Abstract
Petalonia fascia is a widespread brown alga with economic potential due to its use as raw or dried powder, and in the biomedical field. Protoplasts are living plant cells devoid of cell wall with a wide range of applications in basic and applied research, especially in crop improvement, and as a seedstock in seaweeds. Protoplasts have been previously isolated from P. fascia, but, their regeneration ability, an important prerequisite for protoplast applications, has not been explored. In this work, we report the protoplast isolation and successful regeneration from P. fascia using the commercially available cellulase “Onozuka” RS (1%) and alginate lyase (4 U mL−1). Protoplast production was enhanced under increased osmolarity (2512 mOsm L−1 H2O), with chelation pre-treatment, and short incubation time (4 h). Our protocol produced more than 14 times the number of protoplasts obtained using previously reported protocols. After 4 weeks in culture, protoplasts developed into prostrate and discoid and mixed thallus, as well as cell clumps. Blades mostly emerged from prostrate thalli. Antibiotics were not crucial for improving protoplast regeneration, and temperature did not affect the development of the morphological forms and blades. Our results show that high yields of protoplasts (107 protoplasts g−1 FW) with good regeneration ability can be obtained from P. fascia using a simple mixture of commercial enzymes. This represents the first report of successful protoplast regeneration in P. fascia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Petalonia fascia (O.F.Müller) Kuntze is a brown alga distributed along temperate coasts worldwide. Its thallus is mostly epiphytic and shows a polystichous structure, with blades up to 30 cm high attached to the substratum by small discoid holdfasts (Boo 2010). Cultivation of P. fascia was proposed by Lee et al. (2003) in Korea as a new economic species. They stated that P. fascia could be easily cultivated on ropes and that its market value was promising, either as raw or dried powder. In addition, chemical compounds or extracts from P. fascia has been extensively investigated for biomedical applications and protected with nine patent applications. For example, fucoidan from this species has been used for enhancing stem cell mobilization and proliferation, elastase inhibitor for obtaining a synergy effect with fish-derived collagen, and an extract for enhancing bone mass (Kim and Jung 2019). Thus, P. fascia represents a potential economic brown alga with multiple uses.
Protoplasts are naked living plant cells that can be obtained by enzymatic digestion of the cell walls. These are widely used for studying plant genetics, for breeding, and, more recently, for genome-editing and gene silencing technologies (Davey et al. 2005; Burris et al. 2016). In seaweeds, protoplasts can be also used as seeds for large-scale farming, offering the advantage of continuous supply of plantlets without a waiting period (Gupta et al. 2018). Isolation and regeneration of brown algal protoplasts have been accomplished in 33 species (Fisher and Gibor 1987; Chen and Shyu 1994a; Matsumura 1998; Reddy et al. 2008; Avila-Peltroche et al. 2019; Avila-Peltroche and Won 2020). Considering the economic potential of P. fascia, this species is a good candidate for protoplast technology. High yields of protoplasts have been obtained from P. fascia using a combination of cellulase “Onozuka” RS and macerozyme R-10 (Chen and Shyu 1994a). However, their regeneration ability, an important prerequisite in protoplast technology, has not been assessed.
In this study, we report the protoplast isolation and successful regeneration from P. fascia using a simple mixture of commercial enzymes. We tested the effect of osmolarity, driselase inclusion, chelation pre-treatment, incubation time, and previous isolation protocols on protoplast production. Also, we assessed the effect of antibiotics and temperature in protoplast cultures to determine the best conditions for regeneration.
