90% [91], while gelatin alone yielded moderate purity of 73.7% [92] and 55% [22]. Centrifugation with 17% Nycodenz (new non-ionic iodinated gradient medium) followed by differential plating with fibronectin and poly-D-lysine coating enriched the purity of piglet gonocytes to >90%. Overall, the variations in the purity levels observed between studies are attributed to the developmental stage of the testis, differences in purification protocols, and selection of the markers for the enrichment of the cells [93].
SSC culture in livestock: nascent stage
The development of a cell culture medium capable of maintaining stemness and proliferation is a prerequisite for the establishment of SSCs technology. Even though SSCs have an important role in animal reproduction, the factors that regulate self-renewal, proliferation, and stem cell fate are least understood. There is a gradual decrease in the proliferation of putative SSCs during subculture over a period of time, as differentiation and apoptosis dominate the cellular events [28]. So research is focusing on the supplementation of appropriate additives, growth factors, matrix substrates, and serum-free supplements in the culture media favoring SSC self-renewal and proliferation in vitro [28]. As the culture conditions are unique for each species, recent studies are oriented towards developing the protocols and components for a particular species [27].
Short-term culture
Short-term SSC culture have been reported in caprine (7 days) [94], sheep (14 days) [59], and bovine (11 days to 3 months) [56, 57, 95, 96]. In livestock, SSC culture system was optimized by the addition of culture media with additives and other growth factors [24, 56, 97, 98]. In these studies, serum was used as an important component in the culture medium for survival and self-renewal of culture cells. Undefined factors in serum might induce cell differentiation [18] and at higher concentrations had detrimental effects on SSCs expansion in culture [87]. Hence, defined culture media without serum and feeder have been developed recently to overcome this problem [99].
Long-term culture
Long-term culture systems are essential for propagating SSCs at an exponential rate for downstream biotechnological applications. Long-term SSCs cultures have been developed in rodents, for maintaining the stemness over 2 years with the capability to undergo spermatogenesis after transplantation into the testis [18, 89, 100,101,102]. In livestock, though few long-term SSC culture has been carried out [89, 92, 95], the culture conditions are not optimized when compared to the rodent models [9]. Few studies have reported a long-term culture system that supports continuous proliferation of bovine gonocytes in vitro; however, the culture duration is less from 1 [92] to 3 months [95]. The evidences of epigenetic stability of cell line, transplantation efficiency,and production of donor-derived offspring are lacking in livestock [89] due to the gap in knowledge for the requirement of the culture media for the maintenance and proliferation of SSCs in domestic animals [9, 27, 103, 104].
Culture on feeder system
Sertoli cells are mainly used as a feeder system for SSC culture. Feeder cells facilitate the cell’s attachment and provide paracrine support for the survival of the cells. SSCs co-cultured using Sertoli cell monolayer as feeder layer increased the self-renewal process in bull [56] and buck [24]. Due to the interactions between SSCs and Sertoli cells, the undifferentiated state of SSCs is maintained [56, 105]. These colonies are also able to regenerate new colonies upon subculturing [106]. The other feeder layer generally used are Sandos inbred mice (SIM) embryo-derived thioguanine-and ouabain-resistant, mouse embryonic fibroblast and laminin-coated plates. The SIM embryo feeder layer is efficient for in vitro propagation of bovine testicular germ cells when compared to mouse embryonic fibroblast, bovine Sertoli cells, and laminin-coated plates [96]. The addition of growth factors like glial cell line-derived neurotrophic factor (GDNF) enhanced the self-renewal of the SSCs [56, 105]. Serum supplemented at lower concentration (1%) in culture medium maintained stemness for a week, while at higher concentrations, it had detrimental effects on SSC expansion [87].
Feeder and serum-free culture
To develop a more efficient and long-term SSC culture medium, several feeder- and serum-free culture systems have been tested and found superior to the conventional culture [107]. Feeder and serum-free culture media with additives like lipid-rich albumin (Albumax), fetuin (blood proteins), and knock-out serum replacement improved the culture medium and maintained the stemness of SSCs in culture for 5 months in mice [107]. The enriched undifferentiated bovine spermatogonia maintained on bovine fetal fibroblast-conditioned medium sustained stemness for 1 month [92]. Such culture systems avoid unknown variable factors in the media and prevent the growth of contaminating somatic cells [103].
Additives in SSCs
The survival, stemness, and proliferation of cells in vitro depend on the growth factors, hormones, vitamins, and other additives supplied in the medium [108]. The media such as DMEM, MEMα, and DMEM/F12 are most widely used for the culture of SSCs in domestic animals. Stem cell-specific media like StemPro-34 and knockout serum used for SSC culture in rodents [107] are proved to support SSCs from livestock species [103]. Apart from the basal media, growth factors such as GDNF, epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), leukemia inhibitory factor (LIF), colony-stimulating factor (CSF), vascular endothelial growth factor (VEGF), and insulin-like growth factor 1 (IGF1) are supplied in the SSC culture [108,109,110,111]. Other additives with unique function for cell survival and division are hormones such as FSH [33], insulin [51], beta-estradiol, and progesterone [112]. The energy substances such as D-(1)-glucose, pyruvic acid, DL lactic acid and transferrin, vitamins including ascorbic acid, and amino acids such as minimum essential medium amino acids and L glutamine and buffer Hepes [57], and polyamines like putrescine for cell proliferation are also added. The reducing agents such as sodium selenite and 2-mercaptoethanol are incorporated in the media for preventing the effect of oxygen-free radicals. Further, the culture medium contains antibiotic-antimycotic for preventing microbial contamination. Interestingly, suppression of LH levels in the culture media promotes SSC self-renewal by reducing the expression of WNT5A, and such knowledge can be considered while deciding the composition of the culture media [113].
Growth factor as supplements
Growth factors play a crucial role in regulating the fate of SSCs in the testicular microenvironment. Several factors including GDNF, LIF, EGF, and FGF2 in the culture medium have been reported to positively influence the expansion and stemness of the SSCs [89, 114]. VEGF enhanced SSC proliferation in bovine [115] and mice [110]. Growth factors promote the self-renewal, proliferation, and survival of SSCs through autocrine regulation. The combinations of growth factors appropriate for the culture medium are different for the rodents and bovines [27] and hence need to be studied for a particular species for establishing long-term culture.
Glial cell line-derived neurotrophic factor
GDNF was identified as the major growth factor to maintain stemness and self-renewal of SSCs [56, 105]. The dose of GDNF used for SSC culture ranges from 1 to 100 ng/mL [26, 35, 56, 92]. GDNF knockout mice had depleted spermatogonia, while overexpression of GDNF produced clumps of undifferentiated spermatogonia. Gonocytes proliferate to form clusters of cells in the presence of GDNF and LIF [116]. Supplimentation of these growth factors culture medium resulted in SSC proliferation and long-term culture of SSCs in rodents.
GDNF signals act through GFRα-1 and RET tyrosine kinases in various cell types. GFRα-1 and RET are expressed in gonocytes, SSCs, and differentiated spermatogonia. GDNF produced by Sertoli cells has receptors on the SSCs, acts in paracrine signaling pathways, and mediates SSCs self-renewal [117]. The signaling pathways regulated by growth factor GDNF mainly include Src signaling and Ras signaling pathways. Four Src family kinases are connected in SSC proliferation through RET activation namely Src, Yes, Lyn, and Fyn. Among these, Src and Yes sensitizes the primary SSCs to respond to GDNF. Further, Src activates a PI3K/Akt signaling pathway which in turn leads to N-myc expression and promotes SSC proliferation [117,118,119]. GDNF also activates the Ras/ERK1/2 pathways that result in phosphorylation and activation of transcription factors such as Creb-1, Atf-1, and Crem-1 for the proliferation of SSCs [117]. GDNF and FGF2, in the testicular microenvironment, have been shown to directly control the expression of transcription factors namely Id4, Etv5, and Bcl6b to stimulate self-renewal in SSCs. There is also an intrinsic mechanism that regulates the expression of those factors inhibiting/suppressing the differentiating process [120]. Knockout studies revealed that long noncoding RNAs such as, lncRNA033862 regulate SSC fate, as the lncRNA regulates Gfra1 expression levels by interacting with Gfra1 chromatin [121]. Increased GDNF signaling led to phosphorylation of AKT3 but not of AKT1 or AKT2, in undifferentiated spermatogonia suggesting that AKT3 functions in SSC self-renewal or progenitor cell expansion [88].
In rodents, the long- and short-term culture of SSCs with GDNF are established either alone or in combination with other growth factors or additives [88]. In buffalo, 7–10 [122] and 15 days [123] of SSC culture were carried out. The undifferentiated spermatogonial colonies were maintained in culture for 15 days using the combination of GDNF, EGF, and FGF2 than with the same concentrations of GDNF alone or GDNF plus either EGF or FGF2 [123]. The highest numbers of spermatogonia were obtained in cultures when a combination of growth factors like GDNF, LIF, EGF, and FGF2 was used in the culture medium [57, 96]. FSH hormone was found to stimulate GDNF production by Sertoli cells [124]. The cocktail of growth factors GDNF and FGF2 maintained SSC self-renewal and are used in long-term cultures in rodents [117]; however, no such beneficial effects upon addition of FGF to SSCs culture system in livestock are reported [109].
Epidermal growth factor
EGF has been used in the SSC culture media [57]. The receptors for EGF have intrinsic tyrosine kinase activity, and such receptors are present in SSCs [125]. The EGF and EGFR can promote the overexpression of cyclo-oxygenase 2, which can improve the mitotic activity in SSCs [126]. EGF activates cSrc/STAT pathway of SSCs in vitro and enhances the proliferation of SSCs [127]. The effect of the addition of EGF to the culture medium either as a single growth factor [128], or in combination with other growth factors [59] and additives [129], has been studied with success. Various doses of EGF ranging from 0.1 to 20ng/ml as a single factor or with other factors have been reported to improve stemness and proliferation of the stem cells [128,129,130]. EGF had a positive effect on PLZF expression levels in porcine SSCs [109]. A nonadherent culture system containing growth factors EGF along with GDNF and FGF2 favored in vitro short-term culture in mice and dogs [131].
