Abstract
Purpose
The aim was to study the association between embryonal mitochondrial DNA (mtDNA) content and embryo quality and implantation outcomes.
Methods
A retrospective chart review was performed with data collected from a private IVF center database. The study population included female infertility patients with ages ranging from 31 to 38 years old, and the main outcome measures were embryo quality and transfer outcomes.
Results
From a total of 1510 blastocyst biopsies, the majority of embryos consisted of grade 1 (High), followed by grade 2 (mid), and grade 3 (poor). Embryos with higher mtDNA content were found to be of poorer quality (grade 3) relative to grades 1 and 2 (P = 0.003). Using a logistic model, mtDNA best predicted lowest and highest grades, but not mid-grade embryos. There was no correlation between mtDNA content and the subjects’ age (R2 = 0.0018). In an analysis of only euploid embryos (N = 717), there was no longer an association between mtDNA content and embryo quality (P = 0.834). There was no difference in mtDNA content between groups of embryos that did and did not implant (P = 0.53). There was also no association noted between mtDNA content and ongoing pregnancy. Compared to day 6, day 5 blastocysts contain significantly higher amounts of mtDNA (P = 0.0005), lower rates of aneuploidy (P < 0.001), and were more likely to be high-quality blastocysts (grade 1) (P < 0.001).
Conclusion
Although the mtDNA content shows some association to the morphologic grade of an embryo, this association does not persist in an analysis of only euploid embryos. Mitochondrial DNA content also does not appear to be associated with implantation or ongoing pregnancy. Day 5 blastocysts have significantly higher mtDNA content compared to day 6 blastocysts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maternal age is the most important determinant of fertility, and with many women today opting to delay childbearing, infertility rates are on the rise [1]. As women age, declines in oocyte quantity and quality termed “ovarian aging” results in diminishing pregnancy success [2, 3]. While many ovarian aging factors have been examined, recent studies suggest mitochondrial DNA (mtDNA) may play a key role [4, 5]. Unlike nuclear DNA (nDNA), mtDNA is a small genome approximately 16.5 kb in size inherited through the oocyte cytoplasm. It encodes genes in the electron transport chain, where most of cellular energy is created via oxidative phosphorylation [6]. Because sufficient energy supply is needed to obtain embryo viability, the role of mtDNA in infertility has been investigated [7].
The primordial germ cell houses approximately 200 copies of the mtDNA. Throughout development, the copy number increases and decreases until ultimately a mature oocyte contains greater than 200,000 copies—this number is variable [8, 9]. As women age, the rate of oocyte mtDNA with a deletion increases resulting in a lower viable mtDNA copy number [10,11,12]. Many potential explanations for why ovarian aging results in poorer quality oocytes have been explored. One study by Desquiret-Dumas et al. examined mtDNA content in cumulus granulosa cells (CGCs) surrounding oocytes and demonstrated higher mtDNA content of CGCs resulted in higher quality embryos [13]. Several studies have shown that decreased mtDNA copy number in oocytes results in poor in vitro fertilization outcomes [10, 14,15,16]. To date, only four studies have taken this one step further and looked specifically at embryonal mtDNA content’s impact on implantation. The first study by Fragouli et al. demonstrated that blastocysts with lower amounts of mtDNA had higher implantation rates. This study also suggests that a threshold of mitochondrial DNA exists above which no embryo is able to implant. Furthermore, Fragouli et al. demonstrated that aneuploid blastocysts as well as those from older women have a higher mtDNA copy number. On the contrary, cleavage stage embryos (blastomeres) from younger women were seen to contain higher mtDNA content [17]. A subsequent study by Diez-Juan et al. had similar findings showing that a high mtDNA copy number in euploid embryos is indicative of lower embryo viability and implantation in both blastocysts as well as cleavage stage embryos (blastomeres). Interestingly, Diez-Juan et al. did not find a statistically significant relationship between maternal age and mtDNA copy number in embryos [18]. Victor et al. countered this with data demonstrating no significant difference in implantation rates after applying a novel normalization strategy to mtDNA [19]. Shortly thereafter, Treff et al. performed a double embryo transfer study in order to control for confounding factors and found mtDNA quantity did not differ significantly between those embryos that implanted and those that did not [20].
