Abstract
Purpose
To review the current literature regarding the role of vitamin D status in pregnancy outcomes in women undergoing assisted reproductive technology (ART) and to assess cost-effectiveness of routine vitamin D deficiency screening and repletion prior to initiation of ART.
Methods
A systematic literature review was conducted using PubMed. Relevant study outcomes were compared among the selected studies. A cost-benefit analysis was performed using a decision tree mathematical model with sensitivity analyses from the perspective of direct societal cost. Published data were used to estimate probabilities and costs in 2014 US dollars.
Results
Thirty-four articles were retrieved, of which eight met inclusion criteria. One study demonstrated a negative relationship between vitamin D status and ART outcomes, while two studies showed no association. The remaining five studies concluded that ART outcomes improved after vitamin D repletion.
Conclusion
The majority of reviewed studies reported a decrement in ART outcomes in patients with vitamin D deficiency. Cost-benefit analyses suggested that screening and supplementing vitamin D prior to ART might be cost effective, but further evidence is needed. Given the absence of Level I evidence regarding vitamin D status and ART outcomes, full endorsement of routine vitamin D screening and supplementation prior to ART is premature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physiology of vitamin D
The term vitamin D refers to a group of structurally related, fat-soluble secosteroids that are classically known for calcium-phosphate and bone metabolism. In humans, two key vitamin D compounds are ergocalciferol (D2) and cholecalciferol (D3). D3 is acquired either by dietary intake (fatty fish, egg yolks, and milk) or by endogenous cutaneous synthesis that requires sunlight exposure. Plants produce D2 through the irradiation of ergosterol. Regardless of its starting form, previtamin D undergoes hydroxylation to 25-hydroxyvitamin D (25(OH)D) by 25-hydroxylase, a cytochrome P450-dependent enzyme (Fig. 1). The expression of these enzymes determines the cellular responsiveness of target cells to circulating levels of 25(OH)D. These enzymes are predominantly found in the liver, though they have also been identified in a various other tissues. The final transformation occurs mainly in the kidneys, where 1α-hydroxylase converts 25(OH)D to 1,25-dihydroxyvitamin D (1,25(OH)2D). This molecule, called calcitriol, is the active vitamin D metabolite [1]. Vitamin D modulates intracellular activity through the binding of 1,25(OH)2D to the vitamin D receptor (VDR), a nuclear receptor. Given that vitamin D is endogenously produced in the skin and travels to various tissue targets to activate gene expression, it is classically a hormone with myriad effects. Vitamin D is involved in various non-classical roles in physiologic processes including cell growth, tumorigenesis, hypertension, cardiovascular disease, and autoimmune conditions [2], with mounting evidence that it plays an important role in human reproduction.
Epidemiology of vitamin D deficiency
Several epidemiological studies underscore the widespread prevalence of vitamin D deficiency and insufficiency in the United States and worldwide. Mitchell et al. [3] reported the incidence of vitamin D deficiency (defined as <20 ng/mL) in reproductive age females to be 31 %. Data from the National Health and Nutrition Examination Surveys (NHANES) [4] found that risk varied between racial and ethnic groups, with non-Hispanic blacks demonstrating a higher prevalence of vitamin D deficiency and inadequacy as compared to their white counterparts. These findings were echoed by Forrest et al. [5], who found that blacks had the highest rate of vitamin D deficiency (82.1 %), followed by Hispanics (69.2 %). Despite its prevalence, universal screening for vitamin D deficiency has not been promoted due to the high cost of serum assays. As such, screening has been reserved only for patients at high risk for deficiency [6].
Recommendations for vitamin D intake
Due to its stability, serum 25(OH)D concentration is viewed as the best indicator of vitamin D status. Although there is a lack of consensus on the particular serum level of vitamin D that is considered adequate, >30 ng/mL typically is regarded as sufficient, levels <20 ng/mL are considered deficient, and 21–29 ng/mL is regarded as insufficient [7]. Values exceeding >100 ng/mL are associated with vitamin D toxicity [7]. According to both the 2010 Institute of Medicine (IOM) guidelines [8] and the 2011 Endocrine Society guidelines [7], the average recommended daily allowance (RDA) of vitamin D for individuals 1–70 years of age is 600 IU. Both sets of guidelines recommend this same dose in women who are pregnant or lactating, and in their 2011 practice guidelines, the American College of Obstetrics and Gynecology (ACOG) expressed their agreement [9]. For women with vitamin D deficiency or insufficiency, the Endocrine Society recommends 50,000 IU/week for a total of 8 weeks followed by maintenance therapy of between 1500 and 2000 IU/day to achieve a serum vitamin D level of >30 ng/mL [7].
Vitamin D in reproduction
Male reproduction
Several studies set out to evaluate the association between hypovitaminosis D and reproduction in the male. Early studies revealed decreased spermatogenesis and fertility rates in vitamin D deficient rats compared to their replete male counterparts [10]. Further animal studies corroborated these findings by demonstrating VDR expression in target organs of the male reproductive tract; namely, the testis, epididymis, prostate, and seminal vesicles [11–13], as well as in human sperm [14, 15]. Results have been mixed with respect to VDR expression and semen quality. In vitro studies showed that VDR induction mediated increased intracytoplasmic calcium in spermatozoa, resulting in increased motility and concomitant induction of the acrosomal reaction in capacitated sperm [16]. Furthermore, expression of VDR and CYP24A1 (a cytochrome P450 hydroxylase enzyme) in the annulus of sperm have been linked to all semen parameters, with higher expression of CYP24A1 associated with increased sperm motility in fertile compared to subfertile men [17]. However, two separate cross-sectional studies performed 1 year later failed to show a significant association between low serum vitamin D levels and poor semen quality [18, 19].
