Abstract
Stress and growth rates in microalgae and seaweeds are often evaluated by different methods, making the resulting data often incomparable. This poses significant challenges for basic and applied phycological research and algae industry development. To address this issue, we provide a protocol with quantitative definitions and mathematical formulae for assessing algal stress on biomass productivity in organisms and populations. Our purpose with this protocol is to offer a mathematical model to quantify and compare stressors and strains across algal taxa, going beyond qualitative and species-specific approaches. We have applied our protocol to analyze data from studies on Ulva lactuca L. growth under thermal stress and nutrient limitation to demonstrate our protocol's utility and easiness of use. We also present a new and unified perspective for algal stress ecophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Algae offer unique solutions to major global problems, from climate mitigation and bioenergy to bioplastics and food (da Rosa et al. 2023; Farghali et al. 2023). Applied phycological research is often reliant on accurate measurements of algal biomass productivity to ascertain necessary amounts of wet weight, dry mass, or phycoproducts (Buschmann et al. 2017; Muhammad et al. 2020; Cai et al. 2021; Rehman et al. 2022). Following closely on this data, the algae industry depends on comparing methods and results and then deciding which one is appropriate for a particular production requirement (Rehman et al. 2022; Ayala et al. 2023). However, as shown by Yong et al. (2013), the different formulae available to measure algal growth can lead to very different results upon analyzing the same growth data (Glenn and Doty 1992; Schmidt et al. 2010; Luhan and Sollesta 2010; Hayashi et al. 2011). For seaweeds, whereas Yong et al.'s (2013) proposed formula has proven a widely used method, their framework has no provisions to measure stress and its effect on growth rate. In the microalgal literature recent research on algal growth rates has also resorted to different formulae aimed at more specific situations, such as examining the rate of increase in cell density (Indrayani et al. 2019; Kim et al. 2020) or correlating dry weight increase rates and absorbance (Griffiths et al. 2011; Cheng et al. 2022). However, there still is currently no unified protocol or model to provide for the methodological gap on quantitatively assessing the effect of stress on growth rate and biomass productivity for microalgae and seaweeds. Therefore, researchers and industries still have to bear with productivity data that is often not comparable among studies on the same stressor and that does not relate quantitatively to data from studies on different stressors.
While stress is a foundational concept to organismic and population biology (Sutherland et al. 2013), there is no consensus in the literature regarding its definition. Recent debates have also failed to reach a consensual or working definition, with different perspectives on stress and its ecophysiology remaining entrenched (Borowitzka 2018; Kültz 2020; Makuya et al. 2023; Rosado et al. 2023; Schradin et al. 2023). A profusion of qualitative approaches has also lead to increased skepticism surrounding the operational value of stress in ecophysiology (Schradin et al. 2023). The lack of mathematical treatment in current proposals poses a significant obstacle towards a unified method for stress evaluation.
To address this problem, we offer a protocol to measure stress and growth rate for studies on algal stress ecophysiology. To this end, we present quantitative definitions and mathematical formulae applicable to evaluate organismic and populational biomass productivity rates. Our approach aims to provide a mathematical framework that goes beyond qualitative and species-specific approaches, offering a more quantitative foundation for the study of stress in algal ecophysiology.
New formulae and quantitative definitions for stress and growth
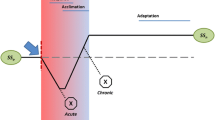
Our quantitative proposal defines growth as all forms of total biomass increase from an organism or population. In our framework, to that effect, growth can happen vegetatively, clonally, colonially, by division, or by reproduction, for example. In the case of growth by division or reproduction, the initial parental biomass should be accounted for as well as that of the progeny cumulatively. This also allows for the continuous modeling of populations that exhibit seasonal breeding and individuals with determinate body size. We define stress as the category of stressors that can affect strain on a given biological unit, such as an organism or a population. We define strain as the reduction in growth compared to a situation where its causal stressor is absent. We posit that stress and strain cannot be presumed as such a priori, but only measured empirically. These definitions are able to be rendered mathematically to model organismal or population growth rate under strain in relation to its biomass productivity. Adapting formulae used for population growth (Frisman et al. 2021), we can modify the Verhulst-Pearl equation to mathematically model idealized growth, where rmax is the constant of the optimal biomass gain for a given organism or population (Eq. 1).