In general, there is very limited data available regarding the influence of mtDNA on embryo implantation. The goal of our study is to determine if a relationship exists between embryonal mtDNA copy number and embryo quality and implantation rates.
Materials and methods
A university institutional review board (IRB) determined that this study was not a “human subject research” study and did not require IRB approval or oversight.
Mitochondrial DNA and embryo quality
We performed a retrospective chart review of subjects whose embryos underwent preimplantation genetic testing for aneuploidy in our center between June 2013 and June 2016. In order to evaluate mtDNA’s relationship with embryonal grade, a total of 259 subjects with 1510 blastocyst biopsies were included. Embryo grade was defined based on the Gardner blastocyst grading system (see Table 1 footnote). Based on this system, grade was defined as follows: 1—High (3 to 6 AA and 4 to 6 AB); 2—Mid (any BB, 1 to 3 AB, and 1 to 2 AA); and 3—Poor (any AC, CA, BC, CB, or CC). “Embryonal mtDNA content” was defined as a ratio of mtDNA to nDNA (see Determining Mitochondrial DNA Content). The primary outcome for this subset of data was to assess the relationship between embryonal mtDNA content to embryo grade. For our secondary outcomes, we compared mtDNA to age and repeated the analysis after excluding embryos with aneuploidy. Statistical analysis was performed using a multinomial logistic regression model of prediction and linear regression. P < 0.05 was considered significant.
Mitochondrial DNA and embryo transfer outcomes
In order to investigate the relationship between mtDNA and embryo transfer outcomes, a total of 153 embryo transfers from 144 subjects were identified. Implantation was defined as a gestational sac observed on ultrasound. We compared mtDNA content between “No Implantation” and “Implantation” groups. We also looked for a potential association between embryonal mtDNA content and ongoing pregnancy. Multinomial logistic regression was used for data analysis between dependent categorical outcomes and continuous dependent variables. Fisher’s exact, Pearson’s chi-squared, or Wilcoxon rank-sum were used as appropriate with P < 0.05 considered significant.
Obtaining blastocysts and biopsies
Subjects underwent controlled ovarian stimulation using standardized protocols. Lupron or hCG trigger was administered once two follicles reached 18 mm in diameter. Fertilization was achieved using intracytoplasmic sperm injection. Standard culture conditions with sequential media were used. Laser-assisted biopsy of blastocyst trophectoderm was performed on culture day 5 or 6, depending on embryo development.
Defining mitochondrial DNA content
Trophectoderm (TE) cells from each embryo first underwent cell lysis, DNA extraction, and then a modified whole genome amplification (WGA) protocol using phi 29 polymerase. Fifty to 100 ng of amplified DNA underwent library preparation (Thermo Fisher Scientific, Waltham, MA). First, the pooled DNA samples underwent enzymatic shearing to produce fragment sizes of approximately 200 bp. The DNA fragments were purified using an AMPure Bead (Beckman Coulter, Sharon Il) wash, stabilized by the ligation of adapters and barcodes at either end of each fragment and size selected using a second AMPure Bead wash. Each DNA fragment was bound to one ion sphere particle (ISP) and amplified using an emulsion polymerase chain reaction (PCR) reaction. The template positive ISPs were recovered using Dynabeads MyOne Streptavidin CI bead washes (Invitrogen, Carlsbad, CA). Following recovery, sequencing primers and Ion Hi-Q sequencing polymerase were added to the samples, and the samples were loaded onto a sequencing chip and analyzed at a depth of 1X across the entire genome by a Personal Genome Machine (PGM) (Thermo Fisher Scientific, Waltham, MA). Sequencing data were processed by a Torrent Browser Server (Thermo Fisher Scientific, Waltham, MA) to provide initial sequencing information and to ensure adherence to the required quality assurance metrics. The data was then transferred to an Ion Reporter Server, version 5.2 (Thermo Fisher Scientific, Waltham, MA) for comprehensive data analysis and interpretation. The PGM sequencing provided a minimum of over 3.5 million reads with a median sequencing fragment length of 186 bp. The sequencing was to a depth of 0.8× across the entire genome. The output data included mitochondrial content calculated as follows: mtDNA content = mtDNA/total genomic DNA. Our assay for mtDNA content has been validated by our qPCR protocol comparing Alu or total genomic DNA to mtDNA with comparable results.