Pregnancy loss
Recent studies demonstrate a link between vitamin D deficiency and pregnancy loss which is possibly mediated by effector CD4+ T helper (Th) cellular responses in the innate (Th1) and adaptive (Th2) immune systems. Vitamin D is postulated to enhance the Th2 response by elevating interleukin (IL)-4, IL-5, and IL-13 and inhibiting the Th1 response of IL-1, IL-2, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). As a micronutrient, it is considered a modifiable factor that is thought to aid implantation by inhibiting the Th1 activation of natural killer (NK) cells by inhibiting the production of IFN-γ [20].
In a retrospective cross sectional study of 133 women diagnosed with recurrent pregnancy loss, Ota et al. [21] reported that 63 out of 133 women (47.4 %) had hypovitaminosis D (less than 30 ng/ml) and that NK cell levels and NK cytotoxicity at effector to target cell (E:T) ratio of 25:1 were significantly higher in the low vitamin D group when compared with subjects with normal vitamin D levels. In addition, in vitro NK cytotoxicity assays demonstrated significant suppression at an E:T ratio of 50:1 when treated with Vitamin D compared to the control group, demonstrating evidence that low Vitamin D may be associated with increased NK cell activity in women with recurrent pregnancy loss. Additionally, in a retrospective cohort study of 115 women with antiphospholipid syndrome (APS), median vitamin D levels in APS patients were significantly lower compared to healthy volunteers (n = 128), although the authors report possible confounding factors related to provider recommendation of increased sunscreen use in the APS cohort [22]. Tavakoli et al. [23] reported similar cytokine profiles and vitamin D expression of whole endometrial cells obtained from women with unexplained recurrent pregnancy loss (n = 8) compared to age matched controls (n = 8), although the authors do not address the limitations of the small sample size. A recent study evaluated the effect of vitamin D treatment with and without low molecular weight heparin on an antiphospholipid antibody-induced inflammatory response of human first trimester trophoblast (HTR8) and primary trophoblast cell lines [24]. This study reported a decrease in the inflammatory response with vitamin D treatment by measurement of IL-8 [24]. Also of note, vitamin D has been associated with increased risk of preeclampsia [25], infants that are small for gestational age (SGA) [26–29], cesarean section [30], and gestational diabetes (GDM) [31].
Endometriosis
Given the presence of VDR in the endometrium as well as the role of vitamin D in immune regulation, a connection has been proposed between vitamin D status and endometriosis—a disease thought to be caused by aberrant immune and inflammatory responses [32]. A study by Agic et al. [33] showed that women with endometriosis overexpressed endometrial VDR and 1α-hydroxylase. Although serum levels of vitamin D did not differ between cases and controls, this study may lend credence to the theory that individuals with endometriosis have immune hypersensitivity to even normal levels of vitamin D. However, another study demonstrated a significantly increased risk of endometriosis at higher serum levels of vitamin D, with even higher levels measured in women with advanced disease [34]. Attempts to identify VDR polymorphisms associated with endometriosis have thus far been unsuccessful.
Polycystic ovarian syndrome
Women with polycystic ovarian syndrome (PCOS) commonly present with insulin resistance and obesity [35]. Interestingly, there is increasing evidence that vitamin D affects insulin and glucose metabolism [36–39], and although the exact pathophysiology remains somewhat elusive, several studies have examined this association. A number of studies have demonstrated an increase in serum parathyroid hormone (PTH) when vitamin D levels are low [40–42]. This increase is suspected to be involved in glucose metabolism and decreased insulin sensitivity. Other studies suggest that vitamin D stimulates the expression of insulin receptors, which in turn augments insulin responsiveness for glucose transport [43, 44]. Another important consideration is that the vitamin D/VDR complex regulates over 300 genes, including genes involved in glucose metabolism [45].
Given the substantial body of evidence supporting a link between vitamin D status and insulin/glucose metabolism, several authors have examined vitamin D status in PCOS patients. While some studies reported lower levels of vitamin D in women with PCOS [46, 47], other studies suggested that levels are comparable [48] or even higher compared to women with PCOS [49]. Several studies have reported an inverse relationship between body mass index (BMI) and vitamin D levels in PCOS patients [50–52]. Considering that vitamin D is a fat soluble hormone, vitamin D sequestration in adipose tissue could explain the increased rates of vitamin D deficiency in women with PCOS [34]. An alternative theory suggests that obese individuals spend less time outdoors exposed to the sun, and consequently have insufficient cutaneous vitamin D biosynthesis [53].
Wehr et al. [46] identified polymorphisms in the VDR gene associated with vitamin D levels in PCOS women, suggesting a role of vitamin D in the pathogenesis of PCOS. In a recent systematic review of 275 publications reporting on the effects of advanced glycation end products (AGEs) on female reproduction, Merhi [54] concluded that AGEs might contribute to the etiology of PCOS and infertility. AGEs, which are modified proteins, lipids, or nucleic acids that are nonenzymatically glycated and oxidized by glucose, damage cellular structures and have been implicated in a number of diseases [55]. Research has demonstrated a strong association between the AGE-RAGE (receptor for AGEs) system and certain aspects of PCOS, including obesity [56], insulin resistance [57] [58], granulosa cell dysfunction [59], and adipocyte dysfunction [60]. In their studies using a human granulosa cell line, Diamanti-Kandarakis et al. [61] have shown that the AGE-RAGE system could be the cause of the ovulation failure commonly seen in PCOS. In particular, the investigators demonstrated that AGEs interfere with the action of luteinizing hormone. This interference impairs normal follicle development and the initiation of ovulation [61].
Vitamin D and female infertility
Studies in female rodents have yielded comparable results to those in males. As in male rats, VDR has been isolated from the female reproductive organs—including the ovaries, fallopian tubes, and endometrium of the uterus [12]—thereby affirming its potential role as a genomic regulator of reproduction. In VDR null mice, uterine hypoplasia, impaired folliculogenesis, and infertility have been noted [62, 63]. One study demonstrated a decline in fertility rates by as much as 75 % in vitamin D deficient female rats, as reflected in decreased litter size [64].
