Abstract
Purpose
To investigate the impact of a 3-month course of intracortical injections of autologous platelet-rich plasma (PRP) upon ovarian reserve markers versus no intervention in women with low ovarian reserve prior to undergoing assisted reproductive technology (ART).
Methods
Prospective controlled, non-randomized comparative study conducted in a private fertility clinic, in Venezuela. Women with abnormal ovarian reserve markers (FSH, AMH and AFC) who declined oocyte donation were allocated to one of the following groups according to patient choice: monthly intracortical ovarian PRP injections for three cycles, or no intervention. Primary outcomes were the change in FSH, AMH and AFC pre- and post-treatment. Secondary outcomes included the number of oocytes collected and fertilized, biochemical/clinical pregnancy rates and miscarriage and live birth rates.
Results
Eighty-three women were included, of which 46 received PRP treatment and 37 underwent no intervention. Overall median age was 41 years (IQR 39–44). There were no demographic differences between the study groups. At the 3-month follow-up, women treated with PRP experienced a significant improvement in FSH, AMH and AFC, whereas there was no change in the control group. Furthermore, overall rates of biochemical (26.1% versus 5.4%, P = 0.02) and clinical pregnancy (23.9% versus 5.4%, P = 0.03) were higher in the PRP group, while there was no difference in the rates of first trimester miscarriage and live birth between groups.
Conclusion
PRP injections are effective and safe to improve markers of low ovarian reserve prior to ART, although further evidence is required to evaluate the impact of PRP on pregnancy outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female age remains by far the principal limiting factor of success in both spontaneous conception and assisted reproductive technology (ART), largely due to a loss of ovarian follicle reserve and oocyte quality as women become older [1]. Indeed, the total number of oocytes in the developing female fetus peaks at 6 million in the second trimester of gestation and steadily declines thereafter [2]. At birth, both ovaries contain 1–2 million oocytes, and more than half will undergo atresia before a woman reaches puberty [3, 4]. The rate of follicle degeneration increases after the age of 37 years, and only 1000 oocytes, on average, are present at the time of the menopause [1, 5]. Alongside a reduction in numbers, aging oocytes are also more prone to errors in DNA synthesis and cell division, resulting in increased rates of aneuploidy and congenital defects in the progeny of older women [6].
There is no known effective treatment to prevent, delay or reverse ovarian senescence. Environmental factors such as cigarette smoking [7, 8], dietary habits [9] and exposure to chemo and radiotherapy [10] are known to irreversibly reduce oocyte numbers and quality, mainly via the excessive production of reactive oxygen species (ROS) [11]. Antioxidant dietary supplements containing vitamins C and E [12, 13], melatonin [14], dehydroepiandrosterone (DHEA) and coenzyme Q10 [15, 16] have thus been used in reproductive medicine to reduce oxidative stress and improve ovarian reserve, but evidence attesting to their overall effectiveness remains sparse, and meta-analyses have been inconclusive [17, 18].
Over the past two decades, regenerative medicine has benefited from significant advances in the field of tissue engineering [19]. The use of platelets in particular has been shown promising due to their role in triggering cell proliferation and tissue differentiation [20]. When activated by external stimuli such as haemorrhage and tissue damage, platelets release multiple bioactive molecules and growth factors that induce clotting, inflammation, neovascularization and local tissue repair [20, 21]. The healing properties attributed to platelet function have led to the use of platelet-rich plasma (PRP), a concentrate derived from centrifuged whole blood with platelet concentrations up to seven times higher than circulating serum, in regenerative medicine [22]. It has been postulated that the heightened regenerative properties of PRP may be explained by higher concentrations of growth factors such as transforming growth factor-β, insulin-like growth factors 1 and 2 (IGF-1 and IGF-2), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor and hepatocyte growth factor (HGF) [20, 22, 23].
In vitro and clinical studies have investigated the applicability of PRP as a therapeutic agent in nerve injuries [24], myocardial infarction [25], cosmetic surgery [26, 27] and eye disease [28]. Furthermore, PRP has been increasingly utilized in sports medicine to treat ligament and tendon lesions due to its association with shortened recovery times and improved functional outcomes, but there is a paucity of good-quality scientific evidence demonstrating its effectiveness [29, 30].