Results
Embryo quality
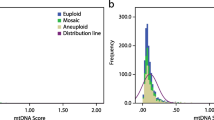
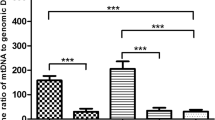
From a total of 1510 blastocyst biopsies, the majority of embryos consisted of grade 1 (N = 951; 62%), followed by grade 2 (N = 331; 21%) and grade 3 embryos (N = 228; 15%) (Table 1). Embryos with high mtDNA content were found to be of poorer quality (grade 3) relative to grades 1 and 2 (RR 1.03 [95% CI 1.01–1.05]; P = 0.003).
Using a logistic model, mtDNA best predicted lowest and highest grades, but not mid-grade (grade 2) embryos. There was no correlation between mtDNA content and the subject age (R2 = 0.0018). After excluding embryos with aneuploidy, a total of 717 euploid embryos were studied. In this subpopulation, a non-statistically significant trend was observed where poor quality embryos had higher mtDNA content (OR 1.16, [95% CI 0.27–5.01]; P = 0.834). Further analysis demonstrated that aneuploid embryos contain higher mtDNA content compared to euploid embryos (0.00629 [0.000367–0.000995] vs 0.0009315 [0.000521–0.00232]; P < 0.0001).
Day 5 vs day 6 blastocysts
From a total of 1510 blastocyst biopsies, 65.3% were biopsied on day 5 (N = 986), and 31.4% were biopsied on day 6 (N = 474). The day of biopsy was not recorded for 50 blastocysts (3.3%). Day 5 blastocysts contained higher amounts of mtDNA compared to day 6 blastocysts (0.00079 [0.00047–0.00154] vs 0.00067 [0.00037–0.00128]; p = 0.0005). Day 5 blastocysts had lower rates of aneuploidy compared to euploidy (N = 459 (32.55%) vs N = 500 (35.46%), respectively; p < 0.001), whereas day 6 blastocysts were more likely to be aneuploid (N = 262 (18.58%) vs N = 189 (13.4%); P < 0.001). Similar results were noted in a euploid only analysis: day 5 blastocysts contained significantly more mtDNA (0.000679 [0.0004195–0.001035] vs 0.000502 [0.000303–0.00081]; P < 0.0001) compared to day 6 (Table 2).
Embryo transfer outcomes
The “Implantation” and “No Implantation” groups were similar with respect to age, BMI, and gravida and para (Table 3). There was no statistically significant difference in mtDNA content between the “Implantation” and “No Implantation” groups (P = 0.53) (Table 3). After adjusting for BMI and age, we observed no significant relationship between embryonal mtDNA content and ongoing pregnancy (coefficient − 21.73, P = 0.292).
Discussion
Aging and mitochondrial DNA content of oocytes and embryos
The physiologic age-related decline in female fertility remains one of the insurmountable barriers to successful pregnancy in older women. This decline is a result of not only decreased oocyte quantity, but decreased quality of the remaining oocytes as well. Even with continued improvements to in vitro fertilization (IVF) techniques, women with diminished ovarian reserve have poor response to ovarian stimulation and therefore less successful pregnancy rates [21]. Many hypotheses exist for potential factors contributing to poor quality oocytes, and growing evidence suggests mitochondria may play a significant role in ovarian aging. Morphologically, mitochondria from older women are more swollen and have disrupted cristae compared to mitochondria of their younger counterparts [22]. Kushnir et al. demonstrated that mitochondria from older mice not only differed morphologically but also had lower mtDNA content than that from younger mice [23]. This relationship of decreasing mtDNA quantity in oocytes of older women has been demonstrated by several studies in humans as well [16, 24, 25]. Additionally, in a study by Reynier et al., the average mtDNA content number was lower in oocytes with fertilization failure compared to those with normal fertilization rates [15].