Given this apparent association between vitamin D deficiency and infertility, it is not surprising that a number of studies have examined the effects of vitamin D status on pregnancy rates. In particular, there has been a recent focus on the effects of vitamin D deficiency on assisted reproductive technology (ART) outcomes. Examining women undergoing ART is an ideal scenario in which to study pregnancy outcomes given the short time-to-pregnancy, which allows for rapid determination of a successful intervention. If the link between vitamin D status and ART outcomes is strengthened, it is very possible that vitamin D supplementation could improve ART outcomes. This systematic review focuses on the current literature regarding the consequences of vitamin D deficiency on ART outcomes, and by means of a cost-benefit analysis, seeks to answer the question: should vitamin D be routinely screened and subsequently repleted in women undergoing infertility treatment?
Materials and methods
Search strategy and selection criteria
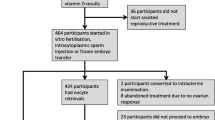
A systematic PubMed search was performed for all available, full-text, English articles published from inception of PubMed to August 11, 2014 in accordance with the Preferred Outcome Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [65]. The following key words were used for the search: “vitamin D,” “cholecalciferol,” “ergocalciferol,” “calcidiol,” “calcitriol,” “25-hydroxyvitamin D,” “25-hydroxycholecalciferol,” “1,25-dihydroxyvitamin D,” “1,25-cholecalciferol,” “assisted reproduction,” “assisted reproductive technology,” “in vitro fertilization,” and “IVF.” Thirty-four articles were identified. Studies were selected if they measured serum and/or follicular fluid (FF) vitamin D levels prior to ART cycles and if they provided outcome data of interest (implantation rates, fertilization rates, clinical pregnancy (CP) rates, and/or live birth rates). Review articles, studies that did not focus on infertile women utilizing ART, and studies that lacked measured vitamin D levels (serum or FF) or outcomes of interest were excluded. Only eight of the 34 articles retrieved from PubMed met criteria (Fig. 2) [66–73]. Reference lists of chosen studies and narrative reviews were cross-referenced to find additional studies. Additionally, an online Google search of the terms “vitamin D” and “IVF” was performed. No further studies were rendered in this manner. A systematic review was subsequently conducted. Currently, data were insufficient to perform a meta-analysis given the absence of published data from RCTs. All substantive data were extracted and distilled systematically into table format.
Cost-benefit analysis
A decision tree mathematical model was devised to compare the cost of a single ART cycle when the subject was either: a) tested, found to have a deficient vitamin D level, and was not repleted (“No treatment” strategy) or, b) the subject was tested, found to have a deficient vitamin D level and was repleted to normal levels before the ART cycle (“Treatment” strategy) (Fig. 3). A societal perspective was employed and only estimated direct costs were considered. Vitamin D deficiency was defined as <20 ng/mL in accordance with the Endocrine Society guidelines [7], and also consistent with the definition utilized by the majority the trials included in this review [67–72]. The study by Polyzos et al. [73] was selected for utilization of clinical pregnancy and ongoing pregnancy rates for mathematical modeling secondary to reporting the largest cohort, recency of publication and attempting to control for embryo quality and quantity by including single blastocyst ART cycles only. CP rates in vitamin D deficient versus vitamin D not deficient women undergoing ART were 41 and 54 %, respectively and ongoing pregnancy rates (utilizing live birth rates reported) of 35 and 48 % respectively. The model assumed that everyone who was screened and found to be deficient was treated with supplementation. Adherence to supplementation, effectiveness of supplementation, and lack of adverse effects (albeit rare) from supplementation was assumed. Additionally, all deficient patients were supplemented equally, regardless of degree of deficiency or patient characteristics (e.g. age, sex, BMI, race, comorbidities, etc.). The prevalence of vitamin D deficiency in US reproductive age females undergoing ART (65 %) was derived from Polyzos et al. [73] as well as previously described by Mitchell et al. [3] (31 %).
Direct medical costs for the model were retrieved from a variety of sources. All costs were adjusted to 2014 US dollars (USD) according to the Consumer Price Index (CPI) [74]. Indirect costs, such as lost wages from missed work during ART treatments, were not taken into account. The cost of a single serum vitamin D assay (2014 US $52.53) was determined from published reimbursement schedules provided by the 2014 Centers for Medicare and Medicaid Services [75]. We estimated that an individual would be tested an average of three times during the course of her treatment—the initial screening, after completing repletion therapy, and an additional test if the patient had either not been adequately repleted initially or to confirm vitamin D sufficiency during her ART treatment. Thus, the total estimated cost of testing was $158 (2014 USD). As discussed previously, the Endocrine Society recommends repletion with 50,000 IU per week for 8 weeks to achieve a serum vitamin D level of >30 ng/mL, followed by a maintenance dose of 2000 IU per day. Based on the average cost from 20 nearby pharmacies [76], the cost of vitamin D supplementation was determined to be $0.029 per 1000 IU. The cost for the initial 8 weeks was thus is estimated to be $11.63 (2014 USD). Assuming that one ART cycle extends for 6 weeks, the cost of maintenance for this period is approximately $2.44.
The cost of a single ART cycle, based on the most recent published data (2006 US $12,513) [77], equated to $14,794 in 2014 USD. After adjusting for CPI, the published cost of a SAB (2006 US $1,500) was $1,831 [78]. All costs hereafter were corrected to 2014 USD. The total cost for the no treatment strategy (single ART cycle plus an initial vitamin D screen) was calculated to be $14,847. The total estimated cost for the treatment strategy (single ART cycle with initial vitamin D screening, repletion following a result indicating deficiency, and retesting twice to confirm replete levels) was $14,967. One-way sensitivity analyses were performed over a range of pregnancy rates and costs to compare a vitamin D tested and treated single blastocyst fresh cycle versus a vitamin D tested and not treated single blastocyst fresh cycle. Ranges were selected broadly to cover the maximum possible range, based on data obtained from individual clinics as well as published United States averages.