Fibroblast growth factor
In rodents, the combinations of bFGF with other growth factors, GDNF and EGF, are required for the long-term culture of germ cells [18, 132]. The concentrations of FGF used in livestock are ranging from 5ng/ml in goats [94] to 10 ng/ml in bulls [133] and porcine [109]. However, studies reported the possibility of the negative effect of FGF on porcine and bovine SSC stemness unlike rodents [27, 109]. In bovines, the addition of FGF2 to the culture system enhanced somatic cell proliferation and induced differentiation of the gonocytes [27], whereas in pigs, FGF decreased the expression of PLZF in the culture system [109], although the exact difference in the mode of action is least understood. FGF can induce the production of GDNF and GFRα1 in neural cells, and these act in autocrine pathways to decrease apoptotic signaling [134] and such mechanisms may determine the fate of the cultured cells [18].
Insulin-like growth factor 1
Insulin-like growth factor-1 (IGF-1) secreted from Leydig and Sertoli cells impart stemness to the spermatogonial cells. The blockage of IGF-1 pathway resulted in reduced alkaline phosphatase activity [51]. IGF1R signaling is essential for the proliferation of SSCs by promoting the G2/M progression of the cell cycle [135]. The molecular mechanism, i.e., the cross talk between IGF1/IGF1R and SDF1/CXXR4 signaling has been elucidated to induce self-renewal of SSCs under the hypoxic microenvironment in mice [136]. Such studies open up a new window for manipulations of the SSC culture system. IGF1 (100 ng/mL) in culture media increased the numbers and diameters of SSCs colonies on day 16 and also improved cryosurvivability in bovine [137]. In combination with GDNF and bFGF, IGF1 improves in vitro proliferation of goat SSCs, while preventing the uncontrolled proliferation of somatic cells [138]. Further, IGF-1 promotes spermatogenesis by increasing the round and elongated spermatids and decreasing the apoptosis of germ cells in the culture of mouse testicular fragments [139].
Leukemia inhibitory factor
LIF in combination with other growth factors such as GDNF, EGF, and FGF supplemented in SSC culture systems [57, 59, 109, 138] enhanced the self-renewal capacity. LIF is known to maintain the pluripotency of SSCs [140]. In humans, the combination of bFGF (1 ng/mL) and LIF (1500 unit/mL) produced the largest diameter of SSC colonies [141]. SSCs co-cultured with Sertoli cells with the addition of LIF enhanced the alkaline phosphatase activity and the expression of SSC specific genes [142].
Advanced culture conditions
Recent studies provided insights for modulating the bioenergetics of SSCs culture. Apart from the use of additives for improvising the SSCs culture conditions, optimization of the oxygen tension and metabolic pathways may improve the SSC proliferation in vitro and transplantation efficiency in vivo. SSC culture conditions with reduced oxygen tension maintained stemness for a long term is a progressive step for maintaining stemness [92]. The exact role of oxygen on SSCs remains unknown; few studies established that hypoxia by modulating the expression levels of Hif genes and reactive oxygen species promoting the self-renewal of SSCs [143]. Optimizing the SSC culture conditions that favor glycolytic activity and modulating similar culture environments is essential for maintaining functional integrity [102]. For instance, omission of lipid from SSCs culture enhances glycolysis pathway, which helps in proliferation of SSCs [102]. The addition of 6-bromoindirubin-3′-oxime, an inhibitor of glycogen synthase kinase-3α, enhanced the proliferation of SSC in long-term culture of SSCs in bovine [95]. Though the doubling time for SSCs in culture is relatively slow, the SSCs can multiply 1:2 to 1:4 every 7 days once the culture micro milieu is optimal. Hence, the time required to generate sufficient SSCs needs careful planning [144]. Advanced systems including a three-dimensional (3D) culture system that mimics the testicular architecture in vitro and provide ECM support for the proliferation are also beneficial. Three-dimensional materials such as nanofiber matrices [145] and soft agar culture system [146] are also used for the culture of the SSCs. ECM from homologous species provides the best bioenvironment for SSCs culture in vitro [147].
SSC cryopreservation and transplantation
Successful cryopreservation and transplantation of SSCs are essential procedures that need to be standardized in livestock for wider application of SSC technology and research has been focused in this direction in rodents [148, 149]. The extent of application of SSCs depends on the success of long-term storage of cultured cells by employing the cryopreservation technique. During cryopreservation, cooling and thawing process induce damage to cells and tissue which directly affect their viability [150]. The selection of appropriate cryoprotectant and the cryopreservation protocol is necessary for preserving the cell structural and functional integrity. The permeating cryoprotectants are small molecules less than 100 dalton including DMSO, ethylene glycol, glycerol, glucose, propylene glycol, diethyl glycol, proline, etc. which can penetrate the plasma membrane and prevent excessive dehydration of cells during freezing [151]. The non-permeating cryoprotectants are large in size and can protect the cells from cryoinjury are usually of polymers, polyvinylpyrrolidone, polyethylene glycol, trehalose, sucrose, etc. [152, 153]. Further, the time taken for freezing the cells from room temperature also affects the viability of SSCs. In this direction, cryopreservation of SSCs using cryoprotectants by employing slow freezing with rapid thawing protocol has been successfully tested [148, 154]. Appropriate selection of cryopreservation protocol for SSC storage should reduce DNA fragmentation, mitochondrial damage, oxidative stress, and osmotic stress during cryopreservation [155, 156].
The success of cryopreservation may also depend on species-wise optimization of cryoprotectants, additives, freezing method and selection of sample type, either cultured cells or testicular tissue. On comparing fast freezing, slow freezing and vitrification methods in livestock species, slow freezing has been suggested as an ideal freezing protocol for testicular tissue cryopreservation in terms of protecting cellular integrity and resumption of spermatogenesis [153, 157, 158]. However, vitrification was found effective than slow freezing in rodents [159] humans [160] sheep [161] and cats [162]. The post-thaw recovery and transplantation efficiency were better in testicular tissue freezing as compared to cell freezing in bovine and porcine [153, 158].
In bovine, DMSO either alone [163] or in combination with sucrose [148] or trehalose, [153] have been used for cryopreservation of SSCs. DMSO, with sucrose as cryoprotectants in bovine [148], sheep [164], and pig [165], yielded 70% post-thaw viability and cultured with optimum prolificacy. DMSO in combination with FBS and 200 mM trehalose as compared with sucrose and polyethylene glycol are effective cryopreservation media in terms of post-thaw recovery and transplantation efficiency in bovine [153] and porcine [158]. DMSO and glycerol combination was effective for the vitrification of cat testicular tissue [162]. The combination of DMSO (10%), knockout serum replacement (10%) with trehalose (20%) using slow-freezing maintained cell viability, reduced apoptosis, and cell integrity of bovine SSCs [166]. Cryomedia containing DMSO (10%) and knockout serum replacement (KSR) (5–10%) along with FBS (5%) are also used for cryopreservation of bovine SSCs [167]. In horse, DMSO-based cryomedia coupled with the slow-freezing method is equally good for maintaining the structure, function, or colony-forming abilities when compared with ethylene glycol and freezing (fast freezing, vitrification) protocols [168]. Testicular tissue from piglets vitrified in cryomedia containing ethylene glycol, polyvinyl pyrrolidone, and trehalose did not affect the viability. In this tissue, sperm have been produced following xenotransplantation and offspring through assisted reproductive technique [169]. Recombinant human serum albumin (5%) as serum replacement in the cryopreservation media improved proliferation potential and maintained SSC characteristics, and after post-thaw, the cells were cultured and colonized in the testis upon transplantation of SSCs in mice [170].
In rodents, many additives such as quercetin [156], caffeic acid [171], vitamin E [172], catalase [173], and hypotaurine [155] have been incorporated to reduce oxidative stress and improve cyotolerance, post-thaw proliferation, and transplantation capacity. Additives such as vitamin E and C were used for the cryopreservation of sheep SSCs [174]. Incorporation of apoptosis inhibitors as additives during cryopreservation resulted in varied success in terms of the post-thaw recovery rate of SSCs, as one study reported beneficial [175], whereas another study reported no effect [155]. The effect of apoptosis inhibitors in livestock SSCs is yet to be elucidated. To reduce oxidative stress, additives such as melatonin are used in goat SSC cryomedia [176].
In livestock, there is a need for refinement of cryopreservation technique in terms of identifying the best combination of freezing methods and the cell or the tissue type. The ideal method of freezing to be adopted for cryopreservation of testicular cells or tissue is still not clear. Further, spermatogenesis capability of cryopreserved SSC following transplantation, and the genetic and epigenetic stability needs to be established in livestock.
Donor-derived spermatogenesis was possible through SSC transplantation in cattle, sheep, and goats, and live progeny has been produced from sheep and goats [74, 106, 177]. Xenotransplantation of bovine SSCs could colonize and proliferate in the seminiferous tubules of the mouse but did not progress through the complete process of spermatogenesis [153, 178]. The development of appropriate recipients is also essential for colonization of donor SSCs in the recipient testis, donor-derived spermatogenesis, and production of live offspring through the transplantation of SSCs. NANOS2 gene knock models were produced in mice, goats, pigs, and cattle which can be used as an ideal surrogate recipient for the spermatogonial stem cells transplantations [15]. The knockout males lacked the endogenous SSCs and other germ cell population but had intact seminiferous tubule and other somatic cell populations [179, 180]. Donor-derived spermatogenesis through allogenic transplantation was possible in mice, pigs, and goats [15]. Other than germ cell–depleted genome-edited animals, chemotoxic drugs such as busulfan and localized testicular X-ray irradiation can be used for developing recipients for germ cell transplantation [149, 153]; however, these treatments also damage the other cells.
