Abstract
In the present study swine wastewater diluted at the different percentages (10%, 20%, 60%, and 100%) was used to cultivate the red alga Porphyridium purpureum to evaluate microalgal growth, nutrient removal and the production of polysaccharide and phycoerythrin. The results showed the dilution level significantly affected algal growth and nutrient removal. The best growth was in the swine wastewater diluted at 60% resulting in the highest biomass concentration of 9.44 ± 0.44 g L−1, and the corresponding removal efficiency of COD, total nitrogen (TN), total phosphorus (TP), and ammonia nitrogen (NH4+-N) reached up to 94.85 ± 0.08%, 92.69 ± 0.09%, 96.08 ± 0.02%, and 100 ± 0.00%, respectively. Moreover, the algal cells cultured in the wastewater produced high concentration of polysaccharide and phycoerythrin. Microalgae cultured in 10% swine wastewater produced the highest polysaccharide concentration of 2.16 ± 0.02 g L−1. The highest phycoerythrin concentration of 54.45 ± 4.76 mg L−1 was observed in the 60% swine wastewater group. The results showed that P. purpureum has great potential in treating wastewater and producing high-value byproducts, which is conducive to the development of the bio-circular economy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Swine wastewater is generally considered as one of the most polluting wastewaters as it usually contains high concentrations of nitrogen, phosphorus, and COD (Feng et al. 2020). If not handled properly, the high concentrations of nitrogen and phosphorous in the wastewater can result in water eutrophication, groundwater contamination and air pollution by ammonia volatilization (Cai et al. 2013a; Fridrich et al. 2014). Thus it is necessary to treat swine wastewater effectively so as to avoid environment pollution. At present, aerobic activated sludge-based treatment process and membrane filtration are mostly used for wastewater treatment (Alshabib and Onaizi 2019; Siddiqui et al. 2021; Zhang et al. 2021). However, these approaches have several drawbacks such as instability, long process time and excessive sludge discharge. They are also likely to cause the secondary pollution (Villegas et al. 2016; Bhatnagar and Anastopoulos 2017). Instead, microalgal-based biological treatment of wastewater has attracted extensive attention (Li et al. 2019a).
Microalgal-based wastewater treatment has been used to treat different kinds of wastewater, such as swine wastewater, municipal wastewater, aquaculture wastewater, etc. (Lee et al. 2015; Iasimone et al. 2018; Moungmoon et al. 2020; Nagarajan et al. 2020). It boasts high removal efficiency of nitrogen, phosphorus and other pollutants from wastewater thanks to the fast growth of microalgal cells and strong adaptability to the wastewater (Whitton et al. 2015; Prandini et al. 2016; Qu et al. 2021). Wang et al. (2015) found that the highest removal efficiency of COD and NH4+-N for 20-fold diluted wastewater treated by Chlorella vulgaris was 72.6% and 91.3%, respectively. Zhou et al. (2012) also found that the removal efficiencies of TP, NH4+-N, TN and COD from concentrated municipal wastewater by Auxenochlorella protothecoides were 98.48%, 100%, 90.60%, and 79.10%, respectively.
It is well-recognized that the resource recycling of nutrients is more economical and sustainable than simply removing them from the wastewater (Adams et al. 2013; Cai et al. 2013a). Nutrients in wastewater can be considered as a special form of available resource (Abinandan and Shanthakumar 2015; Shi et al. 2021; Yan et al. 2021) by transforming them to biomass and corresponding natural active products, such as lutein, astaxanthin and docosahexaenoic acid (Yin et al. 2018; Chen et al. 2019; Pan et al. 2021). The red microalga Porphyridium purpureum produces polysaccharides, phycoerythrin and unsaturated fatty acids (Li et al. 2020). The polysaccharide produced by P. purpureum has functions of preventing cardiovascular diseases, lowering blood lipid and treating diseases of liver, gallbladder, and pancreas. Thus, it has potential to be applied to the health care and animal feed industries (Arad and Levy-Ontman 2010; Jiao et al. 2011; Kavitha et al. 2016). In addition, phycoerythrin is used as a biomolecular marker in fluorescence immunoassay, flow cytometry, fluorescence microscopy and diagnostics (Spolaore et al. 2006; Milledge 2011). Therefore, P. purpureum also has a good prospect of being applied to such industries as medicine, cosmetics and biotechnology (Coward et al. 2016; Su et al. 2016). Presently, research about P. purpureum mainly focuses on the optimization of culture conditions to promote growth and bio-active substance accumulation (Gaignard et al. 2019; Wang et al. 2021a). Numerous studies have demonstrated that culture mode, nutrient concentration, light intensity, temperature and salinity significantly affect the production of phycoerythrin and polysaccharide (Guihéneuf and Stengel 2015; Soanen et al. 2016; Sanchez-Saavedra et al. 2018; Su et al. 2016; Jiao et al. 2018). For example, Jiao et al. (2018) found that the polysaccharide productivity of P. purpureum cultivated in mixotrophic mode was higher than that obtained in photoautotrophic mode. Soanen et al. (2016) observed that 25℃ was the most suitable temperature for the growth of P. purpureum, and 28℃ was conducive to the production of polysaccharides. Nuutila et al. (1997) reported that there was no significant difference in the growth of P. purpureum grown in the range of 35–46 g L−1 NaCl concentration. However, the highest yield of polysaccharides was achieved at NaCl concentration of 41.8 g L−1. In addition, sufficient nitrogen sources in the medium can greatly boost the accumulation of phycoerythrin in P. purpureum; while nitrogen deficiency was beneficial to the accumulation of polysaccharides (Guihéneuf and Stengel 2015). However, to date, there is little research about the feasibility of using P. purpureum to produce high-value products by resource recycling from wastewater. Fortunately, the recent study of Arashiro et al. (2020) demonstrated that P. purpureum could both efficiently treat food-industry wastewater and produce phycobiliproteins. Nevertheless, still more effort should be made in the field of resource recovery from wastewater by microalgae to produce high-value products, which is sustainable and environment-friendly.