Very few studies have investigated the potential applicability of PRP in ovarian tissue regeneration [31]. Bakacak et al. demonstrated a significant effect of PRP in preventing ischemia and reperfusion damage in rats following bilateral adnexal torsion and surgical detorsion, mainly through an increase in VEGF [32]. Other small case series evaluating the role of PRP in women with a thin endometrium [33], recurrent implantation failure [34] and poor response to controlled ovarian stimulation [35] have since been published with encouraging results, but there have been no controlled clinical studies investigating the effectiveness of PRP in women with low ovarian reserve. It is thought that PRP may be beneficial in delaying follicle atresia and oocyte degeneration, but conclusive evidence is lacking.
This study aimed to evaluate the effectiveness of PRP compared with no intervention in women with known low ovarian reserve prior to undergoing ART.
Materials and methods
Study design
This prospective non-randomized comparative pilot study was conducted between February 2015 and February 2018 in a private fertility clinic in Caracas, Venezuela. We included women who fulfilled the following criteria prior to undergoing ART: (i) female age 38 years old and above, (ii) baseline follicle-stimulating hormone (FSH, day 3 of the menstrual cycle) > 12 mIU/mL, (iii) anti-Müllerian hormone (AMH) < 0.8 ng/mL and (iv) normal uterine cavity as demonstrated by recent hysteroscopy. The following exclusion criteria were applied: (i) previous history of pelvic inflammatory disease, (ii) known clinical/biochemical hyperandrogenism or polycystic ovaries, (iii) tubal factor infertility, (iv) endometriosis, (v) known platelet or thromboxane synthesis disorder and (vi) known severe male factor infertility.
Ethical approval
This study was designed, conducted and reported in accordance with the principles of Good Clinical Practice guidance and with the 1964 Helsinki declaration and its later amendments. Prospective ethical approval was granted by a local Institutional Review Board (IRB) and the Venezuelan Health Ministry (IRB reference number #0940), and written consent was obtained from all participants.
Patient allocation
Women planning to undergo fertility treatment (timed intercourse, IUI or IVF/ICSI), who fulfilled the inclusion criteria, were initially informed about the trial and allocated to one of the following groups according to patient choice: ovarian injection with autologous PRP or no intervention. Baseline antral follicle count (AFC) on transvaginal ultrasound and serum levels of FSH and AMH were obtained from all participants on day 3 of menstrual cycle 1.
Ovarian PRP injection
Participants who opted for PRP injections received treatment once between days 7 and 9 of the menstrual cycle for three consecutive cycles (cycles 1, 2 and 3). The decision to undertake three treatment cycles derived from the knowledge that antral follicle development takes approximately 90 to 120 days. We postulated that repeated platelet stimulation would maximise the number of growing follicles exposed to the intervention.
Platelet-rich plasma was initially obtained from whole blood collected on the day of injection. A total of 5 blood collection tubes containing sodium citrate 3.8% were filled with 4.5 mL of blood each and centrifuged at 270g for 10 min. Following centrifugation, 100 μL of the platelet-rich supernatant were transferred from each of 4 of the original blood tubes and mixed with 0.1 mL of 10% calcium chloride. The blood in the remaining fifth tube was not mixed with calcium chloride to allow for quantification of the total number of platelets in the sample.
On the day of blood collection (i.e. day 7, 8 or 9 of the cycle), 200 μL of PRP were injected into the cortex of each ovary using a single lumen aspiration needle (Cook Medical, USA) under transvaginal ultrasound guidance and sedation. Each ovary was punctured once only, with the single lumen needle being inserted into the ovarian cortex superficially, and a total of 200-μL PRP injected into the subcortical area of the ovary.
FSH, AMH and AFC measurements were repeated on day 3 of the cycle following the last round of treatment (i.e. cycle 4) and compared with pre-treatment results (i.e. cycle 1). Following the completion of treatment with PRP, participants were advised to undergo IVF/ICSI, IUI or timed intercourse as soon as the next menstrual cycle started.
Controls
Women who volunteered to participate in the study but declined to receive treatment with PRP were allocated to the control group, in whom no intervention was carried out apart from measuring ovarian reserve parameters in cycles 1 and 4.
