Abstract
Purpose
Mosaicism is a prevalent characteristic of human preimplantation embryos. This retrospective cohort study aimed to investigate pregnancy outcomes after transfer of mosaic or euploid embryos.
Methods
The embryos, which had been transferred as “euploidy,” were processed using array-based comparative genomic hybridization (aCGH). The original aCGH charts of the transferred embryos were reanalyzed. Mosaic and control euploid embryos were defined according to log2 ratio calls.
Results
Overall, 102 embryos were determined to be mosaic, of which 101 were estimated to harbor no more than 50% aneuploid mosaicism. Additionally, 268 euploid embryos were matched as controls. The rates of live birth (46.6% vs. 59.1%, odds ratio (OR) 0.60, 95% confidence interval (CI) 0.38–0.95), and biochemical pregnancy (65.7% vs. 76.1%, OR 0.60, 95% CI 0.37–0.99) per transfer cycle were significantly lower after mosaic embryo transfer than after euploid embryo transfer. The rates of clinical pregnancy and pregnancy loss and the risks of obstetric outcomes did not differ significantly between the two groups.
Conclusions
Compared with euploid embryo transfer, mosaic embryo transfer is associated with a lower rate of live birth, which is mainly attributed to a decreased rate of conception. However, as mosaic embryo transfer yielded a live birth rate of 46.6%, patients without euploid embryos could be counseled regarding this alternative option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryonic mosaicism, a condition in which two or more chromosomally constitutional distinct cell lines present within an embryo, is a prevalent characteristic of human preimplantation embryos [1,2,3]. Mosaicism mainly results from mitotic errors during post-zygotic cell division, including alterations to cell cycle checkpoints, aberrations of the centrosome, and a failure of chromatid cohesion [4]. Mosaic embryos can be divided into three main categories by cell composition: (1) diploid/polyploid mosaics, which contain polypoid cells; (2) aneuploid mosaics, which contain different aneuploid cell lines without diploid cells; and (3) diploid/aneuploid mosaics, which contain both diploid and aneuploid cell lines [5].
Presumably, mosaicism has varied effects on embryonic development. Aneuploid cells in mosaic embryos are not oriented to differentiate into the inner cell mass (ICM) or trophectoderm [6,7,8]. Polyploid cells in embryo are considered a natural process during embryonic development [9]. Aneuploid mosaics have no potential for further development due to a lack of diploid cells. By contrast, diploid/aneuploid mosaics contain diploid cells and may thus implant and develop to the infant stage. Previous researches suggest that mosaicism is related to miscarriage, fetal malformation and adverse perinatal outcomes [10,11,12,13].
Array-based comparative genomic hybridization (aCGH) has been widely used in preimplantation genetic testing (PGT) [14,15,16]. This high-throughput technology provides high-resolution data and allows the simultaneous detection of all 24 chromosomes [14, 17,18,19]. In addition to whole-chromosome aneuploidy, aCGH can be used to detect segmental abnormalities and translocations [20]. Along with trophectoderm biopsy, aCGH can identify blastocyst stage embryos as either euploid and thus suitable for subsequent transfer, or aneuploid, which have little or no reproductive potential.
Recent studies have reported that aCGH and emerging next-generation sequencing (NGS) methodologies may distinguish uniform aneuploidies from mosaic diploid/aneuploid aneuploidies in trophectoderm samples biopsied from blastocysts [13, 21,22,23]. Additionally, a case series reported the births of healthy babies after mosaic embryo transfer [21]. However, studies have shown that compared with euploid embryo transfer, mosaic embryo transfer yields poorer pregnancy outcomes, particularly in cases involving whole chromosome mosaics and embryos with three or more mosaic chromosomes [22, 23]. Furthermore, a prospective study showed that mosaic blastocysts containing more than 50% aneuploid cells yielded significant reductions in pregnancy outcomes when compared with euploid blastocysts, whereas mosaics with less than 50% aneuploidy yielded similar clinical outcomes as euploid blastocysts [24].
In this retrospective study, we re-analyzed the aCGH plots of blastocysts that were initially designated as euploid transfers after PGT in our center and categorized these entities into mosaic and control euploid groups according to their log2 ratio. Accordingly, we were able to compare the outcomes of assisted conception and obstetrics between the two groups.