In the present study, P. purpureum was cultivated in swine wastewater of different dilution concentrations. The algal growth, nutrients removal, and production of polysaccharide and phycoerythrin were investigated. The study intended to explore the feasibility of resource recovery from the widespread swine wastewater by using P. purpureum cultivation to convert nutrients in the wastewater into biomass and biologically active substances. In the end, the challenges of the application of microalgal products generated by re-utilization of wastewater are discussed.
Materials and methods
Microalgae strain
Porphyridium purpureum was obtained from State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology. Algal cells were pre-cultivated with ASW medium in transparent conical flasks (Li et al. 2019c) at 80 μmol photons m−2 s−1, and 25℃.
Compositions and pretreatment of swine wastewater
Swine wastewater (SW) was obtained from the pig farm of Ningbo Mingsheng Agricultural Technology Development Co., Ltd located at Fenghua District, Ningbo, Zhejiang Province, China. Large particles in the wastewater were filtered with sieve filter papers.
Experimental design
To evaluate the microalgae growth and nutrients removal, P. purpureum was cultivated in diluted swine wastewater. The swine wastewater with four different dilutions (10%, 20%, 60% and 100%, SW:water, v/v) (Table 1). Artificial seawater salt was added into each diluted wastewater to maintain consistent salinity. The prepared wastewater medium was sterilized at 121℃ for 20 min.
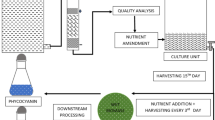
Microalgae for inoculation were collected by centrifugation at 7500xg for 5 min when cells were cultivated at logarithmic growth phase. The harvested cells were washed wit sterilized deionized water, centrifuged and selected again, and finally inoculated into a lab-scale column photobioreactor with 1 L working volume. The total volume of photobioreactor was 1.2 L with a length of 40 cm and a diameter of 6.2 cm. The initial microalgal biomass concentration was 670 mg L−1. Microalgal cells were cultivated at 25 °C with the initial pH of 7.2. The photobioreactor was provided with the light intensity of 80 μmol photons m−2 s−1 and air with 2.5% CO2 continuously aerated at a flow rate of 1.0 vvm. Culture samples were collected from the photobioreactor to measure the microalgal cell concentration, nutrient concentration, polysaccharide and phycoerythrin content.
Dry cell weight and chlorophyll fluorescence
Microalgal biomass concentration was determined by the dry cell weight method (Marjakangas et al. 2015). Specifically, 10 mL culture was sampled and then centrifuged at 4000 rpm for 10 min and the pellet was washed once with sterilized deionized water. The microalgae were transferred to a preweighed dish and dried at 110℃ until constant weight. Specific growth rate (μ) was calculated by the following equation:
where, DWt2 (g L−1) and DWt1 (g L−1) refer to the dry cell weight at culture time t2 (h) and t1 (h), respectively.
Chlorophyll fluorescence was determined using a PAM fluorometer AquaPen-C AP-C 100 (Markou et al. 2016). After 30 min of dark adaptation the values of Fv/Fm were obtained by PAM fluorometer.
Determination of water quality indicators
A 100 mL culture sample was collected and centrifuged at 9000 xg for 10 min. The supernatant was used to determine the water quality. The concentrations of total phosphorus (TP) and COD were measured with a Water Quality Tester (Lianhua company, Shanghai, China). It should be noted that P. purpureum cells are able to produce exopolysaccharides that will lead to the increase of COD in the wastewater. In order to determine the variation concentration of COD introduced by the origin wastewater only and exclude the one induced by exopolysaccharides, the value of COD caused by exopolysaccharides was subtracted from the total COD measured in the wastewater. NH4+-N content was determined by Nessler reagent spectrophotometry. Total nitrogen (TN) was determined by alkaline potassium persulfate digestion-UV spectrophotometry (Purcell and King 1996).
Determination of exopolysaccharide
For exopolysaccharides content determination, 10 mL of microalgal culture was collected and harvested by centrifugation at 4000 rpm for 10 min. Three times the volume of anhydrous ethanol was added to the supernatant, which then precipitated overnight at 4℃. The supernatant was collected by centrifugation at 9000 rpm for 10 min to measure polysaccharide content. The content of polysaccharides was measured by the phenol–sulfuric acid method (DuBois et al. 1956).
Measurement of phycoerythrin
To measure phycoerythrin, 5 mL of microalgal culture was centrifuged at 7500 rpm for 5 min and the pellet was washed twice with deionized water. An equal volume of 0.1 M PBS buffer with pH of 7 was added to the pellet and then frozen at -80℃ for 30 min and thawed at room temperature for 1 h. The freeze and thaw procedure was repeated three times. Next, the cell suspension was centrifuged at 10,000 rpm for 10 min to obtain the supernatant. The absorbance of the supernatant extract was measured at 455 nm, 564 nm and 592 nm. The content of B-phycoerythrin (B-PE) was estimated according to the following formula (Sampath-Wiley and Neefus 2007).
where CPE is the concentration of B-phycoerythrin, and OD values refers to the absorbance at different wavelengths.