Conception
Women in both groups were followed up for a total of 12 months while undergoing subsequent ART. Details of fertility treatment following participation in the study were gathered.
IVF and embryo transfer were carried out following a short GnRH-antagonist protocol. Specifically, all patients received recombinant FSH (rFSH) from day 3 of the cycle, with doses varying from 225 to 300 IU, in addition to 75 to 150 IU of human menopausal gonadotrophin (hMG) for 10–12 days, depending on age, BMI, basal FSH and antral follicle count. Furthermore, cetrorelix acetate (Cetrotide®, Merck KGaA, Darmstadt, Germany) was administered subcutaneously at a dose of 0.25 mg once daily starting from days 5 to 7 of the cycle, according to follicle size on ultrasound, to prevent premature ovulation, until the day of hCG injection. As soon as one or more follicles measuring ≥ 17 mm were identified on transvaginal ultrasound, a fixed dose of recombinant human chorionic gonadotrophin (rhCG) 500 mcg (Ovidrel®, Merck KGaA, Darmstadt, Germany) or 5000 IU (Pregnyl®, MSD, Brussels, Belgium) was administered subcutaneously to induce oocyte maturation. Oocyte collection was performed 36–37 h post-rhCG, and 1 or 2 embryos were transferred between days 2 and 5 depending on patient response and embryo quality.
The local IUI protocol entailed the administration of 100 to 150 IU of rFSH and 75 IU of hMG from day 3 of the cycle for 10–12 days. Once one or more follicles measuring ≥ 17 mm were observed on ultrasound, a fixed dose of rhCG 250 mcg (Ovidrel®, Merck KGaA, Darmstadt, Germany) or 5000 IU (Pregnyl®, MSD, Brussels, Belgium) was used to induce oocyte maturation. IUI was carried out 42 h after rhCG injection, followed by a second insemination 24 h later.
All participants undergoing timed intercourse received 75 IU of rFSH for 5–10 days. Where no follicles measuring ≥ 17 mm were visualized with transvaginal ultrasound following rFSH injection, a further 75 IU of HMG for 5–10 days was administered. As soon as one of more follicles measuring ≥ 17 mm were identified, ovulation was triggered with rHCG 250 mcg (Ovidrel®, Merck KGaA, Darmstadt, Germany) or 5000 IU (Pregnyl®, MSD, Brussels, Belgium), followed by sexual intercourse over a period of 3 days.
Outcome variables
The primary outcome variables were the AFC (defined as all follicles measuring 3–8 mm) on repeat transvaginal ultrasound and the serum levels of FSH and AMH as a measure of ovarian reserve. A single operator (CN) performed the ultrasound assessment of AFC pre- and post-intervention (i.e. in cycles 1 and 4, respectively), using the same equipment. All samples containing baseline ovarian reserve markers prior to treatment were frozen and re-analysed in cycle 4 (i.e. after completion of the three treatment cycles in the PRP group) using the same assay to avoid clinician, operator and assessor bias. AMH quantification was performed using the Ultra-Sensitive AMH/MIS ELISA assay AL-105-I (Ansh Labs, Texas, USA), and FSH quantification was carried out with a Reactiva Search FSH kit (One Global Search, Florida, USA).
Secondary outcomes included number of oocytes collected and fertilization rates during IVF/ICSI; rates of biochemical (diagnosed by the detection of beta hCG in serum [> 5 mIU/mL] or urine), clinical (diagnosed by ultrasonographic visualization of ≥ 1 gestational sac) and ongoing (12 weeks’ gestation and above) pregnancy per participant; and rates of first trimester miscarriage (< 12 completed weeks’ gestation) and live birth (≥ 24 completed weeks’ gestation) per participant.
Data collection and statistical analysis
Data were recorded prospectively on all participants. Details of pregnancy outcomes were obtained from hospital obstetric records.
Continuous variables were assessed for normality with the Shapiro-Wilk test, and results were expressed as the median and interquartile range (IQR) or range. A Mann-Whitney U test was used to assess for differences in continuous variables between the two groups, whereas a Wilcoxon signed-rank test was used to establish univariate comparisons before and after treatment with PRP or no intervention within the same group. For categorical data, significant differences were identified with a chi-square (χ2) test or Fisher’s exact test.