Materials and methods
Isolation and culture of the strain
Petalonia fascia was isolated from crude cultures of filamentous brown algae collected in Geoje Island, Gyeongsangnam-do, Korea, on March 16, 2018. Young germlines were cultured in 60×15 mm Petri dishes containing PES medium (Provasoli 1968) under 14:10-h light/dark photoperiod at 20 °C with light intensity 40 μmol photons m−2 s−1 of blue LED (DyneBioCo. Korea). The medium was renewed weekly. In this condition, prostrate thalli were obtained. Erect thalli (blades) developed upon cultivation in 1 L flat-bottomed round flasks filled with 1 L PES medium under aeration with a light intensity of 40–72 μmol photons m−2 s−1. Temperature and photoperiod were the same as indicated above. The air was sterilized using 0.22 μm SFCA syringe filters (Corning, Germany). The medium was renewed every 2 weeks. After a month in culture, blades reached about 8 cm in length before starting the formation of plurilocular sporangia. Representative specimen was deposited in the herbarium of Chosun University (CUK19258, =MBRB0029TC19258).
Identification of the culture strain
Taxonomic identification was performed using morphological characters (Boo 2010) from blades maintained in 1 L flat-bottomed round flasks with aeration. Photomicrographs were taken using a Leica inverted microscope (DMi8; Leica, Germany) equipped with a Leica DFC450C camera. Genomic DNA extraction, PCR amplification, DNA purification, and sequencing were performed according to Bustamante et al. (2016) using cultured samples. The plastid rbcL was amplified using the primer combinations described by Kogame et al. (1999). The amplified gene sequences were compared to the GenBank nucleotide database using the BLAST program (Altschul et al. 1997).
Protoplast isolation
The commercially available cell wall lytic enzymes used for this study included cellulase “Onozuka” RS (Yakult Co. Ltd., Japan), alginate lyase, and driselase (Sigma-Aldrich, USA). Different enzyme combinations and conditions are shown in Table 1.
Protoplast isolation was carried out as previously described (Coelho et al. 2012) with some modifications. Briefly, approximately 100–300 mg of explants of 4–6 mm2 from cultured blades of P. fascia (about 8 cm in length) were incubated in a 0.22 μm filter-sterilized enzymatic solution (400 mM NaCl, 130 mM MgCl2·6H2O, 22 mM MgSO4, 160 mM KCl, 2 mM CaCl2, and 10 mM MES; pH 6; 1570 mOsm L−1 H2O) containing cellulase “Onozuka” RS and alginate lyase, either with or without driselase, at 20 °C with shaking at 70 rpm in the dark. Vegetative blades were used in all experiments. The osmolarity of the enzymatic solution was tested in two levels: normal osmolarity (1× = 1570 mOsm L−1 H2O) and increased osmolarity (1.6× = 2512 mOsm L−1 H2O). Osmolarity was increased by increasing the component concentrations in the enzymatic solution keeping their same proportions. The inclusion of driselase was assessed together with osmolarity. Also, we tested the effect of chelation pre-treatment and incubation time on protoplast yields. Pre-treatment, which was conducted with a calcium-chelating solution [665 mM NaCl, 30 mM MgCl2·6H2O, 30 mM MgSO4, 20 mM KCl, and 20 mM ethylene glycol-bis(β-amino-ethyl ether)-N,N,N′,N′-tetraacetic acid tetrasodium salt (EGTA-Na4) as the calcium chelator; pH5.5] for 20 min prior to enzymatic digestion (Coelho et al. 2012). Protoplast isolation was repeated four times in each treatment.
Protoplast isolation was also performed following the Chen and Shyu’s (1994a) with 4% cellulase “Onozuka” RS and 2% macerozyme R-10 and Kevekordes’ (1993) non-enzymatic method (only chelation pre-treatment; Kevekordes et al. 1993) in order to determine the best protocol under the same laboratory conditions. Their protoplast yields were compared to the values obtained using our protocol under optimal conditions.
Protoplast purification was performed according to Avila-Peltroche and Won (2020). Protoplast yield was estimated by using a hemocytometer (Marienfeld, Germany) and expressed as protoplasts g−1 fresh weight (FW). Protoplast size was calculated by using ImageJ 1.46r software (Abràmoff et al. 2004) based on 100 cell measurements for each repetition.
Viability and cell wall removal
The viability of protoplasts and cell wall removal were assessed by the red chlorophyll autofluorescence and staining with calcofluor white M2R (Sigma-Aldrich, USA), respectively, as previously described (Avila-Peltroche et al. 2019).