Statistical analysis
All cultivation experiments were repeated separately for three times, and the mean value with standard deviation (± SD) was calculated. SPSS 26.0 was used for data analysis (ANOVA). Statistical significance of mean differences was considered to be attained with p < 0.05.
Results
Growth of P. purpureum cultivated in diluted swine wastewater
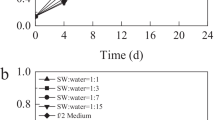
Porphyridium purpureum grew rapidly in 10%, 20%, and 60% swine wastewater (Fig. 1). Specifically, biomass concentration increased markedly initially, then reached a stable level and almost kept constant. However, P. purpureum grew extremely slowly in 100% swine wastewater. The concentrations of COD, NH4+-N, TN and TP in the undiluted swine wastewater were up to 850 ± 12 mg L−1,54.66 ± 2.52 mg L−1, 90 ± 3 mg L−1 and 20 ± 0.3 mg L−1, respectively (Table 1). Such high concentrations of nutrients may create adverse environment conditions for algal growth. Porphyridium purpureum in the 60% swine wastewater showed the best growth with the highest biomass concentration and average growth rate among all the experimental groups (p < 0.05; Table 2). The maximum biomass concentration in the 60% swine water group reached 9.44 g L−1, which was 1.36, 1.23 and 2.67 times higher than that of 10%, 20% and 100% swine wastewater.
The chlorophyll fluorescence parameter was measured to evaluate the activity of photosynthetic system of microalgal cells. The results showed that Fv/Fm of all experimental groups significantly decreased with culture time (Fig. 1b) reflecting that the photosynthesis performance of microalgae slowed down.
Nutrients removal by P. purpureum from diluted swine wastewater
As shown in Fig. 2, the COD concentration of all experimental groups gradually decreased over time. For example, the initial COD concentration of 10% swine water decreased from 85 ± 4 mg L−1 to an extremely low level of 1.52 ± 0.38 mg L−1. As to the undiluted swine water, the COD concentration decreased slowly from the initial 850 ± 12 mg L−1 to 197.56 ± 5.37 mg L−1 in the end. The COD removal of 10%, 20%, 60% and 100% swine wastewater was 98.21 ± 0.04%, 97.11 ± 0.05%, 94.85 ± 0.08% and 76.75 ± 0.04%, respectively. The removal of COD in the diluted wastewater was significantly higher than that in the undiluted wastewater (p < 0.05; Table 3). In addition, it was observed that the removal of COD declined with the increase of COD concentration. Undoubtedly, high initial COD significantly inhibited the microalgal growth, resulting in less organic substance absorbed and utilized by cells, and the high concentration of COD remained in the wastewater. However, it is important to note that the variation of COD showed here only represented the change trend of COD existing in the raw swine water, and it did not contain the one caused by exopolysaccharides secreted from the algal cells. Porphyridium purpureum produces abundant exopolysaccharides during the culture process, thus leading to substantial increase of COD concentration. In fact, due to the accumulation of exopolysaccharides, the total COD concentration of the culture increased over the time of culture (Fig. 1A Supplementary information).
In addition, the concentrations of TN and TP decreased rapidly along with the culture time (Fig. 2), showing that these nutrients can be used efficiently by P. purpureum. After 28 days of cultivation, the TN concentrations in 10%, 20%, 60% and 100% swine wastewater dropped from the initial 9 ± 0.67, 18 ± 0.61, 54 ± 0.82 and 90 ± 1.32 mg L−1 to 1.04 ± 0.26, 1.62 ± 0.29, 3.98 ± 0.33 and 14.92 ± 0.39 mg L−1, respectively. The resultant removal efficiencies of TN were 88.44 ± 0.07%, 91.02 ± 0.11%, 92.62 ± 0.09% and 83.42 ± 0.12%, respectively. The TN removal of diluted wastewater groups was significantly higher than that of the undiluted wastewater group (p < 0.05; Table 3). Furthermore, the concentrations of TP in 10%, 20%, 60% and 100% swine wastewater reduced to 0.21 ± 0.01, 0.21 ± 0.02, 0.47 ± 0.05 and 3.01 ± 0.09 mg L−1 after microalgae cultivation, and the TP removal of each swine wastewater was 89.51 ± 0.04%, 94.75 ± 0.02%, 96.08 ± 0.02% and 84.95 ± 0.02%. The removal of TP in diluted wastewater was significantly higher than that in undiluted wastewater (p < 0.05; Table 3). Furthermore, the removal efficiency of NH4+-N of all experimental groups was high. The NH4+-N in the 10%, 20%, and 60% wastewater could be completely removed by the 28th day. For the undiluted 100% swine wastewater, the removal of NH4+-N reached 86.81 ± 0.06%. Among all, the dilution of 60% was the optimal operation condition for swine wastewater treatment by P. purpureum with high removal of nutrients from wastewater. In terms of the correlation between algal growth and nutrient removal efficiency, the high growth rate of the alga corresponded to the high removal efficiency of nutrients (Wang et al. 2016; Chen et al. 2020b). Moreover, although the nutrients removal efficiency of the undiluted wastewater was the lowest, the residual contents of COD, NH4+-N and TP in the undiluted wastewater were only 197.56 ± 5.37, 7.21 ± 0.2 and 3.01 ± 0.08 mg L−1, respectively, and all of them were lower than the discharge threshold value of 400, 80, and 8 mg L−1, and met the discharge requirements for livestock and poultry pollutants in China (Table 4). In addition, it is worth noting that the salt added in the wastewater should be separated by membrane systems before the discharge. The fresh water species of P. purpureum could be deployed in swine wastewater treatment to avoid the salt addition problem (Oh et al. 2009).