Differences were considered significant where P < 0.05. The statistical software package SPSS 22.0 (IBM, Chicago, IL) was used for the analyses of all data.
Results
Study population
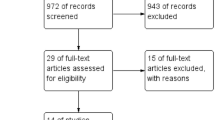
Figure 1 depicts details of patient enrolment in the study, allocation, fertility treatment (IVF/ICSI versus timed intercourse/IUI) and pregnancy outcomes. A total of 120 women were assessed for eligibility. Of these, 15 declined to participate in the study and 22 were excluded due to the following reasons: severely abnormal semen analysis in the male partner, tubal factor infertility, and successful spontaneous conception prior to undergoing treatment. Of the 83 women included in this comparative analysis, 46 underwent treatment with PRP and 37 were subjected to no intervention.
Demographic characteristics, baseline ovarian reserve markers and ultrasound findings of women included in the analysis were similar among the treatment arms and are shown in Table 1. In particular, no significant age difference was identified between the study arms (median age overall 41 years, IQR 39–44) (P = 0.78). No patients were lost to follow-up.
Ovarian reserve parameters
Women treated with PRP had the most significant improvement in biochemical and ultrasound markers of ovarian reserve compared with the control group (Table 2). Notably, AMH levels were on average 63% higher following PRP (P < 0.001) compared with no significant change in the control group (P = 0.15). FSH levels dropped by 33% in the group receiving PRP (P < 0.001) and remained the same in controls (P = 0.23). Finally, there were on average 75% more antral follicles on ultrasound following PRP (P < 0.001), while there was no change in controls at the 3-month follow-up (P = 0.1).
Cycle characteristics
Of the 40 women who underwent IVF/ICSI following participation in the study (Table 3), those with previous PRP treatment yielded on average more than 1.5× the number of oocytes collected in the control group (P < 0.001). The median number of fertilized oocytes and the fertilization rate did not differ between groups (P = 0.38 and P = 0.51, respectively). Nevertheless, the rate of medium- and top-quality embryos in participants who received PRP treatment was significantly higher than in controls (100% versus 55% respectively, P = 0.03). There were no differences between groups in the number of embryos transferred (P = 0.96) and the day of embryo transfer (P = 0.28).
Pregnancy outcomes
An overall comparison of pregnancy outcomes between the two study arms is presented in Table 4. Treatment with PRP was significantly linked with higher biochemical (P = 0.02) and clinical pregnancy rates (P = 0.03), although the rates of first trimester miscarriage and live birth did not differ between treatment groups. A subgroup analysis according to ART modality (timed intercourse/IUI versus IVF/ICSI) did not identify any differences in pregnancy outcomes between those who had previously been treated with PRP and those who had undergone no intervention (Table 5).
Adverse events associated with PRP
There were no significant complications such as allergic reactions, intraabdominal haemorrhage, bowel or bladder injury or infection following treatment with PRP. There were no cases of ovarian hyperstimulation syndrome (OHSS) following IVF/ICSI.
Discussion
This non-randomized controlled pilot study compared the effect of PRP versus no intervention upon ovarian reserve parameters in women with low ovarian reserve prior to ART. Our findings showed that a 3-month treatment course with PRP improved ovarian reserve markers when compared with no intervention. In addition, the use of PRP was associated with a significant increase in biochemical and clinical pregnancy rates. Nevertheless, our data revealed no effect of PRP on the rates of oocyte fertilization in IVF/ICSI, miscarriage and live birth.
The paradigm of inevitable ovarian senescence has long been based on evidence that the female gonads lose their ability to generate new oocytes prior to birth. This long-standing belief postulates that a finite number of oocytes in females of reproductive age are arrested in meiosis I and surrounded by a single layer of squamous pre-granulosa cells forming a primordial follicle [36]. A series of complex bidirectional signalling pathways between the surrounding ovarian tissue and the oocyte involving molecules such as TGF-β, PDGF and IGF-1 lead to the recruitment of some primordial follicles which develop into primary, secondary and antral follicles during the female reproductive years [23, 36,37,38]. While most growing follicles undergo apoptosis and atresia, ~ 400 will reach full development and release mature oocytes throughout the course of a woman’s reproductive life [39]. Once the pool of primordial follicles is exhausted, folliculogenesis comes to a halt and women enter the menopause, usually after 50 years [40, 41]. When the depletion of primordial follicles occurs earlier than 40 years, a loss of ovarian function ensues, leading to premature ovarian insufficiency (POI), a condition affecting 1% of women [42].
