Abstract
Anti-diabetic potential of a brown alga, Padina tetrastromatica, using bioassay-guided purification approach yielded the most active α-glucosidase inhibitor agents as fatty acids. Initially, α-glucosidase inhibition of the MeOH and 80% MeOH extracts were evaluated via a colorimetric assay. The liquid–liquid fractionation of 80% MeOH extract, as the most potent α-glucosidase inhibitor, resulted in four fractions: n-hexane, ethyl acetate, n-butanol and water. The hexane and ethyl acetate fractions were selected for further study with IC50 values of 38.0 ± 0.3 µg mL−1 and 53.7 ± 2.6 µg mL−1, respectively. α-Glucosidase inhibition of the sub-fractions from the hexane fraction using flash column chromatography gave F18-21 as the most potent enzyme inhibitor. After further purification of F18-21 by semi preparative HPLC, the mentioned fraction and two purified compounds, 8-octadecenoic acid (8) and all-cis-5,8,11,14-eicosatetraenoic acid (10) were identified by GC–MS analysis, resulting fatty acids 1-12. In addition, 1D and 2D NMR evaluations were performed for characterisation of 8-octadecenoic acid. Furthermore, three fatty acids, all-cis-8,11,14,17-eicosatetraenoic acid (6), cis-9,12-octadecadienoic acid (7), and all-cis-5,8,11,14,17-eicosapentaenoic acid (11), were isolated from the ethyl acetate fraction and identified by HPLC and GC–MS, respectively. Finally, α-glucosidase inhibition percent of the purified fatty acids were evaluated in two concentrations in microplates, showing their great potential for further investigation as anti-diabetic agents, in comparison with acarbose as the positive control. Furthermore, molecular docking analysis and MD simulation were used to investigate the structure activity of the purified compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2019 the World Health Organization (WHO) defined diabetes mellitus as the ninth leading factor of mortality with over one million deaths per year (WHO 2014). One of therapeutic methods to control diabetes is the reduction of intestinal glucose via inhibiting some key enzymes such as ⍺-glucosidase. To this end, α-glucosidase inhibitors such as acarbose and voglibose have been used clinically in spite of some adverse effects like diarrhoea, flatulence and abdominal pain (Martin and Montgomery 1996; Alam et al. 2021). Due to the major role of α-glucosidase inhibitors to decrease the rise of blood glucose level after meals in people with type 2 diabetes (T2DM) and to overcome the above-mentioned complications of the existing drugs, investigations are needed to find new efficient enzyme inhibitors.
Recently marine algae have attracted attention as anti-diabetic nutraceuticals via diminishing blood glucose levels after algal consumption (Lee et al. 2010; Tanemura et al. 2014; Bocanegra et al. 2021; Shannon et al 2023). Phytochemical analyses of the bioactive algae have resulted in the detection of secondary metabolites with potent anti-diabetic activity (Agarwal et al. 2021; El-Beltagi et al. 2022). For instance, polyunsaturated fatty acids (PUFAs) showed various biological activities such as improving insulin sensitivity in human to manage T2DM (Chellappan et al. 2023; Sinha et al. 2023). Furthermore, essential fatty acids such as omega-3 fatty acids are prescribed as an effective adjuvant for controlling glycaemic level in diabetic people and decrease complications (Triggiani et al. 2006; Behl et al. 2019). In addition, short chain fatty acids raise sodium and water absorption in the colon and are effective for the treatment of diarrhoea, one of the prevalent α-glucosidase inhibitors side effects (Meier et al. 2003; Binder 2010). Three saturated and unsaturated fatty acids including cerotic acid, n-octacos-9-enoic acid, and 11-eicosenoic acid were isolated from a brown alga Dictyopteris hoytii by bioassay-guided approach and n-octacos-9-enoic acid inhibited α-glucosidase activity, significantly (Ur Rehman et al. 2019). Tetradecanoic acid (TDA) was isolated from a methanol extract of the brown alga Sargassum wightii which showed the best inhibition against α-amylase. TDA was found to be an α-amylase and α-glucosidase inhibition agent in vitro and in silico tests (Lakshmanasenthil et al. 2018). Additionally, 37 compounds, including mostly fatty acids with some diols, alkenes, alcohols, amides and aldehydes, were extracted from the alga Halymenia durvillei and identified by GC–MS (Tassakka et al. 2021).
The Persian Gulf is an exceptional habitat for growth and cultivation of macroalgae, providing them hot-sunny weather and resulting salty water. In addition, the high traffic of oil carrying ships may affect the growth conditions and environment of the algae which can cause possible changes in their chemical constituents in response to both oil pollution and new living species brought by the ships. Among different biological activities reported for the marine algae of the region, including antioxidant and anticancer potentials (Jassbi et al. 2013; Pirian et al. 2017; Moheimanian et al. 2021), our focus was on anti-diabetic agents of the brown algae, exploring their α-glucosidase activities and in vivo anti-diabetic activity to lower the blood glucose levels in mice after meals (Moheimanian et al. 2022).