SSC colony characterization and cell morphology
Spermatogonia are round cells with a high nucleus/cytoplasmic ratio. These cells have a spherical nucleus containing one to three dense nucleoli and many cytoplasmic inclusions that are mostly concentrated at one side of the cell in humans [182], rodents [183], and livestock [59]. During the first week of culture, the spermatogonia appeared as single, paired, aligned, and cluster forms [59]. The morphology of 6-week-old mice SSCs cultured with 0.2% BSA plus 10% KSR were grape-shaped colonies. These colonies were positive for specific germ cells markers, αGENA and TRA98. Culturing of SSCs on mouse embryonic fibroblast feeder cells with 10% KSR, substantial cell growth was observed with a doubling time of 5.5±2.7 days, while the addition of 0.2% BSA with 2 or 10% KSR advanced the doubling time to approximately 4 days [184]. Pig SSC colonies were maintained with undifferentiated morphology for more than 2 months and passaged more than 8 times with doubling time between 6 and 7 days. The colony characteristics were similar to mice SSCs [35]. These results revealed the fact that the growth rate of the colony is influenced by the supplements and their concentration in culture.
Maintenance of stemness: problems and possible solutions
The major challenge in establishing the long-term SSC culture system is the maintenance of stemness and preventing differentiation and apoptosis over a period of time. Understanding the pathways influencing stemness is of importance for the in vitro modification of the culture system for successful self-renewal. In addition, the knowledge on SSC differentiation and apoptosis pathways are essential to adopt better strategies for the long-term maintenance of stemness. Few pathways associated with stemness in SSC have been identified in the recent past. For example, the p38 MAPK pathway has a key role in maintaining the self-renewal in mouse mGSCs [185]. Myc-mediated glycolysis increases the SSC self-renewal and proliferation rate of germline stem cells [186]. Blocking of Ras/ERK1/2 pathway by MEK1/2 inhibitor (PD0325901) prevents the proliferation of goat SSCs and downregulated the ETV5 and BCL6B genes [187]. In mice, constitutive WNT signaling from the gonocytes stage onwards stimulates their proliferation, while forced WNT signaling in germ cells causes spermatocytes to undergo apoptosis [188]. SSCs cultured in the presence of BMP4 underwent differentiation (BMP4/Smad signaling pathway), characterized by downregulation of SSC self-renewal markers such as PLZF and upregulation of SSC differentiation marker like c-kit [189].
Several microRNAs, genes and proteins have been upregulated in testicular stem cells and regulating the pathways towards the maintenance of SSCs stemness (Table 1). MicroRNA (miR-224) overexpression increases the expression of SSCs markers such as PLZF and GFRα1 [190] and enhances the proliferation of cultured SSCs [191]. The pathway regulated by matrix mellatoprotein2 and mediated via Chd1l-miR-486-MMP2 regulatory axis increased β-catenin nuclear translocation and stemness gene expression [192]. So far the complete pathways involved in the maintenance of stemness and inducing differentiation remain elusive particularly in livestock. In-depth analysis on stemness regulators may help to alter the SSCs fate in terms of self-renewal, differentiation, or apoptosis.
Epigenetic modifications in SSCs
Many studies have demonstrated that epigenetic modification plays an important role in maintaining pluripotency of SSCs and its lineage differentiation [193, 194]. A previous study in mice has reported that embryonic stem cells (ESCs) cultured in commercial media gave rise to abnormalities in offspring, which may be due to alteration in imprinting genes [195]. It was predicted that SSCs grown culture may be more prone to epigenetic changes due to exposure to growth factors and media [196]; however, there is no experimental evidence to support the epigenetic changes in SSCs cultured in vitro. Both DNA methylation [197] and histone modification [198] are major changes reported during the isolation and expansion of SSCs. Histone modifications are involved in the differentiation of SSCs to more advanced sperm cells, but only during S-phase the canonical histone synthesis take place, which plays a key role during the cell cycle [199]. Many locus regions involved in pluripotency maintenance and function show a stage-specific differential methylation pattern [200]. Spermatogenesis can proceed with the transcription of certain DNA methylated promoters [201]. However, during spermiogenesis core histone proteins are replaced by transition proteins which are further replaced by protamines resulting in hyper-compaction of chromatin [202].
SSCs platforms: an inexorable technology for future
SSC platforms may play a pivotal role in the male fertility research in humans and for improving productive and reproductive performance in animal husbandry sectors. SSC researches on transgenesis, genomic-level modifications, and in vitro sperm production are the thrust areas in the near future. For the first time, transchromosomic mice were produced by the introduction of megabase-sized large DNA fragments into SSCs using the retro-microcell-mediated chromosome transfer method, with superior chromosomal stability when compared to embryonic stem cells [186]. In vitro sperm production may have significant implications in farm animal industries, human fertility restoration, preservation of endangered species, and many other technologies related to mammalian reproduction. The economics of animal management in the cattle industry can be reduced if renewable SSC pools from elite bulls produce high numbers of sperm from small biotechnological facilities [103].
Conclusion
Though SSC research started decades back, the pace of advancement of the technology is very slow, especially in mammals. In-depth knowledge on species-specific factors affecting stemness and differentiation of SSC is required for the development of the long-term culture system and in vitro spermatogenesis in livestock. The identification of stemness regulators that may alter the SSCs fate in terms of self-renewal, differentiation, or apoptosis in the culture system is very much essential. Epigenetic changes definitely can happen during the culture and differentiation of SSCs in vitro; however, the impact of such alterations on fertility needs to be investigated. The success rate of SSCs transplantation using the cultured and cryopreserved SSCs is low. In vitro differentiation of sperm from SSC culture though developed in rodent model remains a challenge in human and farm animals.
References
Hai Y, Hou J, Liu Y, Liu Y, Yang H, Li Z, He Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin Cell Dev Biol. 2014;29:66–75.
Chen SR, Liu YX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction. 2015;149(4):R159-67.
de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121(3):347. https://doi.org/10.1530/rep.0.1210347.
Thibier M, Wagner HG. World statistics for artificial insemination in cattle. Livest Prod Sci. 2002;74(2):203–12.
Giassetti MI, Ciccarelli M, Oatley JM. Spermatogonial stem cell transplantation: insights and outlook for domestic animals. Annu Rev Anim Biosci. 2019;7:385–401. https://doi.org/10.1146/annurev-animal-020518-115239.
Honaramooz A, Yang Y. Recent advances in application of male germ cell transplantation in farm animals. Vet Med Int. 2011;657860:1–9.
Abbasi H, Hosseini SM, Hajian M, Nasiri Z, Bahadorani M, Tahmoorespur M, Nasiri MR, Nasr-Esfahani MH. Lentiviral vector-mediated transduction of goat undifferentiated spermatogonia. Anim Reprod Sci. 2015;163:10–7 (https://www.sciencedirect.com/science/article/pii/S0378432015300130).
González R, Tang L, Dobrinski I. Application of spermatogonial transplantation in agricultural animals. In: Oatley J., Griswold M. (eds) The biology of mammalian spermatogonia. Springer, New York, NY; 2017. pp. 343–377. https://doi.org/10.1007/978-1-4939-7505-1_14.
Sahare MG, Suyatno Imai H. Recent advances of in vitro culture systems for spermatogonial stem cells in mammals. Reprod Med Biol. 2018;17(2):134–42. https://doi.org/10.1002/rmb2.12087.
Binsila BK, Selvaraju S, Somashekar L, Archana SS, Arangasamy A, Ravindra JP, Bhatta R. Molecular advances in semen quality assessment and improving fertility in bulls—a review. Indian J Anim Reprod. 2018;39:1–10 (https://www.researchgate.net/profile/Sarvpreet_Ghuman/publication/318453930_1_).
Kim Y, Selvaraj V, Dobrinski I, Lee H, Mcentee MC, Travis AJ. Recipient preparation and mixed germ cell isolation for spermatogonial stem cell transplantation in domestic cats. J Androl. 2006;27(2):248–56. https://doi.org/10.2164/jandrol.05034.
Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, Matoba S, Ogura A, Ogawa T. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2(1):472. 10.1038/ncomms1478.
Kurita K, Burgess SM, Sakai N. Transgenic zebrafish produced by retroviral infection of in vitro-cultured sperm. PNAS. 2004;101(5):1263–7 (www.pnas.orgcgidoi10.1073pnas.0304265101).
Giudice M, De Michele F, Poels J, Vermeulen M, Wyns C. Update on fertility restoration from prepubertal spermatogonial stem cells: how far are we from clinical practice? Stem Cell Res. 2017;21:171–7 (https://www.sciencedirect.com/science/article/pii/S1873506117300107).
Ciccarelli M, Giassetti MI, Miao D, Oatley MJ, Robbins C, Lopez-Biladeau B, Waqas MS, Tibary A, Whitelaw B, Lillico S, Park CH. Donor-derived spermatogenesis following stem cell transplantation in sterile NANOS2 knockout males. Proc Natl Acad Sci. 2020;117(39):24195–204.
Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–26 (https://www.sciencedirect.com/science/article/pii/S1934590912004754).
Oatley JM. Spermatogonial stem cell biology in the bull: development of isolation, culture, and transplantation methodologies and their potential impacts on cattle production. Soc Reprod Fertil Suppl. 2010;67:133–43 (https://pubmed.ncbi.nlm.nih.gov/21755668/).
Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. PNAS. 2004;101(47):16489–94 (www.pnas.orgcgidoi10.1073pnas.0407063101).
He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82(2):363–72 (https://academic.oup.com/biolreprod/article-abstract/82/2/363/2557980).
Valli H, Gassei K, Orwig KE. Stem cell therapies for male infertility: Where are we now and where are we going? In: Carrell D., Schlegel P., Racowsky C., Gianaroli L. (eds) Biennial review of infertility. Springer, Cham. 2015. pp. 17–39. https://doi.org/10.1007/978-3-319-17849-3_3.