Production of phycoerythrin and polysaccharide by P. purpureum
The phycoerythrin concentration of each experimental group increased rapidly to the maximum value, then decreased gradually and stayed stable (Fig. 3a). It was observed that phycoerythrin production in the undiluted wastewater was clearly the lowest, which was probably caused by the poor microalgal growth and low biomass concentration. However, the undiluted swine wastewater group had the highest phycoerythrin content among all the experimental groups (p < 0.05; Fig. 3b). The highest phycoerythrin concentration of 54.45 ± 4.76 mg L−1 was achieved on the 16th day in the 60% swine wastewater group. In addition, the concentrations of polysaccharides in 10%, 20% and 60% swine wastewater increased rapidly at first, and then stayed at stable levels. However, the polysaccharide concentration in the undiluted swine wastewater did not increase greatly due to the low biomass concentration. The maximum polysaccharide concentration of 2.16 ± 0.08 g L−1 was observed in the 10% swine wastewater group, which is mainly attributed to the high content of polysaccharide within the microalgal cell (p < 0.05; Fig. 4b).
Discussion
In this study the growth of P. purpureum cultivated in swine wastewater of different dilution rates was evaluated. The alga grew rapidly in the diluted swine wastewater, but extremely slowly in the undiluted one, indicating that growth was highly depended on the concentration and dilution rate of swine wastewater. Consistent with our results, other studies also have shown that the dilution remarkably affected microalgal growth in wastewaters (Vargas-Estrada et al. 2021; Wang et al. 2021b). Wen et al. (2017) and Yeh et al. (2010) found that microalgae could hardly grow in the undiluted livestock wastewater owing to the high concentrations of ammonium and turbidity of the wastewater. Microalgal growth would be inhibited at a certain level of ammonium and the degree of inhibition degree varies with species (Cai et al. 2013b; He et al. 2013; Liu et al. 2020). The inhibitory ammonium concentration for the growth of Cyanophyceae Bacillariophyceae, Dinophyceae, and Raphidophyceae has been reported to be 6616 μmol L−1, 725 μmol L−1, 324 μmol L−1, and 635 μmol L−1, respectively (Collos and Harrison 2014). Moreover, it was reported that 100 μmol L−1 ammonium concentration was toxic for 200 species or clones of marine microalgae (Keller et al. 1987). In the undiluted swine wastewater, ammonia nitrogen concentration was 54.66 mg L−1 and the corresponding ammonium concentration was 3904 μmol L−1. Such high concentration may be the primary reason for the poor growth of microalgae.
It was well-recognized that the decrease of Fv/Fm is mostly associated with stressful conditions such as high light and low temperature (Prates et al. 2018). It was observed that Fv/Fm gradually decreased along with the time of culture. Thus, it can be speculated that the stressful condition was slowly formed and the culture environment became adverse to the growth of algal cells. In the present study, the reason for the decrease in Fv/Fm value is considered to be complex and possibly attributed to the comprehensive impact of accumulation of extracellular polysaccharide, deficiency of nutrients, and degradation of phycoerythrin. During the culture process, microalgal cells generated the extracellular polysaccharide (Fig. 4a), which could change the osmotic pressure of the culture. The more extracellular polysaccharide accumulated at the middle and late stages, the higher osmotic pressure would be, which was physiologically harmful to the microalgal cells. It has been reported that the increase of osmotic pressure affected the conductivity and causd the decrease of Fv/Fm values (Abinandan and Shanthakumar 2015). Besides, nutrient depletion can also lead to stressful condition, which would also exert negative impact on the physiological activity of microalgal cells. Tan et al. (2019) found that Fv/Fm of microalgal cells declined in N-starved or P-starved cultures. However, the change of Fv/Fm was also possibly associated with the phycoerythrin of microalgal cells. It has been reported that Fv/Fm decreased when the phycoerythrin content of microalgal cells decreased (Pancha et al. 2014; Li et al. 2020). Therefore, it is difficult to determine the reasons why Fv/Fm decreases during the culture process.
To date, the predominant microalgae species for treating swine wastewater are Chlorella spp., Arthrospira (Spirulina) spp. and Scenedesmus spp. (Park et al. 2010; Ayre et al. 2017; Lu et al. 2020). For example, Chen et al. (2020a) employed Chlorella sorokiniana to purify swine wastewater and found the removal efficiency of COD, TN and TP was 90.1%, 97.0% and 92.8%, respectively. Lu et al. (2020) cultivated Arthrospira (Spirulina) platensis in diluted swine wastewater and observed TN and TP removal efficiencies of 85.86% and 78.69%, respectively. Furthermore, marine microalgae recently also have been tested to treat municipal wastewater, food wastewater, wastewater and other wastewater (Li et al. 2019b; Lavrinovičs et al. 2020). Daneshvar et al. (2018) cultivated Tetraselmis suecica in dairy wastewater and found removal efficiencies of TN and TP was 86.21% and 89.83%, respectively. Katayama et al. (2020) used aquaculture wastewater to culture Amphora coffeiformis for the production of arachidonic acid (EPA) and eicosapentenoic acid (AA), and found that EPA and AA accounted for 18.5% and 8.1% of total fatty acids. It was reported that the removal efficiency of COD, NH4+-N, TN and TP were in the range of 75.29–92.17%, 76.90–99.90%, 78.79–92.51% and 84.55–100% for swine wastewater by microalgae treatment (Wang et al. 2015, 2016; Xu et al. 2015; Chen et al. 2021; Lee et al. 2021). Depending on the dilution level, the removal efficiency of COD, NH4+-N, TN and TP in the swine wastewater were in the range of 76.75–98.21%, 86.81–100% 83.42–92.62% and 84.95–96.08%. The nutrient removal efficiency is comparable to or even higher than those previously reported, indicating that P. purpureum was an alternative microalgae species for efficient treatment of swine wastewater.