Padina tetrastromatica, a brown alga commonly found in South Asia and reported from Iranian coastlines of the Persian Gulf (Hauck 1886; Sohrabipour and Rabii 1999), is a rich source of polyphenols, polysaccharides, fucoxanthin and fatty acids, with some biological activities (Sharma and Baskaran 2021). For instance, this alga collected from the Indian costal area showed various biological activities such as anti-diabetic, anti-inflammatory, anti-hypertensive and antioxidant activity, with high total phenolic contents (Antony and Chakraborty 2019). The acidified methanolic extract of P. tetrastromatica showed antioxidant and anti-diabetic properties (Naveen et al. 2021). Phytochemical analyses resulted in isolation of antioxidant and anti-diabetic dolabellane and dolastane diterpenoids from an ethyl acetate–methanol extract of Indian P. tetrastromatica (Antony et al. 2021). Furthermore, some sulphated polysaccharides from the algae, showed cardioprotective activity (Lekshmi and Kurup 2019). In another investigation,the methanolic extract of P. tetrastromatica, from Bangladesh had a high total phenolic content and high antioxidant activity (Sobuj et al. 2021).
In the present study, due to the mentioned biological activities of P. tetrastromatica, it was studied to find α-glucosidase inhibitors.
Materials and methods
Reagents and instrumentation
Yeast ⍺-glucosidase from Saccharomyces cerevisiae, (EC 3.2.1.20), acarbose and p-nitrophenyl-⍺-D-glucopyranoside (PNPG) were from Sigma Aldrich. All solvents were obtained from Merck. All used reagents were of analytical grade. NMR spectra of compound 8, were achieved on a Bruker Avance III 400 NMR, operating at resonance frequencies of 400 MHz for 1H and 100 MHz for 13C, respectively. It was applied for measuring 1H NMR, 13C APT, 1H-1HCOSY, 1H-13C HSQC and 1H-13C HMBC spectra. EI-MS spectra were recorded on an Agilent 5975 C inert GC–MS instrument. Various chromatographic techniques were used for isolation, including silica gel open column chromatography (70–230 mesh), and TLC using pre-coated aluminium sheets (silica gel 60 F254, Merck) with 0.25 mm film thickness. Reversed-phase (RP-18) HPLC analyses were carried out for more purification, using a Knauer semi-preparative HPLC (K-1050 pump) and K-2600 UV detector set at λ 210 nm (Jassbi et al. 2014). A Phenomenex RP-18 column (250 × 10 mm) was used for the semi-preparative HPLC, eluting with 90% acetonitrile (solvent B) and 10% in ultrapure water (solvent A). The separations, on analytical HPLC, were developed on an Azura analytical HPLC with a quaternary low-pressure mixing pump P 6.1L with degasser module, HPLC Knauer column (C18, 250 × 4.6 mm ID (internal diameter), Germany) and UVD 2.1L UV detector set at λ 210 nm.
Collection and extraction of the algae
Padina tetrastromatica was collected from TV Park (N 28° 59′ 41.0" E 50° 49′ 44.6") in the coastal region of Bushehr city in the Persian Gulf, Iran, in April 2020. The collected sample was identified by Dr. Sohrabipour.
The algal sample (1.2 kg fresh weight) was first cleaned and sliced, then extracted using maceration technique at about 25 °C for two times each for 24 h successively in 2.5 L 80% MeOH and 2.5 L MeOH, respectively. Finally, each extract was filtered and the solvents were evaporated under vacuum at 38 ºC, to obtain the crude dried 80% MeOH (17.9 g) and MeOH (10.9 g) extracts. The 80% MeOH extract was dissolved in 50 mL distilled water and then subjected to liquid–liquid extraction (LLE), which afforded four different fractions including n-hexane (843 mg), ethyl acetate (590 mg), n-butanol (2.81 g) and water (12.11 g). The fractions were evaluated for α-glucosidase inhibition.
Bioassay guided purification
The hexane fraction (840 mg), as the best enzyme inhibitor, was loaded over silica gel-Flash Column Chromatography (FCC) (30 g, 70–230 mesh, 30 × 2.5 cm). The column was eluted using n-hexane, n-hexane/chloroform, and chloroform/MeOH with gradually increasing polarity to afford 30 fractions. After pooling the similar fractions using TLC analyses into eight new ones, (FH1-4; FH6; FH5,7,8; FH9-13; FH14-17; FH18-21; FH22-26; FH27-30) they were subjected to α-glucosidase inhibitory assay to select the best for further phytochemical analyses. The fraction which was eluted with chloroform/MeOH (100/2), FH18-21, was selected for further isolation due to its significant inhibitory activity. Further purification was performed using RP18 semi preparative HPLC. The analytical condition was concluded from analytical RP18-HPLC. The mobile phase was 90% acetonitrile in ultrapure water and the flow rate of 4.5 mL min−1. Compounds 8 (7 mg) and 10 (3.2 mg) were isolated with retention times of 7.78, and 24.85 min, respectively. Compound 8 was subjected to 1H and 13C NMR spectroscopy followed by GC–MS analysis. Based on the NMR spectral data the fatty acid character for compound 8 was suggested. We then evaluated the compounds again for further α-glucosidase inhibitory potential. In addition to the purified compounds, fraction FH18-21 itself was subjected to GC–MS for the detection of its lipid contents (Table 1).