Materials and methods
Study population
This retrospective study was conducted by re-analyzing the aCGH plots of blastocysts detected between January 2013 and June 2016 at the Reproductive Center of Shandong Provincial Hospital, an academic hospital. Patients who underwent PGT included the following: (1) couples with chromosomal abnormalities, such as maternal or paternal numerical or structural chromosomal abnormality, including reciprocal translocations, Robertsonian translocations and inversions, or those with a history of chromosomally abnormal pregnancy; (2) patients with unexplained recurrent miscarriage (uRM) defined as a history of two or more clinical pregnancy losses without known reasons; (3) patients with repeated implantation failure (RIF) defined as a failure to achieve clinical pregnancy after three or more embryo transfer cycles; (4) couples with an advanced maternal age (AMA), defined as a female partner older than 35 years.
Ethical approval for the use and analysis of information and data from patients who underwent PGT was obtained from the Institutional Review Board of Reproductive Medicine, Shandong University.
Array-CGH procedure and frozen embryo transfer
The protocols for ovarian stimulation were described previously [25]. Intracytoplasmic sperm injection (ICSI) was performed after oocyte aspiration. On day 5 or 6, the blastocysts were graded according to the Gardner blastocyst morphologic scoring system [26]. Approximately 4–6 trophoblast cells were biopsied from good-quality blastocysts [25] by laser-mediated drilling, after which the blastocysts were vitrified and saved. Following whole-genome amplification (WGA) of the biopsied trophoblast samples, PGT was performed in the molecular laboratory of the center using 24sure microarrays (Illumina, Inc. San Diego, CA, USA) according to the manufacturer’s protocol. BlueFuse Multi (BFM) software was used to analyze hybridization images produced by a microarray scanner (InnoScan 900). Previously, an “aneuploid” result was defined as a specific chromosome ratio greater than + 0.3 log2 ratio, indicating trisomy, or a log2 ratio of less than − 0.3, indicating that monosomy. A “euploid” result was defined as chromosome ratios within ± 0.3 log2 ratio calls. After endometrial preparation, one frozen euploid embryo was thawed per transfer. Luteal-phase support was continued until 10 weeks after conception.
Re-analysis of aCGH results
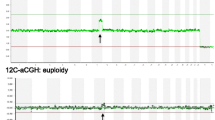
Mosaicism was ascertained from the aCGH results of mixed aneuploid/euploid cells. Briefly, euploid (46,XX) and aneuploid (47,XX,+ 9 or 46,XY,-9) cells were mixed at different ratios and processed by aCGH after WGA by Greco et al. [21]. When re-analyzing the original BFM plots of previously transferred “euploid embryos,” a mosaic embryo was defined as a chromosomal ratio level exceeding 20% of the aneuploid cells. Meanwhile, the log2 ratios of the autosome clones (beside the mosaic signal parts) clustered tightly around the 0 line, with a standard deviation (SD) within 0.1. The log2 ratio of the mosaic parts (both whole and segmental chromosomes) clearly deviated from the 0 line, with a SD within 0.1. The euploid embryo control group was defined as the log2 ratio of all autosome clones clustered tightly around the 0 line, with a SD within 0.1 (Fig. 1). Pregnancy outcomes were compared between cases receiving mosaic embryo and control euploid embryo transfers.
Examples of aCGH plots of re-classified euploid and mosaic embryos. a Plot of a female euploid embryo of which the log2 ratio of all autosome clones clustered tightly around the 0 line, with a standard deviation (SD) within 0.1. b Plot of a male mosaic embryo containing estimated 20% mosaicism of whole chromosome 5 loss
Study outcomes
Biochemical pregnancy was defined as a human chorionic gonadotropin level exceeding 10 mIU/ml at 12 days after blastocyst embryo transfer. Clinical pregnancy was defined as the detection of a gestational sac by transvaginal ultrasound at 32 days after embryo transfer. Beyond 12 weeks of gestational age, the patients were seen at the local obstetric clinic. A follow-up was performed by telephone at 6 weeks after the expected date of delivery. Live birth was defined as the birth of a viable infant after 28 complete weeks of gestational age.
Statistical analysis
Continuous data were represented as means ± SD and were compared between groups using the independent-samples t test. Categorical data were expressed as frequencies with percentages and compared between groups using the chi-square analysis. A P value < 0.05 was defined as statistically significant. All analyses were performed using SPSS software, version 23 (IBM Corp., Armonk, NY, USA).