Wu Y, Zhou H, Fan X, Zhang Y, Zhang M, Wang Y, Xie Z, Bai M, Yin Q, Liang D, Tang W. Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Res. 2015;25(1):67–79. https://doi.org/10.1038/cr.2014.160.
Goel S, Reddy N, Mandal S, Fujihara M, Kim SM, Imai H. Spermatogonia-specific proteins expressed in prepubertal buffalo (Bubalus bubalis) testis and their utilization for isolation and in vitro cultivation of spermatogonia. Theriogenology. 2010;74(7):1221–32 (https://www.sciencedirect.com/science/article/pii/S0093691X10002797).
Mahla RS, Reddy N, Goel S. Spermatogonial stem cells (SSCs) in buffalo (Bubalus bubalis) testis. PLoS One. 2012;7(4):e36020. https://doi.org/10.1371/journal.pone.0036020.
Pramod RK, Mitra A. in vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus). J Assist Reprod Genet. 2014;31(8):993–1001. https://doi.org/10.1007/s10815-014-0277-1.
Paul RK, Bahire SV, Kumar D. Isolation and biochemical characterization of ovine spermatogonial stem cells. Indian J Small Rumin (The). 2017;23(2):186. https://doi.org/10.5958/0973-9718.2017.00041.1.
Lee K, Lee W, Kim J, Yoon M, Kim N, Uhm S, Kim D, Chung H, Song H. Characterization of GFR α-1-positive and GFR α-1-negative spermatogonia in neonatal Pig testis. Reprod Domest Anim. 2013;48(6):954–60 (https://onlinelibrary.wiley.com/doi/abs/10.1111/rda.12193).
Sahare M, Otomo A, Komatsu K, Minami N, Yamada M, Imai H. The role of signaling pathways on proliferation and self-renewal of cultured bovine primitive germ cells. Reprod Med Biol. 2015;14(1):17–25. https://doi.org/10.1007/s12522-014-0189-x.
Zheng Y, Zhang Y, Qu R, He Y, Tian X, Zeng W. Spermatogonial stem cells from domestic animals: progress and prospects. Reproduction. 2014;147(3):R65–74. https://doi.org/10.1530/REP-13-0466.
Clermont Y. Spermatogenesis in man: a study of the spermatogonial population. Fertil Steril. 1966;17(6):705–21.
Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290(2):193–200. https://doi.org/10.1016/0027-5107(93)90159-d.
Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54(6):1192–9.
Paniagua R, Codesal J, Nistal M, Rodriguez MC, Santamaría L. Quantification of cell types throughout the cycle of the human seminiferous epithelium and their DNA content. J Anat Embryol. 1987;176(2):225–30.
Narenji Sani R, Tajik P, Yousefi MH, Movahedin M, Qasemi-Panahi B, Shafiei S, Ahmadi Hamedani M. Follicle stimulating hormone increases spermatogonial stem cell colonization during in vitro co-culture. Vet Res Forum. 2013;4(1):37–41 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4293895/).
Binsila KB, Selvaraju S, Ghosh SK, Parthipan S, Archana SS, Arangasamy A, Prasad JK, Bhatta R, Ravindra JP. Isolation and enrichment of putative spermatogonial stem cells from ram (Ovis aries) testis. Anim Reprod Sci. 2018;196:9–18 (https://www.sciencedirect.com/science/article/pii/S0378432017309430).
Han SY, Gupta MK, Uhm SJ, Lee HT. Isolation and in vitro culture of pig spermatogonial stem cell. Asian-Aust J AnimSci. 2009;22(2):187–93 (www.ajas.info).
Yang Y, Yarahmadi M, Honaramooz A. Development of novel strategies for the isolation of piglet testis cells with a high proportion of gonocytes. Reprod Fertil Dev. 2010;22(7):1057. https://doi.org/10.1071/RD09316.
Bryant JM, Meyer-Ficca ML, Dang VM, Berger SL, Meyer RG. Separation of spermatogenic cell types using STA-PUT velocity sedimentation. J Vis Exp. 2013;80:e50648 (https://www.jove.com/video/50648/separation-spermatogenic-cell-types-using-sta-put-velocity).
Bellvé AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113.
Liu Y, Niu M, Yao C, Hai Y, Yuan Q, Liu Y, Guo Y, Li Z, He Z. Fractionation of human spermatogenic cells using STA-PUT gravity sedimentation and their miRNA profiling. Sci Rep. 2015;5(1):1–1.
Bellve AR, Cavicchia JC, Millette CF, O’brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse isolation and morphological characterization. Int J Cell Biol. 1977;74(1):68–85.
Habas K, Brinkworth MH, Anderson D. Diethylstilbestrol induces oxidative DNA damage, resulting in apoptosis of spermatogonial stem cells in vitro. Toxicology. 2017;382:117–21.
Shah MA, Xu C, Wu S, Zhao W, Luo H, Yi C, Liu W, Cai X. Isolation and characterization of spermatogenic cells from cattle, yak and cattleyak. Anim Reprod Sci. 2018;193:182–90.
Chen X, Che D, Zhang P, Li X, Yuan Q, Liu T, Guo J, Feng T, Wu L, Liao M, He Z. Profiling of miRNAs in porcine germ cells during spermatogenesis. Reproduction. 2017;154(6):789–98.
Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279(1):114–24.
Ogawa T, Ohmura M, Tamura Y, Kita K, Ohbo K, Suda T, Kubota Y. Derivation and morphological characterization of mouse spermatogonial stem cell lines. Arch Histol Cytol. 2004;67(4):297–306. https://doi.org/10.1679/aohc.67.297.
Koruji M, Shahverdi A, Janan A, Piryaei A, Lakpour MR, Sedighi MA. Proliferation of small number of human spermatogonial stem cells obtained from azoospermic patients. J Assist Reprod Genet. 2012;29(9):957–67.
Bahadorani M, Hosseini SM, Abedi P, Hajian M, Afrough M, Azhdari Tafti Z, Azizi H, Hosseini SE, Vahdati A, Baharvand H, Nasr-Esfahani MH. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J Cytol Histol. 2011;2(6):126.
Bahadorani M, Hosseini SM, Abedi P, Hajian M, Afrough M, Azhdari Tafti Z, Azizi H, Hosseini SE, Vahdati A, Baharvand H, Nasr-Esfahani MH. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J Cytol istol. 2011;2(6):126.
Aponte PM, De Rooij DG. Biomanipulation of bovine spermatogonial stem cells. Anim Reprod (AR). 2018;5(1):16–22.
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Bühring HJ, Mattheus U, Mack A. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456(7220):344–9 (https://www.nature.com/articles/nature07404).
Huang YH, Chin CC, Ho HN, Chou CK, Shen CN, Kuo HC, Ling TY. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1-dependent pathway. FASEB J. 2009;23(7):2076–87. https://doi.org/10.1096/fj.08-121939.
Bai Y, Zhu C, Feng M, Wei H, Li L, Tian X, Zhao Z, Liu S, Ma N, Zhang X, Shi R. Previously claimed male germline stem cells from porcine testis are actually progenitor Leydig cells. Stem Cell Res Ther. 2018;9(1):1–5.
Jafarnejad A, Aminafshar M, Zandi M, Sanjabi MR, Kashan NE. Optimization of in vitro culture and transfection condition of bovine primary spermatogonial stem cells. S Afr J Anim Sci. 2018;48(1):108–16.
Wang J, Cao H, Xue X, Fan C, Fang F, Zhou J, Zhang Y, Zhang X. Effect of vitamin C on growth of caprine spermatogonial stem cells in vitro. Theriogenology. 2014;81(4):545–55.
Sharma A, Shah SM, Saini N, Mehta P, Kumar BB, Dua D, Singh MK, Singla SK, Palta P, Manik RS, Chauhan MS. Optimization of serum-free culture conditions for propagation of putative buffalo (Bubalus bubalis) spermatogonial stem cells. Cell Reprogram. 2019;21(1):1.
Aponte P, Soda T, Van De Kant H, de Rooij DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology. 2006;65(9):1828–47 (https://www.sciencedirect.com/science/article/pii/S0093691X05004498).
Aponte P, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136(5):543–57 (https://rep.bioscientifica.com/view/journals/rep/136/5/543.xml).
Giassetti MI, Goissis MD, de Barros FRO, Bruno AH, Assumpcao MEOA, Visintin JA. Comparison of diverse differential plating methods to enrich bovine spermatogonial cells. Reprod Domest Anim. 2016;51(1):26–32. https://doi.org/10.1111/rda.12641.
Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, Behzadi B. Isolation identification and culture of goat spermatogonial stem cells using c-kit and PGP95 markers. J Assist Reprod Genet. 2012;29(10):1029–38. https://doi.org/10.1007/s10815-012-9828-5.
Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod. 2007;77(1):127–37 (https://academic.oup.com/biolreprod/article-abstract/77/1/127/2629746).
Kim YH, Choi YR, Kim BJ, Jung SE, Kim SM, Jin JH, Yun MH, Kim SU, Kim YH, Hwang S, Pang MG. GDNF family receptor alpha 1 is a reliable marker of undifferentiated germ cells in bulls. Theriogenology. 2019;132:172–81.
Shinohara T, Avarbock MR, Brinster RL. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. PNAS. 1999;96(10):5504–9. https://doi.org/10.1073/pnas.96.10.5504.
Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod. 2011;85(2):347–56.
Guo Y, Hai Y, Gong Y, Li Z, He Z. Characterization, isolation, and culture of mouse and human spermatogonial stem cells. J Cell Physiol. 2014;229(4):407–13.
Zhou W, Shao H, Zhang D, Dong J, Cheng W, Wang L, Teng Y, Yu Z. PTEN signaling is required for the maintenance of spermatogonial stem cells in mouse, by regulating the expressions of PLZF and UTF1. Cell Biosci. 2015;5(1):1.