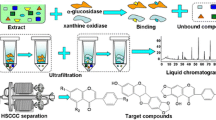
The bioassay showed the ethyl acetate fraction (590 mg) as the second potent fraction of the algal extract and therefore it was subjected again to RP18 semi preparative HPLC with the same analytical conditions mentioned above. Compounds 6 (1.1 mg), 7 (1.5 mg) and 11 (0.8 mg) were isolated with retention times of 8.36, 9.70 and 14.67 min, respectively. The compounds again were subjected to GC–MS analyses to ascertain their purification and their chemical characterization. In each step, the α-glucosidase inhibition activity test was performed as a guide and to measure the potential of the resulting final products as illustrated in the schematic diagram (Fig. 1).
Schematic diagram of isolating anti-diabetic compounds from P. tetrastromatica, by bioassay-guided purification. At each step, the items with greater α-glucosidase inhibition were selected for the next isolations. LLE: Liquid–liquid extraction; FCC: Flash column chromatography; HPLC: High-performance liquid chromatography
α-Glucosidase inhibition assay
The α-glucosidase test was performed as described by Moheimanian et al. (2022). Briefly, 5 μL of each extract, fraction, or isolated compound was mixed with 90 μL potassium phosphate buffer (0.1 mM, pH 6.8), in 96-well microplates. Then 20 μL of α-glucosidase (0.25 U mL−1) in phosphate buffer solution was added to the mixture, followed by dark incubation at 37 ºC for 10 min. After incubation, 15 μL of 2.5 mM p-nitrophenyl-α-D-glucopyranoside (a chromogenic substrate) was added and after 30 min storage at 37 ºC the reaction was stopped by adding 80 μL Na2CO3 (0.2 M) into each well. The solutions and the control were prepared in DMSO (0.4% final concentration). The change in absorbance was measured at a wavelength of 405 nm. Acarbose was used as a positive control. Calculation of the % inhibition, was done by the following formula:
where Aextract and Acontrol are the absorbance of the sample and the control, respectively. The half-maximal enzyme inhibitory concentration (IC50, µg mL−1) values were evaluated by a dilution series of the samples, from 8 µg mL−1 to 120 µg mL−1 using Curve Expert 1.4 software (three replicates).
Gas chromatography–Mass spectrometry analysis
Based on the NMR and TLC analytical results we suggest the fatty acids structure of the compounds in the purified bioactive fractions. To characterize the bioactive fatty acids, they were analysed by GC–MS as performed earlier (Jassbi et al. 2013). The GC–MS was operated on an Agilent5975C GC plus HP-6890 mass spectrometer operating in electron impact mode (EI, 70 eV). The GC column was a HP-5MS (30 m × 0.25 mm i.d., 0.25 µm film thickness, J &W Scientific column). The oven temperature was programmed at 160˚C (held for 2 min), surged to 230˚C at 5˚C min−1 and held stable for 20 min at the final temperature. Helium, as the carrier gas, was applied with a flow rate of 1 mL min−1. The injector temperature was fixed at 250˚C, with the injection volume of 0.1 μL and a split ratio of 1:10. (Heidary Jamebozorgi et al. 2019).
Derivatization of the fatty acids to their methyl esters
Since the fatty acids are not well resolved on the above capillary column, the fraction (FH18-21) or their pure compounds were derived to their methyl esters for a better chromatographic resolution (Jassbi et al. 2013). Briefly, 1 mg of sub-fraction FH18-21 or pure compounds was dissolved in 250 μL 20% BF3 in MeOH in a sealed glass vial followed by heating in a boiling water bath for 1 h. Afterwards, 1 mL distilled water was added to the solution and the organic layer was separated by extracting the mixture with 2 mL n-hexane (three times). The extracting solvent was evaporated using a stream of nitrogen gas after drying with anhydrous Na2SO4, The resulting samples were dissolved in 1 mL hexane and used for GC–MS analyses (Jassbi et al. 2013).
Statistical analysis
The data are expressed as mean ± standard error (SE). Statistical analysis was done by SPSS software (Ver. 16) using one-way analysis of variance (ANOVA). Inhibitory concentration (IC50) values were estimated using Excel 2016 and CurveExpert1.4 software. P-values ≤ 0.05 were considered to be significant.
Molecular docking study
The structure used in this study was ⍺-glucosidase from Saccharomyces cerevisiae. The chemical activities of the fatty acids obtained from P. tetrastromatica were estimated against this enzyme. The fatty acids with the highest and lowest inhibitory activity were considered. The 3D structures of the enzyme (UniProt: P53341) was obtained from the EBI Alpha Fold database (https://alphafold.ebi.ac.uk) (Jumper et al. 2021) and prepared using the protein preparation module of the Schrödinger Suite (Poustforoosh et al. 2022a). Accordingly, the lost hydrogen atoms were added and the molecules of water were deleted from the system. After that, an H-bond network was produced and finally, the system was minimized by implementing the OPLS3e force field (Poustforoosh et al. 2022b). To obtain more trustworthy results, the active binding site of the enzyme was determined employing the SiteMap of Schrödinger and a receptor grid was generated around the active site. The structure of the fatty acids was obtained from the PubChem database as SDF files. The accurate protonation states for ligands were generated by using the LigPrep module of Schrödinger (Poustforoosh et al. 2022c). Ultimately, the molecular docking was operated using the Glide of Schrödinger suites.