Cost per ongoing pregnancy was estimated for each strategy using a data driven mathematical decision tree probability model based on the inputs described. Sensitivity analyses were performed varying the ongoing pregnancy rates for the vitamin D treatment group over the range 25–95 % to cover all potential rates while holding the ongoing pregnancy rate for the not treated group at 35 %. A sensitivity analysis was also performed varying the cost of vitamin D treatment and single ART cycle from $17,000 to $28,000 while setting the cost for a non-treated cycle at $14,847.
Results
Systematic review on effects of vitamin D on ART outcomes
The main characteristics and relevant findings of the eight studies that examined that impact of vitamin D status on ART outcomes are summarized in Table 1. Ozkan et al. [69] were the first to report a positive correlation between vitamin D levels and IVF outcomes. In their prospective cohort study, women achieving CP demonstrated significantly higher FF vitamin D levels compared to women who did not achieve CP (34.42 ng/mL ± 15.58 versus 25.62 ng/mL ± 10.53, p = 0.013). Interestingly, they reported that each ng/mL increase in FF vitamin D corresponded to a 7 % increase in CP rate (p = 0.013). When vitamin D status was evaluated by tertiles, a significant increase in implantation and CP rates was observed across the tertiles (p = 0.041 and p = 0.029, respectively). Furthermore, patients in the highest tertile were nearly four times more likely to achieve CP than those in the lowest tertile (OR 3.83, 95 % 1.20–12.28, p = 0.024). Ozkan et al. did not observe a difference between vitamin D concentration and ovarian reserve or ovarian response parameters (e.g. age or follicle stimulating hormone levels, duration of controlled ovarian stimulation, number of follicles, number of eggs retrieved, or maximal estradiol levels). This finding, considered in the setting of improved implantation and CP rates, corroborates the theory that vitamin D exerts its effects mainly through endometrial pathways.
Garbedian et al. [72] confirmed these findings in their study of a group of infertile Canadian women who subsequently underwent oocyte retrieval. Women who were vitamin D deficient were grouped together with those who were vitamin D insufficient, due to an inadequate sample size in the deficient group (1.2 and 53.8 %, respectively). Women with sufficient vitamin D levels had significantly higher rates of CP per ART cycle than women with insufficient/deficient levels (52.5 % versus 34.7 %, p < 0.001). Vitamin D status was determined to be an independent predictor of CP even after adjusting for age, BMI, and date of embryo transfer in a logistic regression analysis. Implantation rates were also higher in the sufficient group, though this finding did not reach statistical significance (34.5 % versus 25.6 %, p = 0.06).
In the largest and most recent study, Polyzos et al. [73] sought to determine the effect that vitamin D deficiency has on pregnancy rates among women undergoing IVF/ICSI (intracytoplasmic sperm injection) with Day 5 single embryo transfer (SET). In their study, serum vitamin D concentrations were measured retrospectively in 368 infertile women who underwent SET on Day 5. The authors of this study attempted to improve upon earlier research by limiting their study only to women undergoing IVF/ICSI and Day 5 SET. Whereas this study utilized a single embryo transfer policy, previous studies employed no such policy, with patients receiving up to four embryos per transfer. As the authors point out, this could be a potential confounding factor in the previous studies. Another strength of their study design was including only women who reached the Day 5 embryo transfer stage. Doing so hypothetically eliminated patients with poor embryo quality, effectively minimizing any biases stemming from oocyte/embryo quality. The major limitation of this study was the absence of any data regarding maternal vitamin D levels until delivery and any potential vitamin D intake throughout the pregnancy.
Unlike the previously discussed studies, Polyzos and colleagues [73] only divided women into two divisions: vitamin D deficient (<20 nL/mg) and not vitamin D deficient (≥20 nL/mg). Overall, 46 % of patients achieved CP. CP rates were significantly lower in vitamin D deficient women compared to their non-deficient counterparts (41 % versus 54 %, p = 0.015). Additionally, a logistic regression analysis was performed to control for 16 potential confounding factors. The results of the regression revealed that vitamin D deficiency was independently associated with lower CP rates (OR 0.61, 95 % 0.39–0.95, p = 0.030). Similar to the suggestions made by Ozkan et al. [69], Polyzos et al. postulate that this finding might be due to a deleterious effect on endometrial receptivity.
Unlike the previously discussed studies, Aleyasin et al. [67] found no relationship between serum vitamin D concentration and fertilization, implantation, or pregnancy rates in their study of 82 Iranian women. Similar to Ozkan et al. [69], subjects were evaluated by tertiles according to vitamin D level [67]. Interestingly, the fertilization rates in all tertiles were exceptionally high (79.0, 67.8, and 68.3 %, from lowest to highest tertiles). Although there was an upward trend of implantation rates with increasing tertiles of FF vitamin D, no significant linear association was demonstrated (9.6, 13, and 15.9 %, p = 0.791). Likewise, FF vitamin D levels were related to neither fertilization rates (p = 0.274) nor chemical/clinical/ongoing pregnancies (p = 0.959, 0.995, and 0.604, respectively) across tertiles. When logistic regression analysis was applied, FF vitamin D levels were not shown to be an independent predictor of CP. While the small sample size and largely vitamin D deficient population in this study [67] make it difficult to draw any solid conclusions, the high fertilization rates demonstrated in this population suggests that vitamin D stores may not be a player in infertility.