In addition, the dilution level of swine wastewater also strongly affected the production of polysaccharides and phycoerythrin. Microalgae grown in 10% swine wastewater with the lowest concentration of TN had the lowest phycoerythrin content. While the highest phycoerythrin content and concentration were achieved in the 100% swine wastewater with a very high concentration of TN. Furthermore, it was observed that the removal of TN had a positive correlation with the increase of phycoerythrin. The fast accumulation of phycoerythrin and rapid consumption of nitrogen both occurred in the early stage of the culture process. However, the phycoerythrin content slowly decreased in the late stage. The decline of phycoerythrin may provide nitrogen source for algal cells, maintaining cell division and growth under the nitrogen-depletion condition (Pancha et al. 2014; Li et al. 2020). Consistent with our results, other studies also have found that nitrogen starvation significantly inhibited the synthesis of phycoerythrin, while sufficiency of nitrogen could substantially enhance the phycoerythrin content (Guihéneuf and Stengel 2015). It is recognized that nitrogen is an important component of nitrogenous compounds such as key enzymes, photosynthetic pigments and genetic materials of microalgae (Pancha et al. 2014; Li et al. 2016). Thus, sufficient nitrogen would promote the growth of microalgae and accumulation of a large amounts of proteins, whereas nitrogen limitation was beneficial to the biosynthesis of polysaccharides.
It is worth noting that although the sampling during the experiment reduced the volume of culture, it would have little impact on the microalgae growth, nutrient removal and bio-active substances accumulation as the light intensity, aeration rate and temperature were almost kept the same for each experiment group. Furthermore, compared with the PBR with full level of culture, the light regime almost would not change when the culture level was low by sampling because the LED lights were installed on the side of photobioreactors rather than above them.
In recent years there have been a few studies about the production of polysaccharides and phycoerythrin by P. purpureum (Table 5). Li et al. (2019c) reported that the contents of biomass, phycoerythrin and polysaccharide were 5.54 g L−1, 193 mg L−1 and 0.342 g L−1, respectively, under 25℃ and 350 μmol photons m−2 s−1. Velea et al. (2011) observed that the biomass and polysaccharide concentration remarkedly increased to 15.2 and 4.5 g L−1 by increasing the NaHCO3 content in ASW medium and the light intensity. Although the swine wastewater was used in the study, the concentrations of polysaccharides and phycoerythrin were still comparable to or even higher than those previously reported (Table 5), indicating that P. purpureum has great potential in the production of phycoerythrin and polysaccharide by using wastewater.
The use of microalgae to re-utilize nutrients in wastewater and produce byproducts is considered to be an important way to support the biological cycle economy (Noguchi et al. 2021; Ahmed et al. 2022). However, the application of high value-added products produced in wastewater must consider bio-safety because the harmful substances in wastewater may be transferred to biological products, which will pose high potential risks (Nagarajan et al. 2019; Sharma and Arivalagan 2021). In the current study, the phycoerythrin and polysaccharide produced by microalgae cultured in the swine wastewater cannot be directly applied to human consumption. However, bioactive substance produced from microalgae grown in wastewater can be possibly used in the fields of feed and aquaculture (Madeira et al. 2017). It is expected that the potential pollutants in biological products can be reduced or even basically eliminated in the future. Hence, the application scope of high-value products sourced from microalgae cultivated in wastewater can be expanded to more industries.
Conclusion
This study explored the potential of P. purpureum cultured in swine wastewater of different proportions to realize nutrient removal and produce biomass and high-value products. The results confirmed that the COD removal efficiency could reach up to 98.21% by P. purpureum cultured in 10% swine wastewater. The highest removal of TN and TP was 92.62% and 96.08%, respectively, in 60% swine wastewater with corresponding biomass concentration of 9.44 g L−1. Overall, the results demonstrated that P. purpureum can effectively treat the swine wastewater with the production of phycoerythrin and polysaccharide, encouraging further investigation in the feasibility of this process to explore the synergistic mechanism which is related to nutrients removal and natural bioactive substance accumulation.
Data availability
All data generated or analyzed in the present study are available on reasonable request.