This formula represents an idealized maximum biomass growth rate where all stress is absent and all other conditions are kept optimal. However, this formula would be of little use in real cases. It requires an adaptation to model growth under real conditions, where stress is present and needs to be quantified. Following our proposed definitions, algae experiencing strain would exhibit a lower biomass accumulation over a given period compared to another not subjected to such strain. Therefore, the growth rate lost to strain (s) can be quantitatively defined as biomass under optimal growth (Wo) subtracted by biomass under strained growth (Ws) in a given time (Eq. 2):
Therefore, the biomass productivity rate of an organism or population under strain can be defined as (Eq. 3):
However, determining the optimal growth rate is unrealistic. Therefore, the formula needs to be changed to determine the effect of a stressor in relation to a realistic biomass gain, substituting r max for a realistic growth rate parameter. We can introduce the actual growth rate parameter (a) as the maximum growth rate (rmax) multiplied by the growth rate loss to stress (s) (Eq. 4):
In real growth conditions algae would be subjected to many stressors, diminishing its biomass accumulation rate (\({r}_{max}>a\)). Therefore, the actual growth rate of algal populations or organisms could be expressed as biomass (W) multiplied by the actual growth rate parameter (a) (Eq. 5):
Due to the relational nature of our definition of strain, we can substitute a comparison to optimal growth for a comparison between a stress treatment and a stress-free control. This can be done if other parameters are kept constant across treatments and controls, since the optimal growth parameters from the stress treatment and stress-free control would cancel out if we compare them by taking their ratio. On rearranging the formulae, we can thus quantify directly the effect of algal stressors. Biomass lost to strain (s) can be defined as the ratio between the treatment group actual growth rate (at) and the control group actual growth rate (ac), as other parameters would cancel out (Eq. 6):
In this way, our mathematical framework enables quantitative comparisons of stressor effects on organismic and populational biomass growth rate, both on continuous or discrete data.
Protocol applications
We used to data from two studies by Peruzzi (2008) on the seaweed Ulva lactuca L. growth rate under different stressors to showcase our protocol's application.
Example
Ulva lactuca under thermal stress. In the absence of thermal stress (control), U. lactuca exhibited a growth rate of 0.59 g day-1 (ac). When subjected to thermal stress under low temperatures (treatment), the growth rate dropped to 0.02 g day-1 (at).
-
Controls:
$${a}_{c}=0.59$$ -
Treatment:
$${a}_{t}=0.02$$
To quantify the strain caused by thermal stress, we calculate s as follows:
In this case, the actual biomass productivity rate (ac) was multiplied by 0.0339 (s). This resulted in 96.6% of the growth rate lost to strain, due to a thermal stressor.
Example
Ulva lactuca under nutritional stress. Ulva lactuca growth rates differed under nutrient saturation (control) and nutrient limitation (treatment). Under nutrient saturation, the growth rate was 1.7 g day-1 (ac), whereas under nutrient limitation, it dropped to 0.13 g day-1 (at).
-
Controls:
$${a}_{c}=1.7$$ -
Treatment:
$${a}_{t}=0.13$$
To quantify the strain caused by nutrient limitation stress, we calculate s as follows:
Under this treatment, strain due to nutrient limitation was responsible for a 95.5% loss in biomass productivity rate, a similar value to the 96.6% loss under thermal stress. In this way, the effects of thermal and nutritional stress are rendered quantitatively comparable in this study. By analyzing data from total biomass productivity in cultures subjected to different treatments, this approach can be applied to microalgae as well. This method of examining total biomass avoids the complications that arise from differences in cell size and morphology due to replication time, which would affect the results if one examined cell density instead (see e.g., Indrayani et al. 2019).