A study conducted by Firouzabadi et al. [66] supported these findings. This prospective cohort study of 180 infertile Iranian women again demonstrated no significant association between serum or FF vitamin D levels and CP rates. Overall, the CP rate was 33.48 %. However, there was no significant difference in the pregnancy rates between the three groups (p = 0.094). Furthermore, FF vitamin D levels did not differ significantly between pregnant and non-pregnant groups. Fertilization rates trended upwards with improvements in vitamin D status, though not significantly (43.17, 53.37, 58.77 %, p = 0.054). Implantation rates similarly were not significantly associated with FF vitamin D levels (17.33, 15.26, and 18.75 %, p = 0.579). Firouzabadi and colleagues [66] also examined embryo quality between deficient, insufficient, and sufficient groups, and found no significant difference (p = 0.372). They attributed their null findings to an inadequate sample size. Additionally, their participants were overwhelmingly vitamin D insufficient or deficient, mirroring findings by Aleyasin et al. [67] in a similar population.
In a retrospective study, Rudick et al. (2012) [70] examined 188 infertile women, and found no association between vitamin D status and fertilization rates. However, when broken down by race, increased serum vitamin D levels were related to higher implantation (p = 0.01), CP (p = 0.01), and live birth (p = 0.01) rates among non-Hispanic white women across vitamin D tertiles. Similar to Ozkan et al. [69], Rudick and colleagues did not observe an association between ovarian stimulation parameters or embryo scores and vitamin D levels, suggesting that vitamin D modulates its effects on fertility through the endometrium.
A follow-up study examining the relationship between recipient vitamin D levels and pregnancy rates in donor-recipient ART cycles was subsequently performed to test this hypothesis [71]. In that study, Rudick et al. [71] found no significant differences between vitamin D levels and ovarian stimulation parameters, fertilization rates, embryo quality markers, or number of embryos transferred. CP rates decreased with worsening vitamin D status, from 74 % in replete recipients to 35 % in recipients who were vitamin D deficient (p = 0.002). After adjusting for potential confounders (including donor/recipient age, recipient BMI, parity, infertility diagnosis, stimulation protocol/ parameters, season of transfer, number of embryos transferred, and markers of embryo quality), significant improvements in CP rates were only seen in repleted individuals (78 % in replete versus 37 % in insufficient/deficient groups, p = 0.004). This association was also observed in adjusted live birth rates (p = 0.04). Implantation rates were not examined in this study. Rudick et al. [71] surmised that since there was no significant difference seen in pregnancy rates between vitamin D insufficient versus deficient groups, there was a “threshold effect” whereby effective IVF outcomes were associated with replete status as opposed to lower concentrations. However, since this study did not focus on donor characteristics, it is possible that some of these unmeasured parameters may have affected the results presented in that study.
As was posited in studies of nondonor IVF patients by Ozkan et al. [69], Polyzos et al. [73] and the previous Rudick et al. [70] publication, this study indicates that the effects of vitamin D are likely at the level of the endometrium. While these previous studies in nondonor IVF patients were mostly speculative in this regard, by using the oocyte donor-recipient model, Rudick et al. [71] directly evaluated the role of the endometrium and its impact on implantation, and is consistent with the postulate that vitamin D modulates NK cell activity. In addition, VDR expression has been demonstrated in the endometrium [12] and up-regulation of HOXA10—a protein essential for embryo implantation and fertility—has been demonstrated following administration of vitamin D. Furthermore, experiments in mice have shown diminished implantation in association with reduced maternal HOXA10 expression [79]. Taken collectively, these findings strongly suggest that vitamin D signaling contributes to successful embryo implantation.
Perhaps the most surprising findings were those by Anifandis et al. [68], which suggested a detrimental effect of increasing FF vitamin D levels on IVF outcomes in their study of 101 infertile Greek women undergoing IVF-ICSI ovarian stimulation cycles. Women with replete FF vitamin D status had lower CP rates per embryo transfer (40, 38.1, and 15.8 %, respectively, p < 0.05) and poorer embryo quality (mean score of embryo quality 5.6 ± 3.6 vs 7.02 ± 2.5 and 7.96 ± 2.6 respectively, p < 0.05) as compared to women with insufficient or deficient levels. Furthermore, FF vitamin D levels were negatively correlated with embryo quality (r = −0.27, p < 0.05). FF glucose levels were also measured, as the authors hypothesized that glucose provides an essential substrate for oocyte energy production, which may influence IVF outcomes. As serum vitamin D concentrations increased, FF glucose levels declined (r = −0.25, p < 0.05). Anifandis et al. theorized that vitamin D may have a physiological effect on insulin and glucose metabolism in a manner that has not yet been elucidated, and that perhaps this interaction decreases the availability of glucose to the oocyte. This suggestion that the effect of vitamin D occurs at the level of the oocyte opposes the findings of Polyzos et al. [73], Ozkan et al. [69], and Rudick et al. [70, 71]. which suggested a deleterious effect of vitamin D deficiency on endometrial receptivity.
Vitamin D deficiency prevalence
All eight of the cohort studies reported the prevalence of vitamin D deficiency amongst their participants, as is depicted in Table 1. However, only six of these categorized serum vitamin D levels according to the ranges set forth by the Endocrine Society [67–72] (vitamin D deficiency <20 ng/ml, insufficiency 20–30 ng/ml, and replete >30 ng/ml). When the prevalence of each category reported by each of these six studies were weighted for sample size and subsequently averaged, 27.7 % were vitamin D deficient, 38.4 % were insufficient, and 33.8 % were replete. This overall prevalence of vitamin D deficiency was slightly lower than what has been reported for the general population of childbearing age women in the US (31 %) [3]. As Vanni et al. [80] reported, a lower prevalence of vitamin D deficiency is to be expected in a population of women seeking IVF, as these women tend to have higher socioeconomic status as well as education level, both of which are factors associated with higher vitamin D levels. Garbedian et al. [72] suggested that the low number of vitamin D deficient women reported in their study (1.2 %) was due to increased usage of daily prenatal vitamins. However, a majority of their participants (55 %) did not meet criteria for vitamin D repletion despite supplementation.