References
Abinandan S, Shanthakumar S (2015) Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: A review. Renew Sust Energ Rev 52:123–132
Adams C, Godfrey V, Wahlen B, Seefeldt L, Bugbee B (2013) Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Bioresour Technol 131:188–194
Ahmed SF, Mofijur M, Parisa TA, Islam N, Kusumo F, Inayat A, Le VG, Badruddin IA, Khan TMY, Ong HC (2022) Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 286:131656
Alshabib M, Onaizi SA (2019) A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep Purif Technol 219:186–207
Arad S, Levy-Ontman O (2010) Red microalgal cell-wall polysaccharides: biotechnological aspects. Curr Opin Biotechnol 21:358–364
Arashiro LT, Boto-Ordóñez M, Van Hulle SWH, Ferrer I, Garfí M, Rousseau DPL (2020) Natural pigments from microalgae grown in industrial wastewater. Bioresour Technol 303:122894
Ayre JM, Moheimani NR, Borowitzka MA (2017) Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res 24:218–226
Bhatnagar A, Anastopoulos I (2017) Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 168:885–902
Cai T, Park SY, Li Y (2013a) Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew Sust Energ Rev 19:360–369
Cai T, Park SY, Racharaks R, Li Y (2013b) Cultivation of Nannochloropsis salina using anaerobic digestion effluent as a nutrient source for biofuel production. Appl Energ 108:486–492
Chen J-H, Kato Y, Matsuda M, Chen C-Y, Nagarajan D, Hasunuma T, Kondo A, Dong C-D, Lee D-J, Chang J-S (2019) A novel process for the mixotrophic production of lutein with Chlorella sorokiniana MB-1-M12 using aquaculture wastewater. Bioresour Technol 290:121786
Chen C-Y, Kuo E-W, Nagarajan D, Ho S-H, Dong C-D, Lee D-J, Chang J-S (2020a) Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour Technol 302:122814
Chen Z, Shao S, He Y, Luo Q, Zheng M, Zheng M, Chen B, Wang M (2020b) Nutrients removal from piggery wastewater coupled to lipid production by a newly isolated self-flocculating microalga Desmodesmus sp. PW1. Bioresour Technol 302:122806
Chen C-Y, Kuo E-W, Nagarajan D, Dong C-D, Lee D-J, Varjani S, Lam SS, Chang J-S (2021) Semi-batch cultivation of Chlorella sorokiniana AK-1 with dual carriers for the effective treatment of full strength piggery wastewater treatment. Bioresour Technol 326:124773
Collos Y, Harrison PJ (2014) Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar Pollut Bull 80:8–23
Coward T, Fuentes-Grünewald C, Silkina A, Oatley-Radcliffe DL, Llewellyn G, Lovitt RW (2016) Utilising light-emitting diodes of specific narrow wavelengths for the optimization and co-production of multiple high-value compounds in Porphyridium purpureum. Bioresour Technol 221:607–615
Daneshvar E, Zarrinmehr MJ, Hashtjin AM, Farhadian O, Bhatnagar A (2018) Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour Technol 268:523–530
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Feng C, Zhang S, Wang Y, Wang G, Pan X, Zhong Q, Xu X, Luo L, Long L, Yao P (2020) Synchronous removal of ammonium and phosphate from swine wastewater by two agricultural waste based adsorbents: Performance and mechanisms. Bioresour Technol 307:123231
Fridrich B, Krčmar D, Dalmacija B, Molnar J, Pešić V, Kragulj M, Varga N (2014) Impact of wastewater from pig farm lagoons on the quality of local groundwater. Agric Water Manag 135:40–53
Gaignard C, Gargouch N, Dubessay P, Delattre C, Pierre G, Laroche C, Fendri I, Abdelkafi S, Michaud P (2019) New horizons in culture and valorization of red microalgae. Biotechnol Adv 37:193–222
Guihéneuf F, Stengel DB (2015) Towards the biorefinery concept: Interaction of light, temperature and nitrogen for optimizing the co-production of high-value compounds in Porphyridium purpureum. Algal Res 10:152–163
He PJ, Mao B, Shen CM, Shao LM, Lee DJ, Chang JS (2013) Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour Technol 129:177–181
Iasimone F, Panico A, De Felice V, Fantasma F, Iorizzi M, Pirozzi F (2018) Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: Biomass production, lipids accumulation and settleability characteristics. J Environ Manage 223:1078–1085
Jiao G, Yu G, Zhang J, Ewart HS (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 9:196–223
Jiao K, Xiao W, Xu Y, Zeng X, Ho SH, Laws EA, Lu Y, Ling X, Shi T, Sun Y, Tang X, Lin L (2018) Using a trait-based approach to optimize mixotrophic growth of the red microalga Porphyridium purpureum towards fatty acid production. Biotechnol Biofuels 11:273
Katayama T, Nagao N, Kasan NA, Khatoon H, Rahman NA, Takahashi K, Furuya K, Yamada Y, Wahid MEA, Jusoh M (2020) Bioprospecting of indigenous marine microalgae with ammonium tolerance from aquaculture ponds for microalgae cultivation with ammonium-rich wastewaters. J Biotechnol 323:113–120
Kathiresan S, Sarada R, Bhattacharya S, Ravishankar GA (2007) Culture media optimization for growth and phycoerythrin production from Porphyridium purpureum. Biotechnol Bioeng 96:456–463
Kavitha MD, Seema Shree MH, Vidyashankar S, Sarada R (2016) Acute and subchronic safety assessment of Porphyridium purpureum biomass in the rat model. J Appl Phycol 28:1071–1083
Keller MD, Selvin RC, Claus W, Guillard RRL (1987) Media for the culture of oceanic ultraphytoplankon. J Phycol 23:633–638
Lavrinovičs A, Mežule L, Juhna T (2020) Microalgae starvation for enhanced phosphorus uptake from municipal wastewater. Algal Res 52:102090