Gassei K, Valli H, Orwig KE. Whole-mount immunohistochemistry to study spermatogonial stem cells and spermatogenic lineage development in mice, monkeys, and humans. InStem Cells and Tissue Repair. New York, NY: Humana Press; 2014. p. 193–202.
Jung H, Roser JF, Yoon M. UTF1 a putative marker for spermatogonial stem cells in stallions. PLoS One. 2014;9(10):e108825.
Lee WY, Lee KH, Heo YT, Kim NH, Kim JH, Kim JH, Moon SH, Chung HJ, Yoon MJ, Song H. Transcriptional coactivator undifferentiated embryonic cell transcription factor 1 expressed in spermatogonial stem cells: a putative marker of boar spermatogonia. Anim Reprod Sci. 2014;150(3–4):115–24.
Fayomi AP, Orwig KE. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018;29:207–14.
Bedford-Guaus S, Kim S, Mulero L, Vaquero J, Morera C, Adan-Milanes R, Veiga A, Raya A. Molecular markers of putative spermatogonial stem cells in the domestic cat. Reprod Domest Anim. 2017;52:177–86. https://doi.org/10.1111/rda.12819.
Goel S, Fujihara M, Tsuchiya K, Takagi Y, Minami N, Yamada M, Imai H. Multipotential ability of primitive germ cells from neonatal pig testis cultured in vitro. Reprod Fertil Dev. 2009;21(5):696–708.
Borjigin U, Davey R, Hutton K, Herrid M. Expression of promyelocytic leukaemia zinc-finger in ovine testis and its application in evaluating the enrichment efficiency of differential plating. Reprod Fertil Dev. 2010;22(5):733–42 (http://www.publish.csiro.au/rd/RD09237).
Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, Chu T, Marshall GR, Orwig KE. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril. 2014;102(2):566-580e7. https://doi.org/10.1016/j.fertnstert.2014.04.036.
Herrid M, Davey RJ, Hutton K, Colditz IG, Hill JR. A comparison of methods for preparing enriched populations of bovine spermatogonia. Reprod Fertil and Dev. 2009;21(3):393–9 (http://www.publish.csiro.au/rd/RD08129).
Zheng Y, He Y, An J, Qin J, Wang Y, Zhang Y, Tian X, Zeng W. THY1 is a surface marker of porcine gonocytes. Reprod Fertil Deve. 2014;26(4):533. https://doi.org/10.1071/RD13075.
Reding SC, Stepnoski AL, Cloninger EW, Oatley JM. THY1 is a conserved marker of undifferentiated spermatogonia in the pre-pubertal bull testis. Reproduction. 2010;139(5):893–903. https://doi.org/10.1530/REP-09-0513.
Abbasi H, Tahmoorespur M, Hosseini S, Nasiri Z, Bahadorani M, Hajian M, Nasiri MR, Nasr-Esfahani MH. THY1 as a reliable marker for enrichment of undifferentiated spermatogonia in the goat. Theriogenology. 2013;80(8):923–32 (https://www.sciencedirect.com/science/article/pii/S0093691X13002914).
Wu J, Song W, Zhu H, Niu Z, Mu H, Lei A, Yang C, Peng S, Li X, Li G, Hua J. Enrichment and characterization of Thy1-positive male germline stem cells (mGSCs) from dairy goat (Capra hircus) testis using magnetic microbeads. Theriogenology. 2013;80(9):1052–60. https://doi.org/10.1016/j.theriogenology.2013.08.003.
Cai Y, Wang J, Zou K. The progresses of spermatogonial stem cells sorting using fluorescence-activated cell sorting. Stem Cell Rev Rep. 2020;16(1):94–102.
von Schönfeldt V, Krishnamurthy H, Foppiani L, Schlatt S, von Schönfeldt V, Krishnamurthy H, Foppiani L, Schlatt S. Magnetic cell sorting is a fast and effective method of enriching viable spermatogonia from djungarian hamster, mouse, and marmoset monkey Testes1. Biol Reprod. 1999;61(3):582–9. https://doi.org/10.1095/biolreprod61.3.582.
Zhang R, Sun J, Zou K. Advances in isolation methods for spermatogonial stem cells. Stem Cell Rev Rep. 2016;12(1):15–25.
Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97(15):8346–51. https://doi.org/10.1073/pnas.97.15.8346.
Kent Hamra F, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. PNAS. 2005;102(48):17430–5 (www.pnas.orgcgidoi10.1073pnas.0508780102).
Piravar Z, Jeddi-Tehrani M, Sadeghi MR, Mohazzab A, Eidi A, Akhondi MM. In vitro culture of human testicular stem cells on feeder-free condition. J Reprod Infertil. 2013;14(1):17–22 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3719359/).
Tiptanavattana N, Techakumphu M, Tharasanit T. Simplified isolation and enrichment of spermatogonial stem-like cells from pubertal domestic cats (Felis catus). J Vet Med Sci. 2015;77(11):1347–53. https://doi.org/10.1292/jvms.15-0207.
Scarpino S, Rita Morena A, Petersen C, Fröysa B, Söder O, Boitani C. A rapid method of Sertoli cell isolation by DSA lectin, allowing mitotic analyses. Mol Cell Endocrinol. 1998;146(1–2):121–7. https://doi.org/10.1016/S0303-7207(98)00190-7.
Bahadorani M, Hosseini S, Abedi P, Hajian M, Hosseini S, Vahdati A, Baharvand H, Nasr-Esfahani MH. Short-term in-vitro culture of goat enriched spermatogonial stem cells using different serum concentrations. J Assist Reprod Genet. 2012;29(1):39–46. https://doi.org/10.1007/s10815-011-9687-5.
Sharma V, Saini S, Aneja B, Kumar A, Thakur A, Bajwa KK, Kumar S, Mohanty AK, Malakar D. 180 Increasing GfrA1-positive spermatogonial stem cell population of goat. Reprod Fertil Dev. 2018;30(1):230. https://doi.org/10.1071/RDv30n1Ab180.
Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2005;69(2):612–6 (https://academic.oup.com/biolreprod/article-abstract/69/2/612/2713013).
Kim YH, Kang HG, Kim BJ, Jung SE, Karmakar PC, Kim SM, Hwang S, Ryu BY. Enrichment and in vitro culture of spermatogonial stem cells from pre-pubertal monkey testes. J Tissue Eng Regen Med. 2017;14(5):557–66. https://doi.org/10.1007/s13770-017-0058-x.
Ahmad S, Xiao Y, Han L, Hua H, Riaz H, Liang A, Yang LG. Isolation, identification and enrichment of type a spermatogonia from the testis of Chinese cross-bred buffaloes (swamp × river). Reprod Domest Anim. 2013;48(3):373–81. https://doi.org/10.1111/j.1439-0531.2012.02159.x.
Oatley MJ, Kaucher AV, Yang QE, Waqas MS, Oatley JM. Conditions for long-term culture of cattle undifferentiated spermatogonia. Biol Reprod. 2016;95(1):14–14. https://doi.org/10.1095/biolreprod.116.139832.
Yang Y, Honaramooz A. Efficient purification of neonatal porcine gonocytes with Nycodenz and differential plating. Reprod Fertil Dev. 2011;23(3):496–505. https://doi.org/10.1071/RD10042.
Zhu H, Liu C, Li M, Sun J, Song W, Hua J. Optimization of the conditions of isolation and culture of dairy goat male germline stem cells (mGSC). Anim Reprod Sci. 2013;137(1–2):45–52. https://doi.org/10.1016/j.anireprosci.2012.12.005.
Kitamura Y, Minami N, Yamada M, Imai H. 192 Culture conditions supporting long-term expansion of bovine spermatogonial stem cells isolated from adult and immature testes. Reprod Fertil Dev. 2018;30(1):236–236 (http://www.publish.csiro.au/rd/fulltext/RDv30n1Ab192).
Nasiri Z, Hosseini SM, Hajian M, Abedi P, Bahadorani M, Baharvand H, Nasr-Esfahani MH. Effects of different feeder layers on short-term culture of prepubertal bovine testicular germ cells In-vitro. Theriogenology. 2012;77(8):1519–28. https://doi.org/10.1016/j.theriogenology.2011.11.019.
Aponte PM, Van Bragt MPA, De Rooij DG, Van Pelt AMM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113(11–12):727–42. https://doi.org/10.1111/j.1600-0463.2005.apm_302.x.
Fujihara M, Kim S, Minami N, Yamada M, Imai H. Characterization and in vitro culture of male germ cells from developing bovine testis. J Reprod Dev. 2011;57(3):355–64 (https://www.jstage.jst.go.jp/article/jrd/advpub/0/advpub_10-185M/_article/-char/ja/).
Kubota H, Brinster RL. Transplantation and culture of spermatogonial stem cells. In The Biology of Mammalian Spermatogonia, Springer New York, NY. 2017; 271–300. https://doi.org/10.1007/978-1-4939-7505-1_11
Helsel A, Oatley MJ, Oatley JM. Glycolysis-optimized conditions enhance maintenance of regenerative integrity in mouse spermatogonial stem cells during long-term culture. Stem Cell Rep. 2017;8(5):1430–41 (https://www.sciencedirect.com/science/article/pii/S2213671117301108).
Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. PNAS. 2005;102(40):14302–7. https://doi.org/10.1073/pnas.0506970102.
Kanatsu-Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, Shinohara T. Serum-and feeder-free culture of mouse germline stem cells. Biol Reprod. 2011;84(1):97–105 (https://academic.oup.com/biolreprod/article-abstract/84/1/97/2530290).
Aponte PM. Spermatogonial stem cells: current biotechnological advances in reproduction and regenerative medicine. World J Stem Cells. 2015;7(4):669 (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc4444608/).