Molecular Dynamics (MD) simulation
The interactions between the fatty acid with the highest inhibitory activity and the enzyme were further assessed dynamically. Desmond software (a package for molecular dynamics simulation) was used to conduct the MD simulation. The complex of the ligand-enzyme obtained from the docking calculations was evaluated by performing the MD simulation. The simulation was performed in an orthorhombic box and the solvent model of transferable intermolecular potential with 3 points (TIP3P) was chosen for the simulation. The proper number of Na+/Cl− ions with a salt concentration of 0.15 M were used to neutralize the system employing the system setup of Schrödinger (Sirin et al. 2014). The simulation was then accomplished for 100 ns with the default relaxation protocol of software and the constant number of atoms, pressure, and temperature (NPT) ensemble. The Nose–Hoover protocol was used to set the temperature to 310.15 K (37 °C) and the pressure was adjusted to 1 atm employing isotropic scaling (Panwar and Singh 2021).
Results
The 80% MeOH extract of P. tetrastromatica was the superior α-glucosidase inhibitor compared to the methanol extract exhibiting a several fold lower IC50 value of 57.1 µg mL−1, with P value < 0.05 (Tables 1, 2). After the LLE procedure and performing α-glucosidase assay the active aqueous methanol extract resulted in two bioactive layers, the hexane and ethyl acetate fractions with IC50 values of 38.0 µg mL−1 and 53.7 µg mL−1, respectively. The more polar fractions, n-butanol and water were less active (Table 2). Acarbose, as a positive control, showed an IC50 value of 283.1 µg mL−1.
All sub-fractions obtained after column chromatography of the active hexane layer were subjected to the bioassay and evaluated for α-glucosidase inhibition at two concentrations, 120 and 60 µg mL−1. Among them, F18-21, F22-26, F27-30 exhibited the highest percent inhibition of 79.9, 40.2 and 53.8%, respectively, at 60 µg mL−1 (Table 3). Therefore, the ethyl acetate fraction and F18-21 were chosen for further isolation as the best inhibitor resulting in the purified compounds (6, 7, 8, 10 and 11). All of these were unsaturated fatty acids (UFA) or poly-unsaturated fatty acids (PUFA). Among them, 3 compounds including 8-octadecenoic acid (8), all-cis-5,8,11,14-eicosatetraenoic acid (10), and cis-5,8,11,14,17-eicosapentaenoic acid (11), demonstrated more than 70% inhibition in a concentration of 0.1 mM (Table 4).
Structure elucidation of fatty acid compounds
Based on 1H- and 13C NMR spectral data of compound 8, it was characterized as a fatty acid (Noorbakhsh et al. 2022). As seen in supplemental Table 1, the olefinic protons signals was detected at δ 5.35 ppm while the H-2 and H-3 and the allylic signals were recorded at δ 2.34 (t, J = 7.5), 2.02 m, and 1.61 ppm, respectively. A broad signal at δ 1.30 is characteristic of the aliphatic methines (CH2). On the other hand, the HMBC spectra demonstrated the presence of a carbonyl carbon at around 177 ppm (C -1) and double bond signals at δ 130.2 (C -8) and 129.9 ppm (C -9), together with 10 signals at 29.2–29.9 (CH2)10. The signals at δ 33.8 (C-2), 24.9 (C-3), 14.3 (C-18) together with two signals at 27.3 and 27.4 were suggested for the allylic positions of the double bonds. Two of the signals at 22.8 and 32.1 were assigned to two aliphatic methylene carbons. The correlation between carbons and protons were confirmed by HSQC cross peaks. The structure of the compound and its double bond position was decided on the basis of its mass spectral data resulting from GC–MS analyses, including RRI on a non-polar column (DB5) and MS spectra after their transformation to their methyl esters.
In total, twelve compounds were identified in FH18-21 resulting from the active hexane fraction, in addition to the CC and HPLC-purified compounds (supplemental Fig. 1 and Table 1). GC–MS analyses results of these fatty acids and their structures are shown in supplemental Fig. 3 and Fig. 2 respectively.
Furthermore, supplemental Fig. 2 shows that after derivatization the chromatogram peaks become more resolved to be identified precisely. As mentioned before, 5 fatty acids were isolated and identified with one of them being a monounsaturated fatty acid, 8-octadecenoic acid. Interpretation of the purified compounds (6-8, 10, 11) was characterized according to the mass fragmentation pattern of their methyl ester derivatives and in comparison with authentic samples reported in literature databases (Pub Chem.ncbi, NIST Chemistry Web Book and SpectraBase) (Syeda 2011; Merlin et al. 2016).
Two saturated fatty acids, TDA and hexadecanoic acid constituting 10.6 and 19.3% of the active fraction were detected in their methyl esters form. The others were PUFAs like omega-3, all-cis-8,11,14,17-eicosatetraenoic acid, all-cis-5,8,11,14,17-eicosapentaenoic acid methyl ester (8.8 and 1.0%) and omega-6; cis-9,12-octadecadienoic acid, all-cis-5,8,11,14-eicosatetraenoic acid methyl ester (8.5 and 4.4%) were detected in fraction F18-21 after derivatization.
Molecular docking
Based on the docking results the binding affinities of the compounds were not significant. Therefore, we decided to compare the interactions constructed between the compounds and the ⍺-glucosidase enzyme by performing molecular docking calculations. For the comparison the compounds with the highest (11) and lowest (6) inhibitory activity were selected. The outcomes introduced the probable residues of the enzyme that can create strong interactions with the compounds. The results of molecular docking calculations are presented in Table 5. The docking pose of compounds 6 and 11 is shown in Fig. 3. The interactions constructed between these compounds and ⍺-glucosidase are presented in Fig. 4. Compound 6 has created a salt bridge with the residues Arg212. Compound 11 has constructed two hydrogen bonds with the residues Ala216 and Glu276. These H-bonds are created with the hydroxyl group of the ligand. Glu276 is an essential residue of the active site of the enzyme.