Lee CS, Lee S-A, Ko S-R, Oh H-M, Ahn C-Y (2015) Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater. Water Res 68:680–691
Lee S-A, Ko S-R, Lee N, Lee J-W, Le VV, Oh H-M, Ahn C-Y (2021) Two-step microalgal (Coelastrella sp.) treatment of raw piggery wastewater resulting in higher lipid and triacylglycerol levels for possible production of higher-quality biodiesel. Bioresour Technol 332:125081
Li T, Xu J, Gao B, Xiang W, Li A, Zhang C (2016) Morphology, growth, biochemical composition and photosynthetic performance of Chlorella vulgaris (Trebouxiophyceae) under low and high nitrogen supplies. Algal Res 16:481–491
Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W, Huo S, Cheng P, Liu J, Addy M, Chen P, Chen D, Ruan R (2019a) Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour Technol 291:121934
Li S, Zhao S, Yan S, Qiu Y, Song C, Li Y, Kitamura Y (2019b) Food processing wastewater purification by microalgae cultivation associated with high value-added compounds production — A review. Chin J Chem Eng 27:2845–2856
Li T, Xu J, Wu H, Jiang P, Chen Z, Xiang W (2019c) Growth and biochemical composition of Porphyridium purpureum SCS-02 under different nitrogen concentrations. Mar Drugs 17:124
Li S, Ji L, Chen C, Zhao S, Sun M, Gao Z, Wu H, Fan J (2020) Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour Technol 309:123362
Liu N, Zhang H, Zhao J, Xu Y, Ge F (2020) Mechanisms of cetyltrimethyl ammonium chloride-induced toxicity to photosystem II oxygen evolution complex of Chlorella vulgaris F1068. J Hazard Mater 383:121063
Lu WD, Lin MJ, Guo XL, Lin ZY (2020) Cultivation of Spirulina platensis using raw piggery wastewater for nutrients bioremediation and biomass production: effect of ferrous sulfate supplementation. Desalin Water Treat 175:60–67
Madeira MS, Cardoso C, Lopes PA, Coelho D, Afonso C, Bandarra NM, Prates JAM (2017) Microalgae as feed ingredients for livestock production and meat quality: A review. Livest Sci 205:111–121
Marjakangas JM, Chen C-Y, Lakaniemi A-M, Puhakka JA, Whang L-M, Chang J-S (2015) Selecting an indigenous microalgal strain for lipid production in anaerobically treated piggery wastewater. Bioresour Technol 191:369–376
Markou G, Depraetere O, Muylaert K (2016) Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: A study on chlorophyll fluorescence and electron transport. Algal Res 16:449–457
Milledge JJ (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biol 10:31–41
Moungmoon T, Chaichana C, Pumas C, Pathom-aree W, Ruangrit K, Pekkoh J (2020) Quantitative analysis of methane and glycolate production from microalgae using undiluted wastewater obtained from chicken-manure biogas digester. Sci Total Environ 714:136577
Nagarajan D, Kusmayadi A, Yen H-W, Dong C-D, Lee D-J, Chang J-S (2019) Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour Technol 289:121718
Nagarajan D, Lee D-J, Chen C-Y, Chang J-S (2020) Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour Technol 302:122817
Noguchi M, Aizawa R, Nakazawa D, Hakumura Y, Furuhashi Y, Yang S, Ninomiya K, Takahashi K, Honda R (2021) Application of real treated wastewater to starch production by microalgae: Potential effect of nutrients and microbial contamination. Biochem Eng J 169:107973
Nuutila AM, Aura A-M, Kiesvaara M, Kauppinen V (1997) The effect of salinity, nitrate concentration, pH and temperature on eicosapentaenoic acid (EPA) production by the red unicellular alga Porphyridium purpureum. J Biotechnol 55:55–63
Oh SH, Han JG, Kim Y, Ha JH, Kim SS, Jeong MH, Jeong HS, Kim NY, Cho JS, Yoon WB, Lee SY, Kang DH, Lee HY (2009) Lipid production in Porphyridium cruentum grown under different culture conditions. J Biosci Bioeng 108:429–434
Pan M, Zhu X, Pan G, Angelidak I (2021) Integrated valorization system for simultaneous high strength organic wastewater treatment and astaxanthin production from Haematococcus pluvialis. Bioresour Technol 326:124761
Pancha I, Chokshi K, George B, Ghosh T, Paliwal C, Maurya R, Mishra S (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 156:146–154
Park J, Jin H-F, Lim B-R, Park K-Y, Lee K (2010) Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour Technol 101:8649–8657
Prandini JM, da Silva ML, Mezzari MP, Pirolli M, Michelon W, Soares HM (2016) Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour Technol 202:67–75
Prates DD, Radmann EM, Duarte JH, de Morais MG, Costa JAV (2018) Spirulina cultivated under different light emitting diodes: Enhanced cell growth and phycocyanin production. Bioresour Technol 256:38–43
Purcell LC, King CA (1996) Total nitrogen determination in plant material by persulfate digestion. Agron J 88:111–113
Qu W, Zhang C, Chen X, Ho SH (2021) New concept in swine wastewater treatment: development of a self-sustaining synergetic microalgae-bacteria symbiosis (ABS) system to achieve environmental sustainability. J Hazard Mater 418:126264
Sampath-Wiley P, Neefus CD (2007) An improved method for estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). J Appl Phycol 19:123–129
Sanchez-Saavedra MD, Castro-Ochoa FY, Nava-Ruiz VM, Ruiz-Guereca DA, Villagomez-Aranda AL, Siqueiros-Vargas F, Molina-Cardenas CA (2018) Effects of nitrogen source and irradiance on Porphyridium cruentum. J Appl Phycol 30:783–792