Medrano JV, Rombaut C, Simon C, Pellicer A, Goossens E. Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertil Steril. 2016;106(6):1539-1549.e8. https://doi.org/10.1016/j.fertnstert.2016.07.1065.
Oatley Jon M, de Avila DM, Reeves JJ, McLean DJ. success. Biol Reprod. 2004;70(3):625–31. https://doi.org/10.1095/biolreprod.103.022483.
Izadyar F, den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68(1):272–81 (https://academic.oup.com/biolreprod/article-abstract/68/1/272/2683752).
Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, Shinohara T. Improved serum-and feeder-free culture of mouse germline stem cells. Biol Reprod. 2014;91(4):88–1 (https://academic.oup.com/biolreprod/article-abstract/91/4/88, 1-11/2434297).
Binsila BK, Selvaraju S, Ghosh SK, Ramya L, Arangasamy A, Ranjithkumaran R, Bhatta R. EGF, GDNF, and IGF-1 influence the proliferation and stemness of ovine spermatogonial stem cells in vitro. J Assist Reprod Genet. 2020;37(10):2615–30.
Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009;138(4):721–31 (https://rep.bioscientifica.com/view/journals/rep/138/4/721.xml).
Tian R, Yang S, Zhu Y, Zou S, Li P, Wang J, Zhu Z, Huang Y, He Z, Li Z. VEGF/VEGFR2 signaling regulates germ cell proliferation in vitro and promotes mouse testicular regeneration in vivo. Cells Tissues Organs. 2016;201(1):1–13.
Yang F, Whelan EC, Guan X, Deng B, Wang S, Sun J, Avarbock MR, Wu X, Brinster RL. FGF9 promotes mouse spermatogonial stem cell proliferation mediated by p38 MAPK signalling. Cell Prolif. 2021;54(1):e12933.
Gharenaz NM, Movahedin M, Mazaheri Z. Three-dimensional culture of mouse spermatogonial stem cells using a decellularised testicular scaffold. Cell J (Yakhteh). 2020;21(4):410.
Tanaka T, Kanatsu-Shinohara M, Lei Z, Rao CV, Shinohara T. The luteinizing hormone-testosterone pathway regulates mouse spermatogonial stem cell self-renewal by suppressing WNT5A expression in Sertoli cells. Stem Cell Rep. 2016;7(2):279–91. https://doi.org/10.1016/j.stemcr.2016.07.005.
Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, Kim JK, Kim KS, Lee DR. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43(4):405–17. https://doi.org/10.1111/j.1365-2184.2010.00691.x.
Caires KC, de Avila J, McLean DJ. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction. 2009;138(4):667.
Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119(7):1001–12 (https://www.sciencedirect.com/science/article/pii/S0092867404010578).
Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288(1–2):95–103 (https://www.sciencedirect.com/science/article/pii/S0303720708001536).
Oatley Jon M, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282(35):25842–51. https://doi.org/10.1074/jbc.M703474200.
He Z, Jiang J, Kokkinaki M, Dym M. Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem Cells. 2009;27(10):2580–90.
Lord T, Oatley JM. Regulation of spermatogonial stem cell maintenance and self-renewal The Biology of Mammalian Spermatogonia. New York: Springer; 2017. p. 91–129. https://doi.org/10.1007/978-1-4939-7505-1_5.
Li L, Wang M, Wang M, Wu X, Geng L, Xue Y, Wei X, Jia Y, Wu X. A long non-coding RNA interacts with Gfra1 and maintains survival of mouse spermatogonial stem cells. Cell Death Dis. 2016;7(3): e2140. https://doi.org/10.1038/cddis.2016.24.
Kala S, Kaushik R, Singh KP, Kadam PH, Singh MK, Manik RS, Chauhan MS. in vitro culture and morphological characterization of prepubertal buffalo (Bubalus bubalis) putative spermatogonial stem cell. J Assist Reprod Genet. 2012;29(12):1335–42. https://doi.org/10.1007/s10815-012-9883-y.
Kadam P, Kala S, Agrawal H, Singh KP, Singh MK, Chauhan MS, Palta P, Singla SK, Manik RS. Effects of glial cell line-derived neurotrophic factor, fibroblast growth factor 2 and epidermal growth factor on proliferation and the expression of some genes in buffalo. Reprod Fertil Dev. 2013;25(8):1149–57 (http://www.publish.csiro.au/rd/rd12330).
Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113(1):29–39. https://doi.org/10.1016/S0925-4773(02)00004-7.
Abé K, Eto K, Abé SI. Epidermal growth factor mediates spermatogonial proliferation in newt testis. Reprod Biol Endocrinol. 2008;6(1):1–3.
Wang KM, Zhang DL, Mi YL, Zeng WD, Zhang CQ. Promoting effects of epidermal growth factor and prostaglandin E1 on the proliferation of mouse spermatogonia. Chin J Cell Biol. 2008;30:537–40.
Chen JX, Xu LL, Wang XC, Qin HY, Wang JL. Involvement of c-Src/STAT3 signal in EGF-induced proliferation of rat spermatogonial stem cells. Mol Cell Biochem. 2011;358(1):67–73.
Yu RJ, Xu SF. Mechanism of epidermal growth factor effects on spermatogonial stem cells. Life Sci Res. 2010;14(1):6–9 (http://en.cnki.com.cn/Article_en/CJFDTotal-SMKY201001004.htm).
Zhang DL, Mi YL, Wang KM, Zeng WD, Zhang CQ. Effects of follicle-stimulating hormone and epidermal growth factor on proliferation of mouse spermatogonial cells. Chinese J Cell Biol. 2007;29:565–8 (https://ncbi.nlm.nih.gov/pmc/articles/PMC4293895/).
Hu JH, Zhang WC, Wang P, Fan ZG, Jiang ZL, Li QW, Qiang Z. Effects of growth-promoting factors on proliferation of mouse spermatogonial stem cells SSCs in vitro. Afr J Biotechnol. 2012;11(14):3482–9. https://doi.org/10.5897/AJB10.1242.
Lee KH, Lee WY, Kim DH, Lee SH, Do JT, Park C, Kim JH, Choi YS, Song H. Vitrified canine testicular cells allow the formation of spermatogonial stem cells and seminiferous tubules following their xenotransplantation into nude mice. Sci Rep. 2016;6(1):21919. https://doi.org/10.1038/srep21919.
Wu Z, Falciatori I, Molyneux LA, Richardson TE, Chapman KM, Hamra FK. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing Rat spermatogonial stem cells. Biol Reprod. 2009;81(1):77–86. https://doi.org/10.1095/biolreprod.108.072645.
Sahare M, Kim SM, Otomo A, Komatsu K, Minami N, Yamada M, Imai H. Factors supporting long-term culture of bovine male germ cells. Reprod Fertil and Dev. 2016;28(12):2039. https://doi.org/10.1071/RD15003.
Lenhard T, Schober A, Suter-Crazzolara C, Unsicker K. Fibroblast growth factor-2 requires glial-cell-line-derived neurotrophic factor for exerting its neuroprotective actions on glutamate-lesioned hippocampal neurons. Mol Cell Neurosci. 2002;20(2):181–97.
Wang S, Wang X, Wu Y, Han C. IGF-1R signaling is essential for the proliferation of cultured mouse spermatogonial stem cells by promoting the G2/M progression of the cell cycle. Stem Cells Dev. 2015;24(4):471–83. https://doi.org/10.1089/scd.2014.0376.
Kuo YC, Au HK, Hsu JL, Wang HF, Lee CJ, Peng SW, Lai SC, Wu YC, Ho HN, Huang YH. IGF-1R promotes symmetric self-renewal and migration of alkaline phosphatase+ germ stem cells through HIF-2α-OCT4/CXCR4 loop under hypoxia. Stem Cell Rep. 2018;10(2):524–37 (https://www.sciencedirect.com/science/article/pii/S2213671117305519).
Qasemi Panahi B, Tajik P, Movahedin M, Moghaddam G, Geranmayeh MH. Study of insulin-like growth factor 1 effects on bovine type A spermatogonia proliferation and viability. Turk J Vet Anim Sci. 2014;38:693–8. https://doi.org/10.3906/vet-1402-70.
Bahadorani M, Hosseini SM, Abedi P, Abbasi H, Nasr-Esfahani MH. Glial cell line-derived neurotrophic factor in combination with insulin-like growth factor 1 and basic fibroblast growth factor promote in vitro culture of goat spermatogonial stem cells. Growth Factors. 2015;33(3):181–91. https://doi.org/10.3109/08977194.2015.1062758.
Yao J, Zuo H, Gao J, Wang M, Wang D, Li X. The effects of IGF-1 on mouse spermatogenesis using an organ culture method. Biochem Biophys Res Commun. 2017;491(3):840–7. https://doi.org/10.1016/j.bbrc.2017.05.125.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0.
Mirzapour T, Movahedin M, Tengku Ibrahim TA, Koruji M, Haron AW, Nowroozi MR, Rafieian SH. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44:41–55.
Rastegar T, Habibi Roudkenar M, Parvari S, Baazm M. The interaction between Sertoli cells and luekemia inhibitory factor on the propagation and differentiation of spermatogonial stem cells in vitro. Iran J Reprod Med. 2015;13(11):679–86.
Morimoto H, Yamamoto T, Miyazaki T, Ogonuki N, Ogura A, Tanaka T, Kanatsu-Shinohara M, Yabe-Nishimura C, Zhang H, Pommier Y, Trumpp A. An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia. Genes Dev. 2021;35(3–4):250–60.
Goodyear S, Brinster R. Culture and expansion of primary undifferentiated spermatogonial stem cells. Cold Spring Harbor Protocols. 2017; 2017(4): pdb-prot094193.
Shams A, Eslahi N, Movahedin M, Izadyar F, Asgari H, Koruji M. Future of spermatogonial stem cell culture: application of nanofiber scaffolds. Curr Stem Cell Res Ther. 2017;12(7):544–53. https://doi.org/10.2174/1574888X12666170623095457.
Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod. 2009;15(9):521–9. https://doi.org/10.1093/molehr/gap052.
Murdock MH, David S, Swinehart IT, Reing JE, Tran K, Gassei K, Orwig KE, Badylak SF. Human testis extracellular matrix enhances human spermatogonial stem cell survival in vitro. Tissue Eng - Part A. 2019;25(7–8):663–76. https://doi.org/10.1089/ten.tea.2018.0147.
Izadyar F, Matthijs-Rijsenbilt JJ, Den Ouden K, Creemers LB, Woelders H, De Rooij DG. Development of a cryopreservation protocol for type A spermatogonia. J Androl. 2002;23(4):537–45. https://doi.org/10.1002/j.1939-4640.2002.tb02276.x.
Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, Hutton K, Hope S, Hill JR. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81(5):898–905 (https://academic.oup.com/biolreprod/article-abstract/81/5/898/2557874).
Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20(6):1676–87.
Whaley D, Damyar K, Witek RP, Mendoza A, Alexander M, Lakey JR. Cryopreservation: an overview of principles and cell-specific considerations. Cell Transplant. 2021;30:0963689721999617.
Yong KW, Laouar L, Elliott JA, Jomha NM. Review of nonpermeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology. 2020;96:1–11.
Kim KJ, Lee YA, Kim BJ, Kim YH, Kim BG, Kang HG, Jung SE, Choi SH, Schmidt JA, Ryu BY. Cryopreservation of putative pre-pubertal bovine spermatogonial stem cells by slow freezing. Cryobiology. 2015;70(2):175–83 (https://www.sciencedirect.com/science/article/pii/S0011224015000358).
Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, Dobrinski I. Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev. 2009;21(3):489–97. https://doi.org/10.1071/rd08235.
Ha SJ, Kim BG, Lee YA, Kim YH, Kim BJ, Jung SE, Pang MG, Ryu BY. Effect of antioxidants and apoptosis inhibitors on cryopreservation of murine germ cells enriched for spermatogonial stem cells. PLoS One. 2016;11(8):e0161372.
Amidi F, Rashidi Z, Khosravizadeh Z, Khodamoradi K, Talebi A, Navid S, Abbasi M. Antioxidant effects of quercetin in freeze-thawing process of mouse spermatogonial stem cells. Asian Pac J Reprod. 2019;8(1):7.
Pukazhenthi BS, Nagashima J, Travis AJ, Costa GM, Escobar EN, França LR, Wildt DE. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PLoS One. 2015;10(4):e0123957.
Lee YA, Kim YH, Ha SJ, Kim KJ, Kim BJ, Kim BG, Choi SH, Kim IC, Schmidt JA, Ryu BY. Cryopreservation of porcine spermatogonial stem cells by slow-freezing testis tissue in trehalose. J Anim Sci. 2014;92(3):984–95.
Radaelli MR, Almodin CG, Minguetti-Câmara VC, Cerialli PM, Nassif AE, Gonçalves AJ. A comparison between a new vitrification protocol and the slow freezing method in the cryopreservation of prepubertal testicular tissue. JBRA Assist Reprod. 2017;21(3):188.
Han S, Zhao L, Yang C, Xu J, Yao C, Huang C, Zhang H, Ji Z, Luo J, Guo Y, Hong Y. Vitrification with microinjection of single seminiferous tubules: an efficient cryopreservation approach for limited testicular tissue. Reprod Biomed Online. 2021. https://doi.org/10.1016/j.rbmo.2021.06.026.
Bebbere D, Pinna S, Nieddu S, Natan D, Arav A, Ledda S. Gene expression analysis of ovine prepubertal testicular tissue vitrified with a novel cryodevice (E. Vit). J Assist Reprod Genet. 2019;36(10):2145–54.
Lima DB, da Silva TF, Aquino-Cortez A, Leiva-Revilla J, da Silva LD. Vitrification of testicular tissue from prepubertal cats in cryotubes using different cryoprotectant associations. Theriogenology. 2018;110:110–5.
Peng Z, Peng-fei H, Ya-guang T, He H, Gui-xue Z. Isolation, purification and cryopreservation of cells from neonatal bovine testis. J Northeast Agric. 2013;20(1):37–42.
Qasemi-Panahi B, Movahedin M, Moghaddam G, Tajik P, Koruji M, Ashrafi-Helan J, Rafat SA. Isolation and proliferation of spermatogonial cells from Ghezel sheep. Avicenna J Med Biotechnol. 2018;10(2):93.
Pan C, Yu S, Zhang P, Wang B, Zhu Z, Liu Y, Zeng W. Effect of sucrose on cryopreservation of pig spermatogonial stem cells. J Integr Agric. 2017;16(5):1120–9. https://doi.org/10.1016/S2095-3119(16)61489-2.
Zhu WQ, Cai NN, Jiang Y, Yang R, Shi JZ, Zhu CL, Zhang BY, Tang B, Zhang XM. Survivable potential of germ cells after trehalose cryopreservation of bovine testicular tissues. Cryobiology. 2021;101:105–14.
Jiang Y, Zhu WQ, Zhu XC, Cai NN, Yang R, Cai H, Zhang XM. Cryopreservation of calf testicular tissues with knockout serum replacement. Cryobiology. 2020;92:255–7.
Costa GM, Avelar GF, Lacerda SM, Figueiredo AF, Tavares AO, Rezende-Neto JV, Franca LR. Horse spermatogonial stem cell cryopreservation: feasible protocols and potential biotechnological applications. Cell Tissue Res. 2017;370(3):489–500. https://doi.org/10.1007/s00441-017-2673-1.
Kaneko H, Kikuchi K, Nakai M, Somfai T, Noguchi J, Tanihara F, Ito J, Kashiwazaki N. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PloS one. 2013;8(7):e70989.
Jung SE, Jin JH, Ahn JS, Kim YH, Yun MH, Kim SH, Kim BJ, Ryu BY. Effect of serum replacement on murine spermatogonial stem cell cryopreservation. Theriogenology. 2021;159:165–75.
Mahdi NS, Azarbani F, Pirnia A, Abbaszadeh A, Gholami M. The effect of caffeic acid on spermatogonial stem cell-type a cryopreservation. Rep Biochem Mol Biol. 2018;7(1):85.
Aliakbari F, Heidari M, Hossini MA, Hosseini J. Increasing of post-freezing quality of Spermatogonial Stem Cells after pretreatment by vitamin E. Men’s Health J. 2019;3(1):e1–e1.
Aliakbari F, Gilani MA, Amidi F, Baazm M, Korouji M, Izadyar F, Yazdekhasti H, Abbasi M. Improving the efficacy of cryopreservation of spermatogonia stem cells by antioxidant supplements. Cell Reprogram. 2016;18(2):87–95.
Shabani H, Zandi M, Ofogi H, Sanjabi MR, Pajooh KH. The effect of combining vitamin E and C on the viability improvement of transfected ovine spermatogonial stem cells after cryopreservation and thawing. Turk J Vet Anim Sci. 2017;41(5):648–55.
Jung S-E, Oh H-J, Ahn J-S, Kim Y-H, Kim B-J, Ryu B-Y. Antioxidant or apoptosis inhibitor supplementation in culture media improves post-thaw recovery of murine spermatogonial stem cells. Antioxidants. 2021;10(5):754. https://doi.org/10.3390/antiox10050754.
Feng TY, Li Q, Ren F, Xi HM, Lv DL, Li Y, Hu JH. Melatonin protects goat spermatogonial stem cells against oxidative damage during cryopreservation by improving antioxidant capacity and inhibiting mitochondrial apoptosis pathway. Oxid Med Cell Longev 2020. https://doi.org/10.1155/2020/5954635.
Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69(4):1260–4 (https://academic.oup.com/biolreprod/article-abstract/69/4/1260/2712666).
Oatley JM, de Avila DM, McLean DJ, Griswold MD, Reeves JJ. Transplantation of bovine germinal cells into mouse testes. J Anim Sci. 2002;80(7):1925–31. https://doi.org/10.2527/2002.8071925x.
Ciccarelli M, Giassetti MI, Miao D, Oatley MJ, Robbins C, Lopez-Biladeau B, Waqas MS, Tibary A, Whitelaw B, Lillico S, Park CH. Donor-derived spermatogenesis following stem cell transplantation in sterile NANOS2 knockout males. Proc Natl Acad Sci. 2020;117(39):24195–204.
Park KE, Kaucher AV, Powell A, Waqas MS, Sandmaier SE, Oatley MJ, Park CH, Tibary A, Donovan DM, Blomberg LA, Lillico SG, Whitelaw CB, Mileham A, Telugu BP, Oatley JM. Generation of germline ablated male pigs by CRISPR/Cas9 editing of the NANOS2 gene. Sci Rep. 2017;7(1):40176. https://doi.org/10.1038/srep40176.
Oatley JM. Recent advances for spermatogonial stem cell transplantation in livestock. Reprod Fertil Dev. 2018;30(1):44–9.
Mirzapour T, Tengku Ibrahim TA, Movahedin M, Nowroozi MR. Morphological and ultrastructural studies of human spermatogonial stem cells from patients with maturation arrest. Andrologia. 2017;49(7):e12700.
Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87(1):27–34.
Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS ONE. 2013;8(10):e77715. https://doi.org/10.1371/journal.pone.0077715.
Niu Z, Mu H, Zhu H, Wu J, Hua J. p38 MAPK pathway is essential for self-renewal of mouse male germline stem cells (mGSCs). Cell Prolif. 2017;50(1):e12314. https://doi.org/10.1111/cpr.12314.
Shinohara Takashi, Kazuki K, Ogonuki N, Morimoto H, Matoba S, Hiramatsu K, Honma K, Suzuki T, Hara T, Ogura A, Oshimura M, Kanatsu-Shinohara M, Kazuki Y. Transfer of a mouse artificial chromosome into spermatogonial stem cells generates transchromosomic mice. Stem Cell Rep. 2017;9(4):1180–91. https://doi.org/10.1016/j.stemcr.2017.08.012.