MD simulation
The ligand–protein complex of compound 11, as the most active compound against ⍺-glucosidase was further evaluated using the MD simulation for 100 ns. The RMSD value of the protein in these simulations converged at about 3 Å (Fig. 5) indicating the stability of the systems after 100 ns. The ligand–protein interactions between ⍺-glucosidase and compound 11 after the simulation time are presented in Fig. 6. The residues with the highest interactions fraction are Arg212, Ile213, Asp214, Thr215, Glu276, and Phe298.
Discussion
Bioassay-guided purification of extracts of the alga P. tetrastromatica was done, showing the high potency of some fatty acids to inhibit α-glucosidase. The results showed that the 80% methanol extract and its resulting hexane and ethyl acetate fractions exhibited α-glucosidase inhibition potential, comparable to that reported for an Indian collection of this alga with an IC50 value of 28.8 µg mL−1 for its acidified methanolic extract (Naveen et al. 2021). In addition, Gunathilaka et al. (2019) reported potent anti-diabetic effects for ethyl acetate fraction of the methanol extract of the red alga, Gracilaria edulis which was free of polysaccharide.
We identified 12 fatty acids in fraction FH18-21 of the hexane fraction of P. tetrastromatica; trans-9-hexadecenoate (4) (19.3%; area normalized%) and 8-octadecenoic acid (8) (21.3%) were the main fatty acids, while hexadecanoic acid (5) (1.8%),octadecanoic acid (9) (2.3%), all-cis-5,8,11,14-eicosatetraenoic acid (10) (4.4%), and cis-5,8,11,14,17-eicosapentaenoic acid (11) (1.0%) were the minor ones. Finally, 5 fatty acids isolated from the alga, inhibited α-glucosidase showing that 8-octadecenoic acid, with the most area percentage, produced the greatest inhibition. These are common polyunsaturated fatty acids (PUFAs) in algae that can be used beneficially in different industries (Menaa et al. 2020). As reported, α-glucosidase inhibitory activity of fatty acids has been confirmed. For instance, cerotic acid, n-octacos-9-enoic acid, and 11-eicosenoic acid isolated from the brown alga D. hoytii inhibit α-glucosidase activity with n-octacos-9-enoic acid showeing the highest inhibition (Ur Rehman et al. 2019). Similar to our result, TDA isolated from S. wightii, showed α-amylase and also α-glucosidase inhibitory activity (Lakshmanasenthil et al. 2018).
Fatty acids as α-glucosidase inhibitors could have beneficial key points in comparison to acarbose or the other inhibitors. They have commercial value and could be used in local businesses due to their reasonable price. In addition, they have potential applications as additives and supplements for diabetic people in the management of type 2 diabetes (Perdana et al. 2021). Different supplements have been proposed for diabetic people until now, such as chromium and cinnamon (Costello et al. 2016a, b). Furthermore, they have biological activities such as antioxidant and anti-inflammatory activity (Laye et al. 2018; Giacobbe et al. 2020). Fatty acids could have another beneficial factor for diabetic people who suffered from fatty liver, due to their useful role to improve the disease (Hliwa et al. 2021; Prabhakar and Bhuvaneswari 2021).
The presence of some fatty acids in P. tetrastromatica has been reported earlier, but this is the first time that they have been isolated by bioassay-guided purification from the alga. For instance, Naveen et al. (2021) characterised cis-9-tetradecenoic acid and cis-9-hexadecenoic acid as the two main fatty acids found in all the lipid fractions of P. tetrastromatica from India. Similarly, palmitic acid was reported as the predominant fatty acid in all three lipid fractions of P. tetrastromatica from India (Narayan and Miyazhita 2005). It is supposed that secondary metabolites in our investigated alga collected from Bushehr city coastal region had different lipid contents compared to those from India, because of the different ecological conditions. Overall, among the characterized fatty acids in the present study, only compounds 2, 4, 7 and 9, were previously reported in P. tetrastromatica (Maheswari et al. 2017; Naveen et al. 2021). Therefore, the remaining fatty acids: 1, 3, 5, 6, 8, 10, 11 and 12 are reported for the first time from this alga. On the other hand, α-glucosidase inhibition potential of compound 7 was reported earlier (Hu et al. 2018), while to the best of our knowledge this is the first report on the enzyme inhibition potential of the other isolated compounds 8, 6, 10 and 11.