Sharma NK, Arivalagan AR (2021) 8 - Algae or bacteria—the future of biological wastewater treatment. In: Rahman ROA, Hussain CM (eds) Handbook of advanced approaches towards pollution prevention and control. Elsevier, London, pp 217–247
Shi X, Ye X, Zhong H, Wang T, Jin F (2021) Sustainable nitrogen-containing chemicals and materials from natural marine resources chitin and microalgae. Mol Catal 505:111517
Siddiqui MA, Biswal BK, Saleem M, Guan D, Iqbal A, Wu D, Khanal SK, Chen G (2021) Anaerobic self-forming dynamic membrane bioreactors (AnSFDMBRs) for wastewater treatment – Recent advances, process optimization and perspectives. Bioresour Technol 332:125101
Soanen N, Da Silva E, Gardarin C, Michaud P, Laroche C (2016) Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour Technol 213:231–238
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Su G, Jiao K, Li Z, Guo X, Chang J, Ndikubwimana T, Sun Y, Zeng X, Lu Y, Lin L (2016) Phosphate limitation promotes unsaturated fatty acids and arachidonic acid biosynthesis by microalgae Porphyridium purpureum. Bioprocess Biosyst Eng 39:1129–1136
Tan L, Xu W, He X, Wang J (2019) The feasibility of Fv/Fm on judging nutrient limitation of marine algae through indoor simulation and in situ experiment. Estuar Coast Shelf S 229:106411
Vargas-Estrada L, Longoria A, Okoye PU, Sebastian PJ (2021) Energy and nutrients recovery from wastewater cultivated microalgae: Assessment of the impact of wastewater dilution on biogas yield. Bioresour Technol 341:125755
Velea S, Ilie L, Filipescu L (2011) Optimization of Porphyridium purpureum culture growth using two variables experimental design: Light and sodium bicarbonate. UPB Sci Bull Ser B: Chem Mater 73:81–94
Villegas LGC, Mashhadi N, Chen M, Mukherjee D, Taylor KE, Biswas N (2016) A short review of techniques for phenol removal from wastewater. Curr Pollut Rep 2:157–167
Wang Y, Guo W, Yen H-W, Ho S-H, Lo Y-C, Cheng C-L, Ren N, Chang J-S (2015) Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour Technol 198:619–625
Wang M, Yang Y, Chen Z, Chen Y, Wen Y, Chen B (2016) Removal of nutrients from undiluted anaerobically treated piggery wastewater by improved microalgae. Bioresour Technol 222:130–138
Wang W-N, Li Y, Zhang Y, Xiang W-Z, Li A-F, Li T (2021a) Comparison on characterization and antioxidant activity of exopolysaccharides from two Porphyridium strains. J Appl Phycol 33:2983–2994
Wang Z, Xia L, Song S, Farías ME, Li Y, Tang C (2021b) Cadmium removal from diluted wastewater by using high-phosphorus-culture modified microalgae. Chem Phys Lett 771:138561
Wen Y, He Y, Ji X, Li S, Chen L, Zhou Y, Wang M, Chen B (2017) Isolation of an indigenous Chlorella vulgaris from swine wastewater and characterization of its nutrient removal ability in undiluted sewage. Bioresour Technol 243:247–253
Whitton R, Ometto F, Pidou M, Jarvis P, Villa R, Jefferson B (2015) Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. Environ Technol 4:133–148
Xu J, Zhao Y, Zhao G, Zhang H (2015) Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl Microbiol Biot 99:6493–6501
Yan Z, Shen T, Li W, Cheng W, Wang X, Zhu M, Yu Q, Xiao Y, Yu L (2021) Contribution of microalgae to carbon sequestration in a natural karst wetland aquatic ecosystem: An in-situ mesocosm study. Sci Total Environ 768:144387
Yeh K-L, Chang J-S, Chen W-M (2010) Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng Life Sci 10:201–208
Yin F-W, Guo D-S, Ren L-J, Ji X-J, Huang H (2018) Development of a method for the valorization of fermentation wastewater and algal-residue extract in docosahexaenoic acid production by Schizochytrium sp. Bioresour Technol 266:482–487
Zhang F, Peng Y, Wang Z, Jiang H, Ren S, Qiu J (2021) Achieving synergetic treatment of sludge supernatant, waste activated sludge and secondary effluent for wastewater treatment plants (WWTPs) sustainable development. Bioresour Technol 337:125416
Zhou W, Min M, Li Y, Hu B, Ma X, Cheng Y, Liu Y, Chen P, Ruan R (2012) A hetero-photoautotrophic two-stage cultivation process to improve wastewater nutrient removal and enhance algal lipid accumulation. Bioresour Technol 110:448–455
Acknowledgements
We deliver sincere gratitude to Pro.Fan at the State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology for providing the microalgae species used in experiments.
Funding
This research was funded by the Nantong social livelihood science and technology project (MS12021024) and the Fundamental Research Funds for the Central Universities (No. B200201001).
Author information
Authors and Affiliations
Contributions
Aihua Zhang: Conceptualization, Investigation, Writing. Bo Feng: Investigation, Writing—Original Draft. Han Zhang: Data curation; Formal analysis. Jinshun Jiang: Investigation, Writing—Original Draft. Yi Du: Investigation, Writing—Original Draft. Zheng Cheng: Investigation, Writing—Original Draft. Daofeng Zhang: Investigation, Writing-Review & Editing. Jianke Huang: Conceptualization, Project administration, Supervision, Writing—Review & Editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, A.H., Feng, B., Zhang, H. et al. Efficient cultivation of Porphyridium purpureum integrated with swine wastewater treatment to produce phycoerythrin and polysaccharide. J Appl Phycol 34, 2315–2326 (2022). https://doi.org/10.1007/s10811-022-02785-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02785-0