While a decline in oocyte mtDNA content with aging has been observed, the relationship to embryonal mtDNA remains less clear [10, 12]. The study by Fragouli et al. found blastocysts from older women had a higher mtDNA copy number; however, the study by Diez-Juan et al. did not find an association between the two [17, 18]. Similarly, our data did not demonstrate an association between embryonal mtDNA content and age. As a woman ages and oocytes await ovulation, their mtDNA can acquire mutations.
Mitochondrial DNA has no protective histone layer and is therefore more vulnerable to the reactive oxygen species released by the mitochondria itself, which can possibly induce mutations [26]. Mitochondrial DNA deletions result in less mtDNA content in the oocyte. Nevertheless, it is unclear why once the oocyte is fertilized and becomes a blastocyst, this age-related decline in mtDNA content is no longer apparent.
Mitochondrial DNA and embryo quality
In attempt to identify good quality embryos prior to implantation, morphologic grading systems have been developed. The Gardner blastocyst grading system defines morphological criteria of higher quality embryos that are believed to result in higher implantation rates. To date, no studies have investigated whether there is a relationship between blastocyst mtDNA content and morphologic grade. Our study demonstrates that embryos with higher amounts of mtDNA are more likely to be of poor quality. Similar to the discussion on ovarian aging and mtDNA, the majority of previous studies exploring mtDNA and quality were focused on oocytes rather than embryos. Most of these studies demonstrated that lower mtDNA content is associated with poorer quality oocytes and decreased ability to be fertilized [10, 13,14,15,16]. In contrast, our data suggest embryos may differ from oocytes in that higher amounts of mtDNA are correlated with poor embryo quality. A better understanding of mtDNA’s replication and division throughout embryo development will likely help explain this difference. Because mtDNA is maternally inherited, fully developed oocytes contain all the mtDNA that will support the embryo during the preimplantation stage. Animal studies have shown that mtDNA copy number does not change during cleavage divisions, and mtDNA does not begin replication until near the time of implantation [27, 28]. However, when the embryo reaches the blastocyst stage, the distribution of the mtDNA into the inner cell mass and trophectoderm varies [29, 30]. Studies in the mouse and hamster have shown that trophectoderm cells inherit more mitochondria than the cells that make up the inner cell mass [31,32,33]. The biopsies in our study were performed on the trophectoderm of days 5 and 6 blastocysts. If a similar process occurs in humans, this unequal distribution of mtDNA is one possible explanation why biopsies from the trophectoderm of blastocysts differ in mtDNA content compared to oocytes. Perhaps the trophectoderm acquiring a higher portion of the mtDNA is a marker for a poorer quality blastocyst.
One consideration is that many of those poor quality embryos containing high mtDNA content included in our analysis are aneuploid. Our study is congruent with the Fragouli study only with regard to the correlation between mtDNA and aneuploidy, which demonstrated aneuploid blastocysts contain higher amounts of mtDNA quantity compared to euploid blastocysts. Fragouli et al. also supports the concept that higher mtDNA content of aneuploid embryos appears to be an independent factor and not dependent on age. Importantly, a second analysis omitting those aneuploid embryos still yielded a similar trend, in that higher mtDNA was seen in poorer quality embryos. This demonstrates that aneuploidy does not appear to be the explanation as to why higher mtDNA content is correlated with poor quality embryos. Over 1500 embryo biopsies were included making this the largest study of its kind. According to our data, embryonal mtDNA content does not appear to have much relevance in objectively defining embryo quality in euploid embryos. Further investigation on how mtDNA content data can be applied as an objective measure of embryo quality is warranted. In order to obtain higher IVF success rates, we must employ new methods identifying high-quality embryos prior to transfer.