Results
After a retrospective re-analysis of the transferred embryos, 102 were determined to be mosaic. An additional 268 embryos were re-confirmed as euploid and served as the matched control group. Detailed data of the mosaic embryos are listed in Supplementary Table S1. In summary, 62 embryos were only whole chromosome(s) mosaic, 28 were only segmental mosaic, and 12 were both whole chromosome(s) and segmental mosaic. Furthermore, 101 of 102 mosaic embryos exhibited less than 50% aneuploidy; the remaining embryo was 80% segmental mosaic.
These 102 mosaic embryos were transferred to 97 patients, and the 268 control embryos were transferred to 251 patients. The baseline characteristics of the patients in the two groups were comparable (Table 1). Similarly, the endometrial thickness before embryo transfer and Gardner grading of blastocysts did not differ between the groups (Supplementary Table S2).
The live birth rate per transfer cycle was significantly lower in the mosaic embryo group than in the control group (46.6% vs. 59.1%), with an odds ratio of 0.60 (95% confidence interval [CI], 0.38–0.95; P = 0.03). The biochemical pregnancy rate was also significantly lower in the mosaic embryo transfer group than in the control group (65.7% vs. 76.1%), with an odds ratio of 0.60 (95%CI, 0.37–0.99, P = 0.043). There were no significant between-group differences in the rates of clinical pregnancy or pregnancy loss (Table 2).
In subgroup analysis, the live birth rate was significantly lower after the transfer of only whole chromosome(s) mosaic embryos than after the transfer of euploid embryos (43.5% vs. 59.1%, P = 0.026). However, the rate of live birth did not differ significantly after the transfer of only segmental mosaic embryos vs. the transfer of euploid embryos (48.3% vs. 59.1%, P = 0.26) (Table 3).
No significant differences in birth weight or risk of preterm delivery, gestational hypertension, gestational diabetes mellitus, or congenital anomaly were observed between the mosaic embryo transfer and euploid embryo transfer groups (Table 4).
After clinical pregnancy was established, three patients who received mosaic embryo transfers underwent amniocentesis, which yielded normal karyotypes. All infants were found to be healthy after a detailed physical examination performed by a local pediatrician after delivery.
Discussion
In this retrospective cohort study, the live birth rate was significantly lower after mosaic embryo transfer, compared with euploid embryo transfer. As the pregnancy loss rates were similar between the groups, the lower biochemical pregnancy rate associated with mosaic embryo transfer probably explains the difference in live birth rates. This is supported by the finding that once implantation had been established, the live birth rates and obstetric outcomes did not differ significantly between the mosaic embryo and control groups. In brief, live birth could be achieved successfully after mosaic embryo transfer.
Our study revealed that 46.4% of mosaic embryos yielded a live birth outcome, consistent with the 40% ongoing implantation rate reported in a previous study [23]. In that multicentric study, the implantation rates (embryos implanted/transferred) and ongoing implantation rates (ongoing pregnancies/embryos transferred) were higher and the fetal loss rates (embryos lost/implanted) were lower after euploid embryo transfer, compared with mosaic embryo transfer. Meanwhile, another retrospective study that adopted a research strategy identical to ours reported that the pregnancy outcomes did not differ between the groups [27].
One prospective study found that mosaic blastocysts containing more than 50% aneuploid cells yielded significant reductions in pregnancy outcomes when compared with euploid blastocysts. By contrast, mosaics with less than 50% aneuploidy had similar clinical outcomes as euploid blastocysts [24]. In our study, 101 of 102 mosaic blastocysts had estimated aneuploid mosaicism levels of 50% or less. However, the results demonstrated significantly lower live birth and biochemical pregnancy rates after the transfer of these mosaic blastocysts, compared with euploid blastocysts.
Notably, the pregnancy outcomes of embryo transfer did not differ between cases implanted with only segmental mosaic embryos vs. euploid embryos. However, the live birth rate decreased significantly after the transfer of only whole chromosome(s) mosaic embryos, consistent with a study by Fragouli and colleagues [22]. In another study, separate trophectoderm and ICM analyses revealed that all initial segmental mosaic embryos with confirmed normal ICM had normal or mostly normal trophectoderm results, compared with 25% of embryos initially identified as whole chromosome(s) mosaics [28]. In other words, only segmental mosaic embryos were more likely to be euploid embryo intrinsic than were whole chromosome(s) mosaic embryos, and this difference resulted in significantly different outcomes after transfer.