Recent studies have introduced the concept of neo-oogenesis by demonstrating that contrary to previously believed, it is possible to obtain mitotically active germ cells from healthy adult ovarian tissue in mice and humans [43, 44]. While our data suggest a resurgence of ovarian activity following the injection of PRP in women older than 40 years, the pathways through which concentrated platelets improve ovarian reserve remain unclear. Mechanistic studies to elucidate the biochemical actions of PRP are lacking, but we speculate that the high levels of PDGF, TGF-β, IGF-1/2, VEGF and EGF identified in platelet concentrates [23, 45] are likely to play a significant role in stimulating the development of pre-antral follicles during the three cycles of treatment, leading to an increase in circulating levels of AMH and in the number of antral follicles generated per menstrual cycle.
The decision to undertake three cycles of treatment with PRP was based on the notion that follicle development takes on average 90 to 120 days from the time of primordial follicle recruitment to the final stages of antral development, when a follicle either becomes atretic or releases an oocyte at the time of ovulation [40, 41]. Crucially, however, the resurgence of ovarian activity following PRP in this cohort is unlikely to do with increased recruitment of primordial follicles, as it would have taken well over 3 months for these to become hormone-sensitive [46]. Instead, we postulate that PRP is likely to stimulate the development of existing pre-antral follicles or prevent atresia. Moreover, we did not demonstrate a higher fertilisation rate following treatment with PRP in women undergoing IVF/ICSI, suggesting that higher numbers do not necessarily translate into better oocyte quality. Still, a higher proportion of medium- or top-quality embryos were created in the intervention group compared with controls, indicating that there may be a positive effect of PRP on embryo development following fertilisation. Nevertheless, the small number of participants precludes us from drawing a relationship of causality between PRP treatment and embryo quality, and larger randomized controlled trials are required to validate our findings.
This is, to our knowledge, the first prospective trial investigating the impact of PRP on ovarian reserve and pregnancy outcomes in women with known low ovarian reserve. Previous research focused on the use of PRP in reducing ischaemia-reperfusion injury in rat ovaries [32], and the endometrial effects of platelet-derived products in humans. The use of intrauterine G-CSF alone has indeed been trialled in unselected women undergoing IVF, and no significant difference was identified in endometrial thickness following the administration of G-CSF versus placebo [47]. A subsequent study demonstrated a significant increase in endometrial thickness and implantation rates in women with a thin endometrium following intrauterine injection of PRP, although with unclear statistical significance [48]. More recently, a small case series of four patients with low ovarian reserve revealed a significant improvement in ovarian function in those who received intraovarian PRP [49].
There have been no studies on the long-term effects of PRP in older women. It would be useful to elucidate the systemic effect of PRP, particularly on circulating estradiol levels, and to investigate whether there are potential benefits on cardiovascular and bone health. Cohort studies are hence required to ascertain whether there is a long-term rise in estradiol following PRP and its potential impact on hypoestrogenic women.
The prospective nature of our study, involving data collected over 2 years, is its main strength. Women were followed individually from recruitment to the time of ART, and, where applicable, detailed pregnancy outcomes were recorded. In addition, the comparative design allowed for a robust assessment of the efficacy of PRP versus a control group with similar demographic baseline characteristics.
The main limitation of this study is that it was non-randomized. Women were assigned to the study groups on a voluntary basis after receiving information on the evidence about the use of PRP in sports and regenerative medicine. This may have been a significant source of selection bias, owing to participants of a higher socio-economic status potentially being more likely to request a self-funded intervention than opting to be allocated to the control group. Overall, however, there were no significant differences in baseline characteristics between the study groups. Another limitation of this study is that our numbers were low, and thus likely insufficient to detect potentially relevant effects on pregnancy outcomes including clinical pregnancy rate, miscarriage rate and live birth rate. Adequately powered randomized parallel studies, with long-term follow-up data, are required to evaluate the continuing impact of PRP on ovarian function and live birth rates in order to corroborate the clinical applicability of our findings.