Implantation and embryonal mitochondrial DNA content
Despite advancing techniques to identify good quality embryos prior to implantation, it is still difficult to determine why some embryos will fail to implant. In an attempt to identify other potential markers for embryo viability, we compared mtDNA content in embryos that implanted versus those that did not. Our data did not demonstrate a statistically significant difference between the two groups. This indicates that contrary to some of the previous studies, embryonal mtDNA may not be a good marker for implantation potential. Some labs are suggesting embryonal mtDNA content could be used to prioritize embryos for transfer, but our data does not support this concept. We did however observe that embryos in the upper percentiles (75%ile and 90%ile) of mtDNA content appeared to be less likely to implant. This suggests that it may be more appropriate to identify a mtDNA content above which embryos will not implant.
Several different methods have been employed in order to quantify mtDNA content including in situ hybridization, quantitative real-time PCR, and next-generation sequencing (NGS). Similar to our process, Fragouli et al. utilized quantitative real-time PCR in conjunction with NGS. Diez-Juan et al. also used quantitative real-time PCR to determine mtDNA number. Both studies employed strategies to normalize mtDNA content in comparison to nuclear DNA. Our normalization strategy involved a ratio of mtDNA content to total nuclear DNA content.
Implantation requires a significant increase in energy needs of the embryo and thus the utilization of mitochondria. Mitochondria react to stress by hyperproliferation, and it is suggested that embryos that are under metabolic stress have a subsequent increase in mtDNA copy number [34]. Therefore, it can be suggested that a higher mtDNA content in the blastocyst may be a marker of an oxidatively stressed embryo. Interestingly, because of heteroplasmy—more than one genome of mtDNA in a particular cell—different alleles may be mutated within the different copies [35]. In order for mitochondrial mutations to cause clinical significance, they must typically be present in sufficient numbers throughout the many copies of the mtDNA genome. When the mtDNA acquires mutations, it produces faulty proteins and resultant oxidative stress in cells [35, 36]. It is possible embryos under more oxidative stress have a particular mutation that is shared throughout most mitochondrial genomes in the embryos. It is unknown whether higher mtDNA content may be representative of poorer quality or mutated mtDNA that does not function properly to supply sufficient energy for implantation. Those embryos that fall into the upper percentiles of mtDNA content are perhaps the more oxidatively stressed embryos and therefore are less likely to implant.
While implantation is important to IVF success, important outcomes of interest also include clinical pregnancy, ongoing pregnancy, and live birth. If mtDNA can be used to predict embryos that will result in a successful pregnancy, its utility will be instrumental. We did not detect an association between the mtDNA content and clinical or ongoing pregnancy, but we suggest that other studies should investigate this clinically relevant outcome as well.
Potential limitations of our study include that it is an observational study with inclusion of a relatively small number of embryo transfers and lack of generalizability given that data was collected from a single center. One potential bias is also that only good quality embryos are chosen for transfer. Given that mtDNA content may possibly be associated with embryo quality, this may have influenced the implantation data results. Importantly, other factors aside from just embryo characteristics may affect implantation including physician technique, patient characteristics, and quality of endometrium. Another limitation of our data is the unequal distribution of ethnicities between the “Implantation” and “No Implantation” groups. The “No Implantation” group has more Hispanics and African Americans, and the “Implantation” group has more Caucasians. Because the population included was limited to infertile patients, we cannot conclude that these results are applicable to the general population. However, to our knowledge, this is the largest study of its kind.
Conclusion
In conclusion, mitochondrial DNA content does not seem to be a good marker for euploid embryo quality or embryo implantation potential. More studies are needed investigating the relationship between embryonal mtDNA content and IVF outcomes. The findings of our study add to the very limited amount of available data on mtDNA obtained from embryos. While some of the previous studies investigating embryonal mtDNA have suggested a role in predicting embryo viability, our data does not show utility in predicting implantation and pregnancy outcomes. Further investigation of mtDNA both on a clinical and molecular level is needed in order to understand how its content can be interpreted and appropriately applied to increase pregnancy success in the infertility population.
References:
Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–31.
Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–9415.
Gleicher N, Weghofer A & Barah DH 2011. Defining ovarian reserve to better understand ovarian aging. Reproductive Biology and Endocrinology.
Babayev E, et al. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas. 2016;14:121–30.