Discussion
Seaweed and microalgal ecology and physiology were historically founded on the analysis of biomass increase and decrease, evaluated chiefly by productivity and growth rate, and by the effects of disturbance (meaning the discrete partial or total removal of biomass) and stress (Strickland 1960; Steneck and Dethier 1994; Airoldi 1998; Pessarrodona et al. 2022). The assessment of biomass productivity and stress in microalgae and seaweeds is also the methodological foundation of the algae products industry (Cai et al. 2021; Rehman et al. 2022). Accurately being able to parameterize growth rate and stress responses (strain) is pivotal to this aim to optimize phycoproduct production methods.
Stress can correlate to many biological responses (Rosado et al. 2023), such as discoloring, sporulation inducement, or increased susceptibility to pathogens. We agree with recent discussions in the ecological literature that state that these particulars are biologically significant and should be investigated (Schradin et al. 2023) with quantitative methods whenever possible. However, our proposal distinguishes itself by providing a universally applicable mathematical framework, usable from microalgal cells to kelp populations, by evaluating biomass differentials (strains) in response to their effectors (stressors), whereas other models fail to parameterize logistic growth rate and are silent on strain evaluation.
In this way, we offer a quantitative alternative to qualitative and species-specific discussions on whether unusually cold winters are stressful, or prolonged drought might render an environment harsher (Rosado et al. 2023; Schradin et al. 2023). We propose that algal stress ecophysiology can be unified by a quantitative approach, by asking two questions: is there strain \((\frac{{a}_{t}}{{a}_{c}} <1)\)? If so, how much (\(s=\frac{{a}_{t}}{{a}_{c}}\))?
By enabling direct comparisons of stressors' effects on growth, our proposal offers a valuable tool to quantify and compare the effects of various stressors across the biological units of interest. This approach was aimed to contribute to the development of an operational quantitative approach for stress and strain, supporting comparative and modeling studies, basic and applied, across stressors and taxa.
Conclusion
This paper presents a novel and comprehensive quantitative protocol for assessing stress in algal ecophysiology, offering clarity and precision in the study of stressors and strains across organisms and populations. By introducing quantitative definitions and mathematical formulae for growth, stress, and strain, our approach goes beyond qualitative and species-specific discussions, providing a unified perspective for stress physiology. With the ability to quantify and compare stressor effects on growth, our framework provides a valuable tool for seaweed and microalgae ecologists, physiologists and applied researchers interested in understanding the effects of stressors on organismic and populational growth rates. This approach supports a more rigorous and unified understanding of stress in phycology, enabling comparative and modeling studies across taxa and stressors in natural and laboratory settings.
References
Airoldi L (1998) Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 79:2759–2770
Ayala M, Thomsen M, Pizzol M (2023) Using quantitative storytelling to identify constraints in resource supply: The case of brown seaweed. J Indust Ecol 27:1567–1578
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Buschmann AH, Camus C, Infante J, Neori A, Israel Á, Hernández-González MC, Pereda SV, Gomez-Pinchetti JL, Golberg A, Tadmor-Shalev N, Critchley AT (2017) Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52:391–406
Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, Diffey S, GarridoGamarro E, Geehan J, Hurtado A, Lucente D, Mair G, Miao W, Potin P, Przybyla C, Reantaso M, Roubach R, Tauati M, Yuan X (2021) Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular No. 1229. FAO, Rome
Cheng H-Y, Shao Z-H, Li S-Y, Lin X, Da H-R, Xu M-Y, Lin L-M, Wu Z-L (2022) Research on the manipulation of iron ions and alkalis in Chlorella vulgaris culture. S Afr J Bot 151:583–590