It remains unclear why mosaic embryo transfer can yield a healthy newborn. However, cell and animal experiments may provide some clues. Compared with euploid cells, aneuploidy altered both gene expression and cellular metabolic properties, leading to impaired cell proliferation and changes in cell volume [29, 30]. One study showed that diploid human embryonic stem cells (HESC) could be derived from mosaic embryos in vitro [31]. During this derivation process, the percentage of aneuploid cells decreased and vanished, and euploid cells became dominant in the culture. In a mouse experiment, the proportions of aneuploid cells in mosaic embryos decreased progressively, and aneuploid cells of different lineages exhibited different fates [32]. During blastocyst development, aneuploid cells originating from the ICM lineage were eliminated by apoptosis, whereas those in trophectoderm lineage exhibited severe proliferative defects. Accordingly, mosaic embryos containing sufficient proportions of euploid cells are fully able to yield healthy progeny [32].
Technique artifacts may also explain why mosaic embryos can yield a healthy newborn [33, 34]. When ICM and multiple trophectoderm samples from embryos with diploid/aneuploid mosaic results in the original trophectoderm biopsy were retested separated, both the ICM and trophectoderm from five of eight embryos were identified as normal euploid [35]. Mathematical models have shown that a single biopsy of five to ten cells cannot be considered representative of the whole blastocyst [36]. Therefore, cases with altered log2 ratios should be subjected to a second biopsy to conform true mosaicism [37].
A mosaic embryo is not a specific characteristic of the PGT procedure. Rather, such entities are prevalent among preimplantation embryos, including those generated via in vitro fertilization and ICSI. After a single blastocyst transfer, cytogenetic analyses of the retained products of conception after a missed abortion found that 1.1% of these cases involved mosaicism [38]. In a retrospective large-scale analysis of chorionic villi (CV) samples, 2.18% of all cases were mosaic, and a follow-up amniocentesis revealed that mosaicism was confined to the placenta in 87% these cases, leaving the incidence of true fetal mosaicism being 13% [39]. However, the pregnancies were advised to undergo CV sampling or amniocentesis because of indications such as fetal ultrasound anomalies or positive first-trimester screening.
Our study showed that despite a decreased live birth rate, 46.6% of mosaic embryo transfers resulted in healthy infants. Therefore, patients with only mosaic embryos could be advised through comprehensive counseling to transfer these embryos rather than initiate a new ovarian stimulation cycle [40].
There were some limitations in this study. First, we re-analyzed aCGH plot results, rather than NGS results. Recently, NGS has been introduced into PGT for aneuploidy because of its increased resolution [41]. When compared with aCGH, NGS provided a 100% concordance rate with the results of 24-chromosome aneuploidy diagnosis [42]. Furthermore, NGS appears better able than aCGH to detect mosaicism because of its increased dynamic range and higher sensitivity [13]. As this study did not aim to analyze the constitutions of embryos, the use of aCGH results was appropriate in this scenario. Second, not all of the infants were subjected to chromosomal analysis. At the time of PGT counseling, all patients were advised to perform amniocentesis after a clinical pregnancy had been established, but the telephone follow-up revealed that only some of the patients complied. Finally, this was a retrospective study, and selection bias might have been present in both groups. An additional well-designed prospective study is needed to confirm our findings.
In summary, live birth could be obtained following mosaic embryo transfer in 46.6% of cycles. After conception was established, the live birth rates of mosaic embryo transfer and euploid embryo transfer were similar. Given these outcomes, patients with no available euploid embryos could be counseled about the possibility of transferring a mosaic embryo. However, prospective studies are needed to confirm our findings.
References
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20(4):571–81. https://doi.org/10.1093/humupd/dmu016.
Delhanty JD, Griffin DK, Handyside AH, Harper J, Atkinson GH, Pieters MH, et al. Detection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in situ hybridisation (FISH). Hum Mol Genet. 1993;2(8):1183–5.
Munne S, Weier HU, Grifo J, Cohen J. Chromosome mosaicism in human embryos. Biol Reprod. 1994;51(3):373–9.
McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33(7):448–63. https://doi.org/10.1016/j.tig.2017.04.001.
Delhanty JD, Harper JC, Ao A, Handyside AH, Winston RM. Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients. Hum Genet. 1997;99(6):755–60.
Evsikov S, Verlinsky Y. Mosaicism in the inner cell mass of human blastocysts. Hum Reprod. 1998;13(11):3151–5.
Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15(8):1781–6.
Capalbo A, Wright G, Elliott T, Ubaldi FM, Rienzi L, Nagy ZP. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod. 2013;28(8):2298–307. https://doi.org/10.1093/humrep/det245.
Bielanska M, Tan SL, Ao A. High rate of mixoploidy among human blastocysts cultured in vitro. Fertil Steril. 2002;78(6):1248–53.
Vorsanova SG, Kolotii AD, Iourov IY, Monakhov VV, Kirillova EA, Soloviev IV, et al. Evidence for high frequency of chromosomal mosaicism in spontaneous abortions revealed by interphase FISH analysis. J Histochem Cytochem. 2005;53(3):375–80. https://doi.org/10.1369/jhc.4A6424.2005.
Forsberg LA, Gisselsson D, Dumanski JP. Mosaicism in health and disease—clones picking up speed. Nat Rev Genet. 2017;18(2):128–42. https://doi.org/10.1038/nrg.2016.145.
Simon C. Introduction: to transfer or not transfera mosaic embryo, that is the question. Fertil Steril. 2017;107(5):1083–4. https://doi.org/10.1016/j.fertnstert.2017.03.025.
Maxwell SM, Colls P, Hodes-Wertz B, DH MC, McCaffrey C, Wells D, et al. Why do euploid embryos miscarry? A case-control study comparing the rate of aneuploidy within presumed euploid embryos that resulted in miscarriage or live birth using next-generation sequencing. Fertil Steril. 2016;106(6):1414–9. https://doi.org/10.1016/j.fertnstert.2016.08.017.
Gutierrez-Mateo C, Colls P, Sanchez-Garcia J, Escudero T, Prates R, Ketterson K, et al. Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril. 2011;95(3):953–8. https://doi.org/10.1016/j.fertnstert.2010.09.010.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24. https://doi.org/10.1186/1755-8166-5-24.
Sermon K, Capalbo A, Cohen J, Coonen E, De Rycke M, De Vos A, et al. The why, the how and the when of PGS 2.0: current practices and expert opinions of fertility specialists, molecular biologists, and embryologists. Mol Hum Reprod. 2016;22(8):845–57. https://doi.org/10.1093/molehr/gaw034.
Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, et al. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26(7):1925–35. https://doi.org/10.1093/humrep/der082.
Alfarawati S, Fragouli E, Colls P, Wells D. First births after preimplantation genetic diagnosis of structural chromosome abnormalities using comparative genomic hybridization and microarray analysis. Hum Reprod. 2011;26(6):1560–74. https://doi.org/10.1093/humrep/der068.
Franasiak JM, Scott RT Jr. Embryonic aneuploidy: overcoming molecular genetics challenges improves outcomes and changes practice patterns. Trends Mol Med. 2014;20(9):499–508. https://doi.org/10.1016/j.molmed.2014.06.006.
Colls P, Escudero T, Fischer J, Cekleniak NA, Ben-Ozer S, Meyer B, et al. Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod BioMed Online. 2012;24(6):621–9. https://doi.org/10.1016/j.rbmo.2012.02.006.
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089–90. https://doi.org/10.1056/NEJMc1500421.
Fragouli E, Alfarawati S, Spath K, Babariya D, Tarozzi N, Borini A, et al. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum Genet. 2017;136(7):805–19. https://doi.org/10.1007/s00439-017-1797-4.
Munne S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;108(1):62–71.e8. https://doi.org/10.1016/j.fertnstert.2017.05.002.
Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril. 2018;109(1):77–83. https://doi.org/10.1016/j.fertnstert.2017.09.025.
Zhang Q, Li G, Zhang L, Sun X, Zhang D, Lu J et al. Maternal common variant rs2305957 spanning PLK4 is associated with blastocyst formation and early recurrent miscarriage. Fertil Steril 2017;107(4):1034–40 e5. doi:https://doi.org/10.1016/j.fertnstert.2017.01.006, 1040.e5.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8. https://doi.org/10.1016/s0015-0282(00)00518-5.
Lledo B, Morales R, Ortiz JA, Blanca H, Ten J, Llacer J, et al. Implantation potential of mosaic embryos. Syst Biol Reprod Med. 2017;63(3):206–8. https://doi.org/10.1080/19396368.2017.1296045.