The higher α-glucosidase potential (30.5 µM) of n-octacos-9-enoic acid isolated from D. hoytii was proposed by its free carboxylic OH interaction with certain amino acid moieties in the active site of S. cerevisiae α-glucosidase (Ur Rehman et al. 2019). In addition, the presence of the double bond makes this molecule and 11-eicosenoic acid to be active but the methylation of the saturated fatty acid, TDA to methyl tetradecanoate made its hydrogen bonding impossible with the interacting residue of the enzyme confirmed by molecular docking studies (Lakshmanasenthil et al. 2018; Ur Rehman et al. 2019). The docking study against both α-amylase and glucosidase enzymes suggested TDA as an effective inhibitor with the same interacting functional group of carboxylic acid which is consistent with the above-mentioned results (Lakshmanasenthil et al. 2018). The potent inhibition of the enzyme caused by the isolated compounds is compatible with the in silico and in vitro enzyme inhibitory tests on the fatty acids obtained from D. hoytii and S. wightii (Lakshmanasenthil et al. 2018; Ur Rehman et al. 2019). Based on information from the Uniprot database and the previously reported data by Ur Rehman et al. (2019), Asp214, Glu276 and Asp349 are the catalytic triad of the enzyme’s active site and targeting these residues could considerably affect the enzyme activity. As could be seen, compound 11 created an H-bond with Glu276 with a length of 2.21 Å. This interaction can effectively decrease enzyme activity. There are also ten hydrophobic contacts between compound 11 and the residues of ⍺-glucosidase. These residues are Trp57, Tyr51, Ile213, Ala216, Val274, Phe298, Phe300, Tyr344, Ala351, and Tyr386. These contacts can increase the binding affinity of the ligand to the enzyme. The results of the MD simulation revealed that compound 11 can construct some interactions with Asp214 and Glu276. Targeting these residues could enhance the inhibitory activity of compound 11 against the enzyme. Furthermore, the fatty acids TDA, hexadecanoic acid, cis-9,12-octadecadienoic acid, and cis-9-hexadecenoic acid with antioxidant and antibacterial activities have been identified in the essential oil of the edible seaweed, Laminaria japonica (Patra et al. 2015). Saturated and unsaturated fatty acids also have been reported as major lipid components of four seaweeds, Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima, making them of interest for use in food supplementation (Neto et al. 2018).
Conclusion
The Persian Gulf brown alga P. tetrastromatica is a rich source of anti-diabetic compounds due to its high α-glucosidase activity. Fatty acids are one metabolite class found in this alga, showing strong α-glucosidase inhibition. α-Glucosidase bioassay-guided fractionation and purification of the algal extract resulted in isolation of five unsaturated fatty acids with high enzyme inhibition power. In silico study suggests that compound 11 could effectively target the catalytic residues of the active site of α-glucosidase. On the other hand, the higher molecular weight of the long chain fatty acid is another positive point to exhibit the better bioactivity via better enzyme-metabolite interaction. The isolation of unsaturated fatty acids in the present study confirms previous studies and suggest these fatty acids for further anti-diabetic investigation. Furthermore, this alga could be recommended as an effective nutritious diet for diabetes treatment, and also for utilization in other therapeutic areas.
Data availability
All data generated or analyzed during this study can be obtained from the authors upon reasonable request.
References
Adams RP (2017) Identification of essential oil components by gas chromatography/mass spectrometry, 5th edn. Texensis Publishing, Gruver, Texas
Agarwal S, Singh V, Chauhan K (2021) Antidiabetic potential of seaweed and their bioactive compounds: A review of developments in last decade. Crit Rev Food Sci Nutr 63:5739–5770
Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T (2021) Diabetes Mellitus: Insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2:36–50
Antony T, Chakraborty K (2019) Pharmacological properties of seaweeds against progressive lifestyle diseases. J Aquat Food Prod 28:1092–1104
Antony T, Chakraborty K, Joy M (2021) Antioxidative dolabellanes and dolastanes from brown seaweed Padina tetrastromatica as dual inhibitors of starch digestive enzymes. Nat Prod Res 35:614–626
Behl T, Grover M, Shah K, Makkar R, Kaur L, Sharma S, Gupta J (2019) Role of omega-3-fatty acids in the management of diabetes and associated complications. In: Watsin RR, Preedy VR (eds) Bioactive Food as Dietary Interventions for Diabetes, 2nd edn. Elsevier, Amsterdam, pp 185–192
Binder HJ (2010) Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 72:297–313
Bocanegra A, Macho-González A, Garcimartín A, Benedí J, Sánchez-Muniz FJ (2021) Whole alga, algal extracts, and compounds as ingredients of functional foods: Composition and action mechanism relationships in the prevention and treatment of type-2 diabetes mellitus. Int J Mol Sci 22:3816