Cree LM, Hammond ER, Shelling AN, Berg MC, Peek JC, Green MP. Maternal age and ovarian stimulation independently affect oocyte mtDNA copy number and cumulus cell gene expression in bovine clones. Hum Reprod. 2015;30(6):1410–20. https://doi.org/10.1093/humrep/dev066.
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65.
Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction. 2012;143:417–27.
Cao L, Shitara H, Horii T, Nagao Y, Imai H, Abe K, et al. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat Genet. 2007;39:386–90.
Cree LM, Samuels DC, de Sousa Lopes SC, Rajasimha HK, Wonnapinij P, Mann JR, et al. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet. 2008;40:249–54.
Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod. 2005;11:843–6.
Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women under- going in vitro fertilization. Fertil Steril. 2002;78(5):1046–8.
Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64(3):577–83.
Desquiret-Dumas V, Clement A, Seegers V, et al. The mitochondrial DNA content of cumulus granulosa cells in linked to embryo quality. Hum Reprod. 2017;32(3):607–14.
Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–91.
Reynier P, May-Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–9.
Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–75.
Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241.
Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril. 2015;104:534–41. e531
Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2016;107:34–42.
Treff NR, Zhan Y, Tao X, OlchaM HM, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;32(4):954–62.
Bancsi LF, Broekmans FJ, Eijkemans MJ, de Jong FH, Habbema JD, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328–36.
Müller-Höcker J, Schäfer S, Weis S, Münscher C, Strowitzki T. Morphological-cytochemical and molecular genetic analyses of mitochondria in isolated human oocytes in the reproductive age. Mol Hum Reprod. 1996, 2(12):951–8.
Kushnir VA, Ludaway T, Russ RB, Fields EJ, Koczor C, Lewis W. Reproductive aging is associated with decreased mitochondrial abundance and altered structure in murine oocytes. J Assist Reprod Genet. 2012;29(7):637–42.
Boucret L, Chao de la Barca JM, Moriniere C, Desquiret V, Ferre-L’Hotellier V, Descamps P, et al. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015;30:1653–64.
Duran HE, Simsek-Duran F, Oehninger SC, Jones HW Jr, Castora FJ. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil Steril. 2011;96:384–8.
Alexeyev M, Shokolenko I, Wilson G, et al. The maintenance of mitochondrail DNA integrity- critical analysis and update. Cold Spring Harb Perspect Biol. 2013;5:a012641.
Piko L, Taylor KD. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987;123:364–74.
Piko L, Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol. 1976;49:1–10.
Ebert KM, Liem H, Hecht NB. Mitochondrial DNA in the mouse preimplantation embryo. J Reprod Fertil. 1988;82:145–9.
Larsson J, Want H, Wilhemsson A, Oldfors P, Rustin M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–6.
Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn. 1996;205(1):64–72.
Van Blerkom J. Mitochondria as regulatory forces in oocytes, preimplantation embryos and stemcells. Reprod BioMed Online. 2008;16(4):553–69.
DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–68.
Monnot S, Samuels DC, Hesters L, Frydman N, Gigarel N, Burlet P, et al. Mutation dependance of the mitochondrial DNA copy number in the first stages of human embryogenesis. Hum Mol Genet. 2013;22:1867–72.
Sobenin IA, Mitrofanov KY, Zhelankin AV, et al. Quantitative assessment of heteroplasmy of mitochondrial genome: perspectives in diagnostics and methodological pitfalls. Biomed Res Int. 2014;2014(292017):9. https://doi.org/10.1155/2014/292017.
van den Ouweland JMW, Lemkes HHPJ, Ruitenbeek W, et al. Mutation in mitochondrial tRMALeu(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992;1(5):368–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Accepted as poster presentations at the ASRM 2017 Meeting in San Antonio, Texas, and PCRS 2018 Meeting in Indian Wells, California.
Rights and permissions
About this article
Cite this article
Klimczak, A.M., Pacheco, L.E., Lewis, K.E. et al. Embryonal mitochondrial DNA: relationship to embryo quality and transfer outcomes. J Assist Reprod Genet 35, 871–877 (2018). https://doi.org/10.1007/s10815-018-1147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1147-z