Niu Z, Zheng L, Wu S, Mu H, Ma F, Song W, Zhu H, Wu J, He X, Hua J. Ras/ERK1/2 pathway regulates the self-renewal of dairy goat spermatogonia stem cells. Reproduction. 2015;149(5):445–52.
Chassot AA, Le Rolle M, Jourden M, Taketo MM, Ghyselinck NB, Chaboissier MC. Constitutive WNT/CTNNB1 activation triggers spermatogonial stem cell proliferation and germ cell depletion. Dev Biol. 2017;426(1):17–27 (https://www.sciencedirect.com/science/article/pii/S0012160616304717).
Li Y, Zhang Y, Zhang X, Sun J, Hao J. BMP4/Smad signaling pathway induces the differentiation of mouse spermatogonial stem cells via upregulation of Sohlh2. Anat Rec. 2014;297(4):749–57. https://doi.org/10.1002/ar.22891.
Cui N, Hao G, Zhao Z, Wang F, Cao J, Yang A. MicroRNA-224 regulates self-renewal of mouse spermatogonial stem cells via targeting DMRT1. J Cell Mol Med. 2016;20(8):1503–12. https://doi.org/10.1111/jcmm.12838.
Li J, Liu X, Hu X, Tian GG, Ma W, Pei X, Wang Y, Wu J. MicroRNA-10b regulates the renewal of spermatogonial stem cells through Kruppel-like factor 4. Cell Biochem Funct. 2017;35(3):184–91. https://doi.org/10.1002/cbf.3263.
Liu SS, Maguire EM, Bai YS, Huang L, Liu Y, Xu L, Fauzi I, Zhang SQ, Xiao Q, Ma NF. A novel regulatory axis, CHD1L-MicroRNA 486-matrix metalloproteinase 2, controls spermatogonial stem cell properties. Mol Cell Biol, 2019; 39(4):e00357–18. https://doi.org/10.1128/MCB.00357-18.
Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7(7):540–6. https://doi.org/10.1038/nrm1938.
Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc B: Biol Sci. 2010;365(1546):1663–78. https://doi.org/10.1098/rstb.2010.0026.
Dean W. Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development. 1998;125(12):2273–82. https://dev.biologists.org/content/125/12/2273.short.
Bahadur G. Ethics of testicular stem cell medicine. Hum Reprod. 2004;19(12):2702–10. https://doi.org/10.1093/humrep/deh538.
Weber M, Schübeler D. Genomic patterns of DNA methylation: targets and function of an epigenetic mark. Curr Opin Cell Biol. 2007;19(3):273–80. 10.1016/j.ceb.2007.04.011.
Lee J, Shinohara T. Epigenetic modifications and self-renewal regulation of mouse germline stem cells. Cell Res. 2011;21(8):1164. https://www.nature.com/articles/cr2011111.
Venkatesh S, Workman JL. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol. 2015;16(3):178–89. https://doi.org/10.1038/nrm3941.
Kubo N, Toh H, Shirane K, Shirakawa T, Kobayashi H, Sato T, Sone H, Sato Y, Tomizawa SI, Tsurusaki Y, Shibata H. DNA methylation and gene expression dynamics during spermatogonial stem cell differentiation in the early postnatal mouse testis. BMC Genomics. 2015;16(1):624. https://doi.org/10.1186/s12864-015-1833-5.
Hammoud SS, Low DH, Yi C, Carrell DT, Guccione E, Cairns BR. Chromatin and transcription transitions of mammalian adult germline stem cells and spermatogenesis. Cell Stem Cell. 2014;15(2):239–53. https://doi.org/10.1016/j.stem.2014.04.006.
Bao JQ, Bedford MT. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction. 2016;151(5):55–70 (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc4896072/).
Liu SS, Bai YS, Feng L, Dong WW, Li Y, Xu LP, Ma NF. Identification of CHD1L as an important regulator for spermatogonial stem cell survival and self-renewal. Stem cells Int. 2016;4069543:1–12. https://doi.org/10.1155/2016/4069543.
Niu Z, Goodyear SM, Rao S, Wu X, Tobias JW, Avarbock MR, Brinster RL. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. PNAS. 2011;108(31):12740–5. https://doi.org/10.1073/pnas.1109987108.
Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011;121(9):3456–66.
Yamaji M, Jishage M, Meyer C, Suryawanshi H, Der E, Yamaji M, Garzia A, Morozov P, Manickavel S, McFarland HL, Roeder RG. DND1 maintains germline stem cells via recruitment of the CCR4–NOT complex to target mRNAs. Nature. 2017;543(7646):568–72.
Niimi Y, Imai A, Nishimura H, Yui K, Kikuchi A, Koike H, Saga Y, Suzuki A. Essential role of mouse Dead end1 in the maintenance of spermatogonia. Dev Biol. 2019;445(1):103–12.
Suzuki A, Niimi Y, Shinmyozu K, Zhou Z, Kiso M, Saga Y. Dead end1 is an essential partner of NANOS 2 for selective binding of target RNAs in male germ cell development. EMBO reports. 2016;17(1):37–46.
Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436(7053):1030–4.
Zheng L, Zhai Y, Li N, Ma F, Zhu H, Du X, Li G, Hua J. The modification of Tet1 in male germline stem cells and interact with PCNA, HDAC1 to promote their self-renewal and proliferation. Sci Rep. 2016;6:37414.
Zhou W, Shao H, Zhang D, Dong J, Cheng W, Wang L, Teng Y, Yu Z. PTEN signaling is required for the maintenance of spermatogonial stem cells in mouse, by regulating the expressions of PLZF and UTF1. Cell Biosci. 2015;5(1):42.
Raju P, Nyamsuren G, Elkenani M, Kata A, Tsagaan E, Engel W, Adham IM. Pelota mediates gonocyte maturation and maintenance of spermatogonial stem cells in mouse testes. Reproduction. 2015;149(3):213–21.
Hu K, Zhang J, Liang M. LncRNA AK015322 promotes proliferation of spermatogonial stem cell C18–4 by acting as a decoy for microRNA-19b-3p. In Vitro Cell Dev Biol Anim. 2017;53(3):277–84.
Zhou F, Yuan Q, Zhang W, Niu M, Fu H, Qiu Q, Mao G, Wang H, Wen L, Wang H, Lu M. MiR-663a stimulates proliferation and suppresses early apoptosis of human spermatogonial stem cells by targeting NFIX and regulating cell cycle. Mol Ther-Nucleic Acids. 2018;12:319–36.
Fu H, Zhang W, Yuan Q, Niu M, Zhou F, Qiu Q, Mao G, Wang H, Wen L, Sun M, Li Z. PAK1 promotes the proliferation and inhibits apoptosis of human spermatogonial stem cells via PDK1/KDR/ZNF367 and ERK1/2 and AKT pathways. Mol Ther-Nucleic Acids. 2018;12:769–86.
He Z, Jiang J, Kokkinaki M, Tang L, Zeng W, Gallicano I, Dobrinski I, Dym M. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells. 2013;31(10):2205–17.
Ma F, Zhou Z, Li N, Zheng L, Wu C, Niu B, Tang F, He X, Li G, Hua J. Lin28a promotes self-renewal and proliferation of dairy goat spermatogonial stem cells (SSCs) through regulation of mTOR and PI3K/AKT. Sci Rep. 2016;6:38805.
Ma F, Du X, Wei Y, Zhou Z, Clotaire DZJ, Li N, Peng S, Li G, Hua J. LIN28A activates the transcription of NANOG in dairy goat male germline stem cells. J Cell Physiol. 2019;234(6):8113–21.
Zheng L, Zhu H, Tang F, Mu H, Li N, Wu J, Hua J. The Tet1 and histone methylation expression pattern in dairy goat testis. Theriogenology. 2015;83(7):1154–61.
Mu H, Li N, Wu J, Zheng L, Zhai Y, Li B, Song W, Wang J, Zhu H, Li G, Hua J. PLZF-induced upregulation of CXCR4 promotes dairy goat male germline stem cell proliferation by targeting Mir146a. J Cell Biochem. 2016;117(4):844–52.
Song W, Mu H, Wu J, Liao M, Zhu H, Zheng L, He X, Niu B, Zhai Y, Bai C, Lei A. miR-544 regulates dairy goat male germline stem cell self-renewal via targeting PLZF. J Cell Biochem. 2015;116(10):2155–65.
Zhu H, Zheng L, Wang L, Tang F, Hua J. MiR-302 enhances the viability and stemness of male germline stem cells. Reprod Domest Anim. 2018;53(6):1580–8.
Lee R, Lee WY, Park HJ, Ha WT, Woo JS, Lee JH, Hong K, Song H. Stage-specific expression of DDX4 and c-kit at different developmental stages of the porcine testis. Anim Reprod Sci. 2018;190:18–26.
Sharma M, Srivastava A, Fairfield HE, Bergstrom D, Flynn WF, Braun RE. Identification of EOMES-expressing spermatogonial stem cells and their regulation by PLZF. Elife. 2019;8:e43352.
Acknowledgements
The authors thank the Director, ICAR-National Institute of Animal Nutrition and Physiology, Indian Council of Agricultural Research, Ministry of Agriculture, Govt. of India, Bengaluru, India, for providing the necessary facilities to undertake the work. This research work is supported by the DST-SERB funded project (ECR/2018/001304), Department of Science and Technology, Government of India. Dr. S. Selvaraju is supported by ICAR-National Fellow Project, ICAR, Ministry of Agriculture, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Binsila, B., Selvaraju, S., Ranjithkumaran, R. et al. Current scenario and challenges ahead in application of spermatogonial stem cell technology in livestock. J Assist Reprod Genet 38, 3155–3173 (2021). https://doi.org/10.1007/s10815-021-02334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02334-7