da Rosa MDH, Alves CJ, dos Santos FN, de Souza AO, Zavareze EdR, Pinto E, Noseda MD, Ramos D, de Pereira CMP (2023) Macroalgae and microalgae biomass as feedstock for products applied to bioenergy and food industry: A brief review. Energies 16:1820
Farghali M, Mohamed IMA, Osman AI, Rooney DW (2023) Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: a review. Environ Chem Lett 21:97–152
Frisman EY, Zhdanova OL, Kulakov MP, Neverova GP, Revutkaya OL (2021) Mathematical modeling of population dynamics based on recurrent equations: Results and prospects. Part I. Biol Bull Russ Acad Sci 48:1–15
Glenn EP, Doty MS (1992) Water motion affects the growth rates of Kappaphycus alvarezii and related red seaweeds. Aquaculture 108:233–246
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Meth 85:119–123
Hayashi L, Faria GSM, Nunes BG, Zitta CS, Scariot LA, Rover T, Felix MRL, Bouzon ZL (2011) Effect of salinity on the growth rate, carrageenan yield, and cellular structure of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultured in vitro. J Appl Phycol 23:439–447
Indrayani I, Moheimani NR, Borowitzka MA (2019) Long-term reliable culture of a halophilic diatom, Amphora sp. MUR258, in outdoor raceway ponds. J Appl Phycol 31:2771–2778
Kim YO, Choi J, Baek SH, Lee M, Oh H-M (2020) Tracking Alexandrium catenella from seed-bed to bloom on the southern coast of Korea. Harmful Algae 99:101922
Kültz D (2020) Defining biological stress and stress responses based on principles of physics. J Exp Zool A 333:350–358
Luhan MRJ, Sollesta H (2010) Growing the reproductive cells (carpospores) of the seaweed, Kappaphycus striatum, in the laboratory until outplanting in the field and maturation to tetrasporophyte. J Appl Phycol 22:579–585
Makuya L, Pillay N, Rimbach R, Schradin C (2023) Suppression of the physiological stress response is not stress. Trends Ecol Evol 38:907–909
Muhammad G, Alam MdA, Xiong W, Lv Y, Xu J-L (2020) Microalgae biomass production: An Overview of dynamic operational methods. In: Alam J-MDA, Xu J-L, Wang Z (eds) Microalgae Biotechnology for Food, Health and High Value Products. Springer, Singapore, pp 415–432
Peruzzi V (2008) Competição por nutrientes e seus efeitos no metabolismo de Ulva spp. Dissertation, Universidade Federal do Rio de Janeiro. https://doi.org/10.17771/PUCRio.acad.14811
Pessarrodona A, Assis J, Filbee-Dexter K, Burrows MT, Gattuso JP, Duarte CM, Krause-Jensen D, Moore PJ, Smale DA, Wernberg T (2022) Global seaweed productivity. Sci Adv 8:eabn2465
Rehman M, Kesharvani S, Dwivedi G, Suneja KG (2022) Impact of cultivation conditions on microalgae biomass productivity and lipid content. Mater Today: Proc 56:282–290
Rosado BHP, Roland H, Moraes YCS (2023) On the biological concept of stress. Trends Ecol Evol 38:905–906
Schmidt EC, Nunes BG, Maraschin M, Bouzon ZL (2010) Effect of ultraviolet-B radiation on growth, photosynthetic pigments, and cell biology of Kappaphycus alvarezii (Rhodophyta, Gigartinales) macroalgae brown strain. Photosynthetica 48:161–172
Schradin C, Makuya L, Pillay N, Rimbach R (2023) Harshness is not stress. Trends Ecol Evol 38:224–227
Steneck RS, Dethier MN (1994) A Functional group approach to the structure of algal-dominated communities. Oikos 69:476–498
Strickland JDH (1960) Measuring the production of marine phytoplankton. Fish Res Bd Canada Bull 122:1–172
Sutherland WJ, Freckleton RP, Godfray HCJ et al (2013) Identification of 100 fundamental ecological questions. J Ecol 101:58–67
Yong YS, Yong WTL, Anton A (2013) Analysis of formulae for determination of seaweed growth rate. J Appl Phycol 25:1831–1834
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
JPGM and VPO contributed equally to the study.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Machado, J.P.G., Oliveira, V.P. Protocol for measuring stressor effects and growth rates of microalgae and seaweeds at organismic and populational levels. J Appl Phycol 36, 1485–1488 (2024). https://doi.org/10.1007/s10811-024-03188-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-024-03188-z