A further limitation is that we did not freeze all samples to perform concomitant serum measurements of AMH and FSH pre- and post-intervention, which would have reduced the risk of bias in our analysis. We did, however, freeze the pre-treatment samples to verify the initial result following intervention and compare it with post-treatment levels. Furthermore, the AMH results were required in real time to assess whether women with previously low ovarian reserve markers were suitable to pursue IVF/ICSI following PRP injection.
The intervention described in this study carries a degree of invasiveness that may not be acceptable to some women, although there were no significant adverse events in our sample. Indeed, the procedure was quick in the majority of patients and performed with bilateral single ovarian punctures to minimise the risk of injury to surrounding structures. Further trials, with larger numbers of participants, are nonetheless required to determine whether the intraovarian injection of PRP using a transvaginal approach proves to be less invasive than some of the techniques proposed by other studies, such as bone marrow stimulation with growth factors, laparoscopic surgery and ovarian artery catheterism [50].
Conclusions
This study revealed that the injection of PRP into human ovaries is safe and improves ovarian reserve markers as measured by antral follicle count and serum levels of AMH and FSH. Nevertheless, further studies are needed to evaluate the impact of PRP on pregnancy outcomes in women undergoing ART.
References
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101:633–34.
Stoop H, Honecker F, Cools M, de Krijger R, Bokemeyer C, Looijenga LHJ. Differentiation and development of human female germ cells during prenatal gonadogenesis: an immunohistochemical study. Hum Reprod. 2005:1466–76.
Macklon NS, Fauser BC. Aspects of ovarian follicle development throughout life. Horm Res. 1999;52:161–70.
Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93.
Fritz R, Jindal S. Reproductive aging and elective fertility preservation. J Ovarian Res. 2018;11:66.
Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–22.
Weigert M, Hofstetter G, Kaipl D, Gottlich H, Krischker U, Bichler K, et al. The effect of smoking on oocyte quality and hormonal parameters of patients undergoing in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16:287–93.
Oboni JB, Marques-Vidal P, Bastardot F, Vollenweider P, Waeber G. Impact of smoking on fertility and age of menopause: a population-based assessment. BMJ Open. 2016;6:e012015.
Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27:716–24.
Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–43.
Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86:27.
Collins GG, Rossi BV. The impact of lifestyle modifications, diet, and vitamin supplementation on natural fertility. Fertil Res Pract. 2015;1:11.
Bahadori MH, Sharami SH, Fakor F, Milani F, Pourmarzi D, Dalil-Heirati SF. Level of vitamin E in follicular fluid and serum and oocyte morphology and embryo quality in patients undergoing IVF treatment. J Family Reprod Health. 2017;11:74–81.
Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. The role of melatonin as an antioxidant in the follicle. J Ovarian Res. 2012;5:5.
Gat I, Blanco Mejia S, Balakier H, Librach CL, Claessens A, Ryan EA. The use of coenzyme Q10 and DHEA during IUI and IVF cycles in patients with decreased ovarian reserve. Gynecol Endocrinol. 2016;32:534–7.
Özcan P, Fıçıcıoğlu C, Kizilkale O, Yesiladali M, Tok OE, Ozkan F, et al. Can coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. 2016;33:1223–30.
Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14:345–57.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. 2017;7.
Okabe K, Yamada Y, Ito K, Kohgo T, Yoshimi R, Ueda M. Injectable soft-tissue augmentation by tissue engineering and regenerative medicine with human mesenchymal stromal cells, platelet-rich plasma and hyaluronic acid scaffolds. Cytotherapy. 2009;11:307–16.
Sánchez-González D, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012.
Qian Y, Han Q, Chen W, Song J, Zhao X, Ouyang Y, et al. Platelet-rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front Chem. 2017;5:89.
Amable PR, Carias RBV, Teixeira MVT, Pacheco IC, Amaral RJFC, Granjeiro JM, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67.