Chellappan DK, Chellian J, Rahmah NSN, Gan WJ, Banerjee P, Sanyal S, Banerjee P, Ghosh N, Guith T, Das A, Gupta G, Singh SK, Dua K, Kunnath AP, Norhashim NA, Ong KH, Palaniveloo K (2023) Hypoglycaemic molecules for the management of diabetes mellitus from marine sources. Diabetes, Metab Syndr Obes 16:2187–2223
Costello RB, Dwyer JT, Bailey RL (2016a) Chromium supplements for glycemic control in type 2 diabetes: Limited evidence of effectiveness. Nutr Rev 74:455–468
Costello RB, Dwyer JT, Saldanha L, Bailey RL, Merkel J, Wambogo E (2016b) Do cinnamon supplements have a role in glycemic control in type 2 diabetes? A narrative review. J Acad Nutr Diet 116:1794–1802
El-Beltagi HS, Mohamed AA, Mohamed HI, Ramadan K, Barqawi AA, Mansour AT (2022) Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar Drugs 20:342
Giacobbe J, Benoiton B, Zunszain P, Pariante CM, Borsini A (2020) The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front Psychiatry 11:122
Gunathilaka TL, Samarakoon KW, Ranasinghe P, Peiris LDC (2019) In-vitro antioxidant, hypoglycemic activity, and identification of bioactive compounds in phenol-rich extract from the marine red algae Gracilaria edulis (Gmelin) Silva. Molecules 24:3708
Hauck F (1886) Ueber einige von JM Hildebrandt im Rothen Meere und Indischen Ocean gesammelte algen: IV. Hedwigia 26:18–21
HeidaryJamebozorgi F, Yousefzadi M, Firuzi O, Nazemi M, Jassbi AR (2019) In vitro anti-proliferative activities of the sterols and fatty acids isolated from the Persian Gulf sponge; Axinella sinoxea. DARU J Pharm Sci 27:121–135
Hliwa A, Ramos-Molina B, Laski D, Mika A, Sledzinski T (2021) The role of fatty acids in non-alcoholic fatty liver disease progression: An update. Int J Mol Sci 22:6900
Hu Y, Zhang B, Zhang J, Zhao L, Wang L (2018) Chemical constituents of fruiting bodies of Coprinus comatus and their activities against diabetes. Mycosystema 37:371–378
Jassbi A, Miri R, Masroorbabanari M, Asadollahi M, Attarroshan M, Baldwin IT (2014) HPLC-DAD-ESIMS analyses of Hyoscyamus niger and H. reticulatus for their antioxidant constituents. Austin Chromatogr 1:1022
Jassbi AR, Mohabati M, Eslami S, Sohrabipour J, Miri R (2013) Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iran J Pharm Res 12:339
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O et al (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589
Lakshmanasenthil S, Vinoth Kumar T, Geetharamani D, ShanthiPriya S (2018) α-Amylase and α-glucosidase inhibitory activity of tetradecanoic acid (TDA) from Sargassum wightii with relevance to type 2 diabetes mellitus. J Biol Act Prod Nat 8:180–191
Laye S, Nadjar A, Joffre C, Bazinet RP (2018) Anti-inflammatory effects of omega-3 fatty acids in the brain: physiological mechanisms and relevance to pharmacology. Pharmacol Rev 70:12–38
Lee HJ, Kim HC, Vitek L, Nam CM (2010) Algae consumption and risk of type 2 diabetes: Korean National Health and Nutrition Examination Survey in 2005. J Nutr Sci Vitaminol 56:13–18
Lekshmi V, Kurup GM (2019) Sulfated polysaccharides from the edible marine algae Padina tetrastromatica protects heart by ameliorating hyperlipidemia, endothelial dysfunction and inflammation in isoproterenol induced experimental myocardial infarction. J Funct Foods 54:22–31
Maheswari MU, Reena A, Sivaraj C (2017) GC-MS analysis, antioxidant and antibacterial activity of the brown algae, Padina tetrastromatica. Int J Pharm Sci Res 41:84–93
Martin AE, Montgomery PA (1996) Acarbose: An α-glucosidase inhibitor. Am J Health Syst Pharm 53:2277–2290
Meier R, Burri E, Steuerwald M (2003) The role of nutrition in diarrhoea syndromes. Curr Opin Clin Nutr Metab Care 6:563–567
Menaa F, Wijesinghe P, Thiripuranathar G, Uzair B, Iqbal H, Khan BA, Menaa B (2020) Ecological and industrial implications of dynamic seaweed-associated microbiota interactions. Mar Drugs 18:641
Merlin K, Manickavasakam K, Mohan S (2016) GC-MS analysis of bioactive components of a Siddha poly herbal drug Adathodai chooranam. Int J Ayurveda Res Pharm 7:4–7
Moheimanian N, Firuzi O, Sohrabipour J, Jassbi AR (2021) Assessment of phenolic contents and antibacterial, cytotoxic, and antioxidant activities of five brown algae from the Persian Gulf. Iran J Sci Technol Trans A 45:1869–1877
Moheimanian N, Mirkhani H, Sohrabipour J, Jassbi AR (2022) inhibitory potential of six brown algae from the Persian Gulf on α-glucosidase and in vivo antidiabetic effect of Sirophysalis trinodis. Iran J Med Sci 47:484–493
Narayan B, Miyazhita K (2005) Lipid composition of Padina tetrastomatica (Dictyotales, Phaeophyta), a brown seaweed of the west coast of India. Indian J Fish 52:263–268
Naveen J, Baskaran R, Baskaran V (2021) Profiling of bioactives and in vitro evaluation of antioxidant and antidiabetic property of polyphenols of marine algae Padina tetrastromatica. Algal Res 55:102250
Neto RT, Marçal C, Queirós AS, Abreu H, Silva AM, Cardoso SM (2018) Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. Int J Mol Sci 19:2987.