Protoplast regeneration
Cells were dispensed into 1 mL of regeneration medium (PES + 285 mM NaCl + 5 mM CaCl2; Mejjad et al. 1992) in 24-well tissue culture test plates, and cultured in the dark at initial protoplast density of 9 × 103 protoplasts mL−1. After 2 days in the dark, osmotic pressure was reduced slowly by adding PES medium (1/5 the volume of the initial regeneration medium). Osmotic pressure was further reduced during the next 2 days by adding PES (2/5 the volume of the initial regeneration medium each day). Protoplasts were exposed to 2–4 μmol photons m−2 s−1 by the second day of osmolarity reduction. Light intensity was increased to 20–25 μmol photons m−2 s−1 by the end of the osmolarity reduction. PES medium was renewed once a week. White fluorescent light was used in all the cultures.
The addition of an antibiotic mixture (50 mg L−1 penicillin G, 25 mg L−1 streptomycin, and 5 mg L−1 chloramphenicol; Coelho et al. 2012) and three temperatures (10 °C, 16 °C, and 20 °C) was evaluated in three repetitions. The percentage (%) of dividing cells was calculated after 3 weeks in culture. Morphological forms (i.e., prostrate thallus, discoid thallus, mixed thallus and cell clumps) obtained in each treatment combination were recorded as the number of forms per well after 4 weeks in culture. The regeneration rate (%) was calculated at this point by counting the number of blades in each well.
Statistical analysis
Normality and homoscedasticity were examined by using the Shapiro-Wilk and Levene tests, respectively, prior to conducting parametric tests. Two-way analysis of variance (ANOVA) was used for the comparison of protoplast yield under driselase inclusion and osmolarity. One-way ANOVA was performed to examine the effect of chelation pre-treatment, incubation time, and isolation protocols on protoplast yields. Effect sizes (Sullivan and Feinn 2012) were presented as ω2 (Field 2009; Lakens 2013) in case of significant results. All these analyses were performed using “car” (Fox and Weisberg 2019) and “userfriendlyscience” (Peters 2018) packages in R.
Tukey’s post hoc test was used when the results were significant. Post hoc comparisons were conducted using “multcomp” (Hothorn et al. 2008) or “userfriendlyscience” (Peters 2018) packages in R.
The percentage (%) of dividing cells and regeneration rate (%) were analyzed as proportions with beta regressions, since beta distribution provides a flexible model for continuous variables restricted to the interval (0, 1) (Ferrari and Cribari-Neto 2004). The analyses were performed using “betareg” package in R (Cribari-Neto and Zeileis 2010). The proportional reduction of error (PRE) statistic was used as the overall model effect size (Smithson and Verkuilen 2006).
The number of each morphological form per well was analyzed using either negative binomial or Poisson regression model. Likelihood ratio test was used for deciding which count regression model to use. If zeros were present, Voung test for non-nested data was carried out to check if a zero-inflated regression was needed (Elhai et al. 2008). The analyses were performed using “pscl” (Jackman 2015) and “MASS” (Venables and Ripley 2002) packages in R (R Core Team 2016).
The significance threshold was set at p = 0.01 in order to reduce the true Type I error rate (at least 7%, but typically close to 15%) (Sellke et al. 2001). All graphs were created in Graphpad Prism 6.0 (GraphPad Software, USA).
Results
Identification
The vegetative characteristics of the cultured blades matched with the description of Petalonia fascia (Boo 2010), although they were somewhat twisted due to culture conditions (suspension cultures; Fig. 1a). Our morphological identification of P. fascia was also confirmed by molecular analysis. A 1333-bp portion of the 1476-bp rbcL gene was sequenced from our strain of P. fascia (Genbank accession number, MW810430). The rbcL sequence of our strain was 99% similar to field samples of P. fascia reported from Japan by Matsumoto et al. (2014).