Serum and follicular fluid levels of vitamin D
Four of the eight studies correlated serum and FF levels of 25(OH)D [66–69]. All four studies found that these two assays of vitamin D stores were significantly and strongly correlated with each other. (Aleyasin et al.: r = 0.77, p < 0.001; Anifandis et al.: r = 0.79, p < 0.001; Firouzabadi et al.: r = 0.83, p = 0.001; Ozkan et al.: r = 0.94, p < 0.001). This finding is important because it suggests that peripheral vitamin D status is, indeed, a reliable indicator of 25(OH)D availability within the ovary [80].
Vitamin D and patient demographics
Most of the included studies analyzed a number of patient demographics for significant differences across groups of vitamin D status. However, the findings were inconsistent and contradictory. According to Rudick et al. [70], women categorized as vitamin D deficient were significantly younger than those not deficient (p = 0.03). However, none of the other studies corroborated this finding [67, 68, 72, 73]. Of the seven studies that reported data on BMI, four found no significant association with vitamin D status [67, 68, 71, 73]. In their most recent study, Rudick et al. [71] found that when serum vitamin D levels were used as a continuous variable, there was an inverse relationship with weight, though no significance was achieved (p = 0.20). In their first study, however, Rudick et al. [70] reported that vitamin D deficient women were significantly heavier (p = 0.03). Of note, all participants in this study had BMI values within the normal range. Similarly, Garbedian et al. [72] found that BMI was significantly higher (p = 0.02) in women with vitamin D insufficiency/deficiency (24.8) as compared to their replete counterparts (23.3), and Ozkan et al. [69] noted a significant inverse correlation between FF vitamin D levels and BMI (r = −0.25, p = 0.035).
Four studies examined the effects of race with regard to vitamin D concentrations and cycle parameters [69–72]. Garbedian et al. [72] did not find a significant association between vitamin D status and race (p = 0.3). Ozkan et al. [69] reported that black patients had lower FF vitamin D levels than nonblack patients (18.88 ± 8.5 ng/mL versus 30.51 ± 12.95 ng/mL, p = 0.001). This finding of lower vitamin D levels in blacks is consistent with previous findings in a non-infertile population [5]. The first Rudick et al. [70] study reported Hispanic whites as having significantly lower serum vitamin D levels than Asians or non-Hispanic whites (p = 0.01). In their second study, however, [71] they reported lower vitamin D levels among Asians and African-Americans compared to Hispanic or non-Hispanic whites (p = 0.02). Previous studies reported that Asian women have lower success rates with IVF treatment [81, 82]. Studies published by Gleicher et al. [83, 84] have pointed to diminished ovarian function among Asian women, which may contribute to poorer IVF outcomes.
Only three studies reported any findings on comorbid conditions amongst patients. Aleyasin et al. [67] reported that PCOS affected 20.6 % of participants, though serum and FF vitamin D levels did not differ significantly between PCOS and non-PCOS patients (p = 0.938 and 0.158, respectively). Ozkan et al. [69] noted lower levels of FF vitamin D levels among women with PCOS and diminished ovarian reserve (DOR) as compared to other diagnosed infertility etiologies. However, these findings did not reach statistical significance (p > 0.05). Rudick et al. [70] found that women deficient in vitamin D were significantly less likely to have a diagnosis of DOR (p = 0.01).
Cost-benefit analysis
The cost per ongoing pregnancy of an untreated, vitamin D deficient ART cycle was estimated to be $42,734 compared to a cost per ongoing pregnancy of $31,410 of a tested and repleted vitamin D ART cycle (2014 USD). After adjusting for a population with a vitamin D deficiency prevalence of 65 % [73], the average cost per OPR was $38,771, while adjusting for a population with a vitamin D deficiency prevalence of 31 % [3] was $34,920. Sensitivity analyses demonstrated that, if the ongoing pregnancy rate (OPR) of a single not-treated vitamin D deficient cycle was set at 35 %, then a vitamin D treated cycle was cost-effective when the OPR achieved by ART was 38 % or greater (Fig. 4). If the OPRs for treated and not-treated cycles are set at the national averages reported in the literature and the cost for a not-treated cycle was set at $14,847, then a treated approach was cost-effective when the cost of a treated cycle was less than $20,500 (Fig. 5). These data suggest that since a sizeable proportion of ART clinics in the US offer services at less than $20,500 per cycle with an OPR of approximately 38 %, in the majority of cases there might be substantially decreased costs for patients with vitamin D assessment and treatment.
Cost-benefit sensitivity analysis varying the ongoing pregnancy rate of an ART cycle with screening and treatment with vitamin D supplementation. The ongoing pregnancy rate of an untreated ART cycle was set at 38 %. Solid black line, treatment strategy; dashed gray line, no treatment strategy. X-axis, ongoing pregnancy rate (OPR); Y-axis, cost per ongoing pregnancy (2014 USD)
Cost-benefit sensitivity analysis varying the cost of single ART cycle with vitamin D supplementation included. The cost for a single ART untreated cycle was set at $14,794. Solid black line, treatment strategy; dashed gray line, no treatment strategy. X-axis, cost per treated cycle (2014 USD), Y-axis, cost per ongoing pregnancy (2014 USD)
A caveat and notable limitation of our cost-benefit analysis is that current evidence regarding vitamin D replacement in the setting of ART is based on limited information. We used the study by Polyzos et al. [73] for modeling the analysis, because it represented the largest cohort, was recently published, and controlled for embryo quality by including single blastocyst ART cycles only. Consequently, our analysis depends greatly on that sole report. However, the strength of the effects of vitamin D deficiency upon pregnancy outcomes at ART is not conclusively proven. Ideally, a cost-benefit analysis would be informed by several studies and a clear consensus of evidence; a situation that does not presently exist for vitamin D and ART. Nonetheless, our analysis underscores the pressing need for additional outcome data, given the potential beneficial effect of replacement in this clinical scenario.