Reddy SHR, Reddy R, Babu NC, Ashok GN. Stem-cell therapy and platelet-rich plasma in regenerative medicines: a review on pros and cons of the technologies. J Oral Maxillofac Pathol. 2018;22:367–74.
Bastami F, Vares P, Khojasteh. Healing effects of platelet-rich plasma on peripheral nerve injuries. J Craniofac Surg. 2017;28:e49–57.
Spartalis E, Tomos P, Moris D, Athanasiou A, Markakis C, Spartalis MD, et al. Role of platelet-rich plasma in ischemic heart disease: an update on the latest evidence. World J Cardiol. 2015;7:665–70.
Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107–10.
Ulusal BG. Platelet-rich plasma and hyaluronic acid–an efficient biostimulation method for face rejuvenation. J Cosmet Dermatol. 2017;16:112–9.
Avila MY, Igua AM, Mora AM. Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. Br J Ophthalmol. 2019;103:648–53.
Miller LE, Parrish WR, Roides B, Bhattacharyya S. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237.
Taylor DW, Petrera M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344–52.
Dawood AS, Salem HA. Current clinical applications of platelet-rich plasma in various gynecological disorders: appraisal of theory and practice. Clin Exp Reprod Med. 2018;45:67–74.
Bakacak M, Bostanci MS, Ínanc F, Yaylali A, Serin S, Attar R, et al. Protective effect of platelet rich plasma on experimental ischemia/reperfusion injury in rat ovary. Gynecol Obstet Investig. 2016;81:225–31.
Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8:1286–90.
Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Ajori L. Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: a pilot study. Int J Reprod Biomed. 2016;14:625–8.
Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pantou A, Vaxevanoglou T, et al. A case series on platelet-rich plasma revolutionary management of poor responder patients. Gynecol Obstet Investig. 2018;22:1–8.
Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131:1007–15.
McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11.
Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–54.
Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–24.
Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–71.
Oktem O, Oktay K. The ovary: anatomy and function throughout human life. Ann N Y Acad Sci. 2008;1127:1–9.
Fenton AJ. Premature ovarian insufficiency: pathogenesis and management. J Midlife Health. 2015;6:147–53.
Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6.
White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–21.
Jain NK, Gulati M. Platelet-rich plasma: a healing virtuoso. Blood Res. 2016;51:3–5.
Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol. 2010;71:132–43.
Barad DH, Yu Y, Kushnir VA, Shohat-Tal A, Lazzaroni E, Lee HJ, et al. A randomized clinical trial of endometrial perfusion with granulocyte colony-stimulating factor in in vitro fertilization cycles: impact on endometrial thickness and clinical pregnancy rates. Fertil Steril. 2014;101:710–5.
Molina A, Sanchez J, Sanchez W, Vielma V. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist Reprod. 2018;22:42–8.
Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol. 2018;34:756–60.
Herraiz S, Pellicer N, Romeu M, Pellicer A. Treatment potential of bone marrow-derived stem cells in women with diminished ovarian reserves and premature ovarian failure. Curr Opin Obstet Gynecol. 2019;31:156–62.
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, et al. Standardization of grading embryo morphology. Fertil Steril. 2010;94:1152–3.
Author information
Authors and Affiliations
Contributions
PM contributed to the study design, data analysis and interpretation and drafted the manuscript. CN contributed to the study design and implementation, patient recruitment, PRP administration, data acquisition and critically revised the manuscript. CJ contributed to the study design and critically revised the manuscript. KC contributed to the study design and critically revised the manuscript. LC developed the concept and design of the study and had overall responsibility for trial registration, seeking ethical approval, data collection and interpretation, manuscript drafting and critical revisions. All authors critically revised the article for intellectual content and gave final approval. All authors accept responsibility for the paper as published.
Corresponding author
Ethics declarations
This study was designed, conducted and reported in accordance with the principles of Good Clinical Practice guidance and with the 1964 Helsinki declaration and its later amendments. Prospective ethical approval was granted by a local Institutional Review Board (IRB) and the Venezuelan Health Ministry (IRB reference number #0940), and written consent was obtained from all participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Melo, P., Navarro, C., Jones, C. et al. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J Assist Reprod Genet 37, 855–863 (2020). https://doi.org/10.1007/s10815-020-01710-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01710-z