Garrisi G, Walmsley RH, Bauckman K, Mendola RJ, Colls P, Munne S. Discordance among serial biopsies of mosaic embryos. Fertil Steril. 2016;106((3):e151. https://doi.org/10.1016/j.fertnstert.2016.07.447.
Durrbaum M, Kuznetsova AY, Passerini V, Stingele S, Stoehr G, Storchova Z. Unique features of the transcriptional response to model aneuploidy in human cells. BMC Genomics. 2014;15:139. https://doi.org/10.1186/1471-2164-15-139.
Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322(5902):703–9. https://doi.org/10.1126/science.1160058.
Lavon N, Narwani K, Golan-Lev T, Buehler N, Hill D, Benvenisty N. Derivation of euploid human embryonic stem cells from aneuploid embryos. Stem Cells. 2008;26(7):1874–82. https://doi.org/10.1634/stemcells.2008-0156.
Bolton H, Graham SJ, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016;7:11165. https://doi.org/10.1038/ncomms11165.
Capalbo A, Rienzi L. Mosaicism between trophectoderm and inner cell mass. Fertil Steril. 2017;107(5):1098–106. https://doi.org/10.1016/j.fertnstert.2017.03.023.
Marin D, Scott RT Jr, Treff NR. Preimplantation embryonic mosaicism: origin, consequences and the reliability of comprehensive chromosome screening. Curr Opin Obstet Gynecol. 2017;29(3):168–74. https://doi.org/10.1097/GCO.0000000000000358.
Popovic M, Dheedene A, Christodoulou C, Taelman J, Dhaenens L, Van Nieuwerburgh F, et al. Chromosomal mosaicism in human blastocysts: the ultimate challenge of preimplantation genetic testing? Hum Reprod. 2018;33(7):1342–54. https://doi.org/10.1093/humrep/dey106.
Gleicher N, Metzger J, Croft G, Kushnir VA, Albertini DF, Barad DH. A single trophectoderm biopsy at blastocyst stage is mathematically unable to determine embryo ploidy accurately enough for clinical use. Reprod Biol Endocrinol. 2017;15(1):33. https://doi.org/10.1186/s12958-017-0251-8.
Capalbo A, Ubaldi FM, Rienzi L, Scott R, Treff N. Detecting mosaicism in trophectoderm biopsies: current challenges and future possibilities. Hum Reprod. 2017;32(3):492–8. https://doi.org/10.1093/humrep/dew250.
Segawa T, Kuroda T, Kato K, Kuroda M, Omi K, Miyauchi O, et al. Cytogenetic analysis of the retained products of conception after missed abortion following blastocyst transfer: a retrospective, large-scale, single-centre study. Reprod BioMed Online. 2017;34(2):203–10. https://doi.org/10.1016/j.rbmo.2016.11.005.
Malvestiti F, Agrati C, Grimi B, Pompilii E, Izzi C, Martinoni L, et al. Interpreting mosaicism in chorionic villi: results of a monocentric series of 1001 mosaics in chorionic villi with follow-up amniocentesis. Prenat Diagn. 2015;35(11):1117–27. https://doi.org/10.1002/pd.4656.
Munne S, Grifo J, Wells D. Mosaicism: “survival of the fittest” versus “no embryo left behind”. Fertil Steril. 2016;105(5):1146–9. https://doi.org/10.1016/j.fertnstert.2016.01.016.
Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, et al. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil Steril. 2014;101(5):1375–82. https://doi.org/10.1016/j.fertnstert.2014.01.051.
Yang Z, Lin J, Zhang J, Fong WI, Li P, Zhao R, et al. Randomized comparison of next-generation sequencing and array comparative genomic hybridization for preimplantation genetic screening: a pilot study. BMC Med Genet. 2015;8:30. https://doi.org/10.1186/s12920-015-0110-4.
Acknowledgements
The authors expressed thanks to Wenjie Jiang, Hongqiang Xie, Hongchang Li, and Ping Li from Reproductive Hospital Affiliated to Shandong University for performing PGT procedures and following up.
Funding
This study was funded by the National Key Research and Development Program of China (grant number 2016YFC1000202), National Natural Science Foundation of China (grant number 81671522), and Innovative Foundation of Reproductive Hospital Affiliated to Shandong University (grant number 20171114).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, L., Wei, D., Zhu, Y. et al. Rates of live birth after mosaic embryo transfer compared with euploid embryo transfer. J Assist Reprod Genet 36, 165–172 (2019). https://doi.org/10.1007/s10815-018-1322-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1322-2