Conclusion
Current data regarding vitamin D status and ART outcomes are conflicting, but the majority of studies support replacement of vitamin D in deficient patients. The inconsistencies between studies are likely attributable to multiple confounding variables and insufficient sample size, and highlight the need for randomized controlled trials (RCTs). At the time of this review, there is an ongoing RCT examining the effects of vitamin D status on ART outcomes [85]; however, no data have been published. Furthermore, future research must examine the benefits of supplementation in different populations, as our review revealed differences across races, BMIs, and ages.
Given the absence of Level I evidence regarding vitamin D supplementation, full endorsement of routine vitamin D screening and supplementation prior to ART cannot be made at this time. Nevertheless, the high prevalence of vitamin D deficiency, the beneficial non-classical role of vitamin D in human reproduction, and the increasing use of screening by primary care physicians during routine annual visits, all provide support for screening and supplementation in ART. Other key motivations for ART practices to consider is that the majority of studies detailed in this review report decreased pregnancy outcomes with vitamin D deficiency and amelioration of deficient vitamin D levels is safe, accessible, and inexpensive. Cost-benefit analysis for a single ART cycle involving fresh single blastocyst embryo transfer suggests that screening and supplementing vitamin D prior to ART might significantly decrease societal cost per ongoing pregnancy by implementing a simple intervention, if the magnitude of the observed effect was confirmed in future studies.
References
Bouillon R, Carmeliet G, Daci E, Segaert S, Verstuyf A. Vitamin D metabolism and action. Osteoporos Int. 1998;8(2 Suppl):S13–9.
Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S–9S.
Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie SA. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract. 2012;18(6):914–23.
Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011;59:1–8.
Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54.
Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85(8):752–8.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academy Press; 2010.
American College of Obstetricians and Gynecologists. Vitamin D: screening and supplementation during pregnancy. Committee opinion no. 495. Obstet Gynecol. 2011;118:197–8.
Kwiecinski GG, Petrie GI, Deluca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989;119(5):741–4.
Jensen MB, Nielsen JE, Jorgensen A, Meyts ER-D, Kristensen DM, Jorgensen N, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25(5):1303–11.
Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical detection and distribution of the 1,25-dihydroxyvitamin D-3 receptor in rat reproductive tissues. Histochem Cell Biol. 1996;105(1):7–15.
Schleicher G, Privette TH, Stumpf WE. Distribuition of soltriol [1,25(OH)2-vitamin-D3] binding sites in male sex organs of the mouse: an autoradiographic study. J Histochem Cytochem. 1989;37(7):1083–6.
Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. 2006;68(6):1345–9.
Aquila S, Guido C, Perrotta I, Tripepi S, Nastro A, Ando S. Human sperm anatomy: ultrastructural localization of 1 alpha,25-dihydroxyvitamin D(3) receptor and its possible role in the human male gamete. J Anat. 2008;213(5):555–64.
Jensen MB, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, Olesen IA, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Obstet Gynecol Surv. 2011;66(9):556–8.
Blomberg Jensen M, Jorgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. 2012;35(4):499–510.
Ramlau-Hansen CH, Moeller UK, Bonde JP, Olsen J, Thulstrup AM. Are serum levels of vitamin D associated with semen quality? Results from a cross-sectional study in young healthy men. Fertil Steril. 2011;95(3):1000–4.
Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14(6):855–9.
Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90.
Ota K, Dambaeva S, Han AR, Beaman K, Gilman-Sachs A, Kwak-Kim J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum Reprod. 2014;29(2):208–19.
Andreoli L, Piantoni S, Dall’Ara F, Allegri F, Meroni PL, Tincani A. Vitamin D and antiphospholipid syndrome. Lupus. 2012;21(7):736–40.
Tavakoli M, Jeddi-Tehrani M, Salek-Moghaddam A, Rajaei S, Mohammadzadeh A, Sheikhhasani S, et al. Effects of 1,25(OH)2 vitamin D3 on cytokine production by endometrial cells of women with recurrent spontaneous abortion. Fertil Steril. 2011;96(3):751–7.
Gysler SM, Mulla MJ, Stuhlman M, Sfakianaki AK, Paidas MJ, Stanwood NL, et al. Vitamin D reverses aPL-induced inflammation and LMWH-induced sFlt-1 release by human trophoblast. Am J Reprod Immunol. 2014.
Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517–22.
Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010;140(5):999–1006.
Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. 2006;174(9):1273–7.
Brooke OG, Brown IRF, Bone CDM, Carter ND, Cleeve HJW, Maxwell JD, et al. Vitamin-D supplements in pregnant Asian women: effects on calcium status and fetal growth. BMJ. 1980;280(6216):751–4.
Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91(3):906–12.
Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940–5.
Poel YHM, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur J Intern Med. 2012;23(5):465–9.
Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10.
Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. 2007;14(5):486–97.
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3.
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31.
Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33–83.
Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65(9):1005–15.
Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5.
Tai K, Need AG, Horowitz M, Chapman IM. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition. 2008;24(3):279–85.
Chiu KC, Chuang LM, Lee NP, Ryu JM, McGullam JL, Tsai GP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49:1501–5.
McCarty MF, Thomas CA. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis-implications for the impact of calcium, vitamin D, and alcohol on body weight. Med Hypotheses. 2003;61:535–42.
Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–55.
Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–91.
Maestro B, Davila N, Carranza MC, Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223–30.
Kim JJ, Choi YM, Chae SJ, Hwang KR, Yoon SH, Kim MJ, et al. Vitamin D deficiency in women with polycystic ovary syndrome. Clin Exp Reprod Med. 2014;41(2):80–5.
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164:741–9.