Noorbakhsh F, Zare S, Firuzi O, Sakhteman A, Chandran JN, Schneider B, Jassbi AR (2022) Phytochemical analysis and biological activity of Salvia compressa Vent. Iran J Pharm Res 21:127031
WHO (2014) Global health estimates: deaths by cause, age, sex and country, 2000–2012, vol 9. World Health Organisation, Geneva
Panwar U, Singh SK (2021) Atom-based 3D-QSAR, molecular docking, DFT, and simulation studies of acylhydrazone, hydrazine, and diazene derivatives as IN-LEDGF/p75 inhibitors. Struct Chem 32:337–352
Patra JK, Das G, Baek K-H (2015) Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules 20:12093–12113
Perdana BA, Chaidir Z, Kusnanda AJ, Dharma A, Zakaria IJ, Bayu A, Putra MY (2021) Omega-3 fatty acids of microalgae as a food supplement: A review of exogenous factors for production enhancement. Algal Res 60:102542
Pirian K, Moein S, Sohrabipour J, Rabiei R, Blomster J (2017) Antidiabetic and antioxidant activities of brown and red macroalgae from the Persian Gulf. J Appl Phycol 29:3151–3159
Poustforoosh A, Faramarz S, Nematollahi MH, Hashemipour H, Negahdaripour M, Pardakhty A (2022a) In silico SELEX screening and statistical analysis of newly designed 5mer peptide-aptamers as Bcl-xl inhibitors using the Taguchi method. Comput Biol Med 146:105632
Poustforoosh A, Farmarz S, Nematollahi MH, Hashemipour H, Pardakhty A (2022b) Construction of bio-conjugated nano-vesicles using non-ionic surfactants for targeted drug delivery: A computational supported experimental study. J Mol Liq 367:120588
Poustforoosh A et al (2022c) The impact of D614G mutation of SARS-COV-2 on the efficacy of anti-viral drugs: A comparative molecular docking and molecular dynamics study. Curr Microbiol 79:241
Prabhakar O, Bhuvaneswari M (2021) Role of diet and lifestyle modification in the management of nonalcoholic fatty liver disease and type 2 diabetes. Tzu Chi Med J 33:135
Shannon E, Conlon M, Hayes M (2023) In vitro enzyme inhibitory effects of green and brown Australian seaweeds and potential impact on metabolic syndrome. J Appl Phycol 35:893–910
Sharma PP, Baskaran V (2021) Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice. Algal Res 54:102187
Sirin S, Pearlman DA, Sherman W (2014) Physics‐based enzyme design: Predicting binding affinity and catalytic activity. Proteins: Struct Funct Bioinf 82:3397–3409
Sinha S, Haque M, Lugova H, Kumar S (2023) The effect of omega-3 fatty acids on insulin resistance. Life 13:1322
Sobuj MKA, Islam M, Mahmud Y, Rafiquzzaman S (2021) Effect of solvents on bioactive compounds and antioxidant activity of Padina tetrastromatica and Gracilaria tenuistipitata seaweeds collected from Bangladesh. Sci Rep 11:1–13
Sohrabipour J, Rabii R (1999) A list of marine algae from seashores of Iran (Hormozgan Province). Qatar Univ Sci J 19:312–337
Syeda FA (2011) Gas chromatography-mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bio-assays of crude extract of Iris germanica. Int J Genet Mol Biol 3:95–100
Tanemura Y, Yamanaka-Okumura H, Sakuma M, Nii Y, Taketani Y, Takeda E (2014) Effects of the intake of Undaria pinnatifida (Wakame) and its sporophylls (Mekabu) on postprandial glucose and insulin metabolism. J Med Investig 61:291–297
Tassakka ACMA et al (2021) Potential bioactive compounds as SARS-CoV-2 inhibitors from extracts of the marine red alga Halymenia durvillei (Rhodophyta)–a computational study. Arab J Chem 14:103393
Triggiani V, Resta F, Guastamacchia E, Sabbà C, Licchelli B, Ghiyasaldin S, Tafaro E (2006) Role of antioxidants, essential fatty acids, carnitine, vitamins, phytochemicals and trace elements in the treatment of diabetes mellitus and its chronic complications. Endocr Metab Immune Disord Drug 6:77–93
Ur Rehman N, Rafiq K, Khan A, Ahsan Halim S, Ali L, Al-Saady N, Hilal Al-Balushi A, Al-Busaidi HK, Al-Harrasi A (2019) α-Glucosidase inhibition and molecular docking studies of natural brominated metabolites from marine macro brown alga Dictyopteris hoytii. Mar Drugs 17:666
Acknowledgements
ARJ is thankful to Alexander von Humboldt foundation for a research stay support at the department of pharmacognosy of Kiel University.
Funding
The authors wish to acknowledge the financial supports of Shiraz University of Medical Sciences (grant number: 18830). This study was part of the Ph.D. thesis of Niloofar Moheimanian.
Author information
Authors and Affiliations
Contributions
Author contribution Nioofar Moheimanian: Conceptualization, Methodology, Investigation, Formal analysis, Writing-original draft preparation. Hossein Mirkhani Supervision, Conceptualization, Writing-review & editing. Najmeh Edraki Methodology, Conceptualization, Writing—review & editing. Alireza Poustforoosh Molecular docking analysis, Conceptualization, Writing-review & editing. Safieh Momeni Collecting the algae, Writing-review & editing. Najmeh Khalighian Collaborating in HPLC analysis, Writing-review & editing. Christian Zidorn NMR analysis, Conceptualization, Writing-review & editing. Jelveh Sohrabipour Identification of the algae, Writing-review & editing. Amir Reza Jassbi Supervision, Methodology, Conceptualization, Resources, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
Amir Reza Jassbi is founder and scientific leader of the startup company: "Aryana Phytochemistry of Tirazis".
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moheimanian, N., Mirkhani, H., Edraki, N. et al. Bioassay-guided purification of α-glucosidase inhibitor fatty acids from Padina tetrastromatica. J Appl Phycol 36, 359–370 (2024). https://doi.org/10.1007/s10811-023-03125-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03125-6