Mazloomi S, Sharifi F, Hajihosseini R, Kalantari S, Mazloomzadeh S. Association between hypoadiponectinemia and low serum concentrations of calcium and vitamin D in women with polycystic ovary syndrome. ISRN Endocrinol. 2012;2012:949427.
Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism. 2011;60(10):1475–81.
Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z. Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril. 2010;93(4):1208–14.
Panidis D, Balaris C, Farmakiotis D, Rousso D, Kourtis A, Balaris V, et al. Serum parathyroid hormone concentrations are increased in women with polycystic ovary syndrome. Clin Chem. 2005;51(9):1691–7.
Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114(10):577–83.
Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280(4):559–63.
Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29(9):3713–20.
Merhi Z. Advanced glycation end products and their relevance in female reproduction. Hum Reprod. 2014;29(1):135–45.
Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–46.
Vazzana N, Guagnano MT, Cuccurullo C, Ferrante E, Lattanzio S, Liani R, et al. Endogenous secretory RAGE in obese women: association with platelet activation and oxidative stress. J Clin Endocrinol Metab. 2012;97(9):E1726–30.
Unoki H, Yamagishi S. Advanced glycation end products and insulin resistance. Curr Pharm Des. 2008;14(10):987–9.
Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2005;62(1):37–43.
Diamanti-Kandarakis E, Piperi C, Patsouris E, Korkolopoulou P, Panidis D, Pawelczyk L, et al. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem Cell Biol. 2007;127(6):581–9.
Jia X, Chang T, Wilson TW, Wu L. Methylglyoxal mediates adipocyte proliferation by increasing phosphorylation of Akt1. PLoS One. 2012;7(5):e36610.
Diamanti-Kandarakis E, Piperi C, Livadas S, Kandaraki E, Papageorgiou E, Koutsilieris M. Interference of AGE-RAGE signaling with steroidogenic enzyme action in human ovarian cells. San Francisco: Endocrine Society; 2013.
Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16(4):391–6.
Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141(4):1317–24.
Halloran BP, Deluca HF. Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr. 1980;110(8):1573–80.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Firouzabadi RD, Aflatoonian A, Modarresi S, Sekhavat L, MohammadTaheri S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement Ther Clin Pract. 2012;18(2):85–8.
Aleyasin A, Hosseini MA, Mahdavi A, Safdarian L, Fallahi P, Mohajeri MR, et al. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):132–7.
Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Bio Endocrinol. 2010;8:91.
Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94(4):1314–9.
Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod. 2012;27(11):3321–7.
Rudick BJIS, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril. 2014;101(2):447–52.
Garbedian KBM, Moody J, Liu K. Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open. 2013;1(2):E77–82.
Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, et al. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod. 2014.
Bureau of Labor Statistics. Consumer price index. Washington, DC: Bureau of Labor Statistics; 2014. Available from: http://www.bls.gov/schedule/archives/cpi_nr.htm.
Centers for Medicare and Medicaid Services. 2014 clinical laboratory fee schedule. Baltimore, MD: Centers for Medicare and Medicaid Services, 2014. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html.
Drug Price Search. https://www.rxpricequotes.com. Accessed 11 Aug 2014.
Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–94.
You JH, Chung TK. Expectant, medical or surgical treatment for spontaneous abortion in first trimester of pregnancy: a cost analysis. Hum Reprod. 2005;20(10):2873–8.
Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000;7(16):1378–84.
Vanni VS, Vigano’ P, Somigliana E, Papaleo E, Paffoni A, Pagliardini L, et al. Vitamin D and assisted reproduction technologies: current concepts. Reprod Biol Endocrinol. 2014;12:47.
Purcell K, Schembri M, Frazier LM, Rall MJ, Shen SH, Croughan M, et al. Asian ethnicity is associated with reduced pregnancy outcomes after assisted reproductive technology. Fertil Steril. 2007;87(2):297–302.
Fujimoto VY, Luke B, Brown MB, Jain T, Armstrong A, Grainger DA, et al. Racial and ethnic disparities in assisted reproductive technology outcomes in the United States. Fertil Steril. 2010;93(2):382–90.
Gleicher N, Weghofer A, Li J, Barad D. Differences in ovarian function parameters between Chinese and Caucasian oocyte donors: do they offer an explanation for lower IVF pregnancy rates in Chinese women? Hum Reprod. 2007;22(11):2879–82.
Gleicher N, Kim A, Weghofer A, Barad DH. Differences in ovarian aging patterns between races are associated with ovarian genotypes and sub-genotypes of the FMR1 gene. Reprod Biol Endocrinol. 2012;10:77.
Karolinska University Hospital. Vitamin D during in vitro fertilisation (IVF)—a prospective randomized trial. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2014 Aug 17]. Available from: http://clinicaltrials.gov/show/NCT01019785 NLM Identifier: NCT01019785.
Acknowledgments
The authors thank Dr. Elizabeth E. Puscheck and Dr. Alan H. DeCherney for their generous support, as well as J.C. Sanchez for graphical design assistance.
Funding
This study was funded, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, and ZIA HD-008737 to JHS.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Vitamin D has been shown to play important roles in reproduction and vitamin D deficiency is a prevalent condition. A systematic review of vitamin D and ART outcomes yielded five studies that reported ART outcomes improved after vitamin D repletion, two studies found no association, and one study reported a negative association. Currently, Level I evidence is insufficient and a recommendation for routine vitamin Dscreening and supplementation prior to ART cannot be supported. There is a pressing need for additional studies of vitamin D replacement in this clinical scenario.
Michelle M. Pacis and Chelsea N. Fortin Contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Pacis, M.M., Fortin, C.N., Zarek, S.M. et al. Vitamin D and assisted reproduction: should vitamin D be routinely screened and repleted prior to ART? A systematic review. J Assist Reprod Genet 32, 323–335 (2015). https://doi.org/10.1007/s10815-014-0407-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0407-9