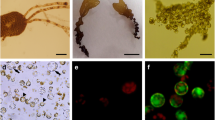
Protoplast isolation and regeneration from Petalonia fascia. a A blade from suspension culture in 1 L flat-bottomed round flask with aeration. b Protoplasts from the cortex (arrows) and medulla (arrowhead) c True protoplasts showing red chlorophyll autofluorescence. The blue fluorescence indicates cell wall material. d A protoplast starting cell wall formation after 24 h of culture. e Complete cell wall regeneration after 4 days of culture. Bright blue fluorescence indicates cellulose deposition. f Three-celled stage after 3 weeks of culture. G Prostrate thallus with a phaeophycean hair (arrow) after 4 weeks of culture. The asterisks in f and g indicate the initial protoplast. h Discoid thallus. i Mixed thallus showing prostrate filaments and disc thallus. j A cell clump. k A “spore” (arrowhead) being released from a group of cells derived from a protoplast-cell. l Germination of “spores” (arrowheads) and germination of a protoplast (arrow). m A blade formed from prostrate thallus after 4 weeks of culture. Scale bars: a = 1 cm; b, f, j, k = 10 μm; c, d, e = 5 μm; g, l = 25 μm; h = 50 μm; i, m = 100 μm
Protoplast isolation using enzymes
Protoplast yields ranged from 28–85 × 106 protoplasts g−1 FW. Mixture C (cellulase ‘Onozuka” RS, alginate lyase and driselase with 1.6× osmolarity) produced the highest number of protoplasts (85.16 ± 29.62 × 106 protoplasts g−1 FW), followed by mixture D (cellulase “Onozuka” RS and alginate lyase with 1.6× osmolarity) with 48.80 ± 12.45 × 106 protoplasts g−1 FW. The effect of osmolarity and driselase inclusion is shown in Fig. 2a. Osmolarity increase had a positive effect on protoplast production (p = 0.008; ω2 = 0.36). Under increased osmolarity (1.6×), protoplast yields were around 1.8 times higher than in normal osmolarity (1×). Although there was a tendency toward higher protoplast yields when driselase was included, this was not statistically significant (p = 0.011). The interaction between both factors did not have a significant effect (p = 0.999).
Effects of different isolation conditions on protoplast yield from Petalonia fascia. a Effect of osmolarity and driselase inclusion. b Effect of chelation pre-treatment. c Effect of incubation time. d Effect of three different isolation protocols: CRS+AL (this study); non-enzymatic (Kevekordes et al. 1993); and CRS+MR-10 (Chen and Shyu 1994a). Independent data points and averages (horizontal lines) are shown (n = 4). Error bars represent 95% confidence intervals. Different letters indicate significant differences between means (p < 0.01). CR, cellulase “Onozuka” RS; AL, alginate lyase; MR-10, macerozyme R-10; ns, no significant difference (p > 0.01)
In an effort to simplify our protoplast isolation protocol, chelation pre-treatment and different incubation times were tested. Our experiments showed that pre-treatment had a significant effect on protoplast yield (p = 0.002; ω2 = 0.77; Fig. 2b). Also, incubation time could be reduced to 4 h without compromising the protoplast numbers (Fig. 2c).
When comparing protoplast protocols, 98.58 ± 10.27 × 106 protoplasts g−1 FW were isolated from our protocol 1% cellulase “Onozuka” RS and 4 U mL−1 with chelation pre-treatment). In contrast, 6.66 ± 8.55 × 106 protoplasts g−1 FW were isolated from Chen and Shyu’s (1994a) protocol (4% cellulase “Onozuka” RS and 2% macerozyme R-10), and 1 ± 0.99 × 106 protoplasts g−1 FW from Kevekordes’ (1993) non-enzymatic method (Fig. 2d).
Explants were totally digested after 2–4 h in the enzymatic mixture. Numerous protoplasts were isolated from the cortex and medulla of the blades (Fig. 1b). Protoplasts were pale yellow-brown, spherical shape with a single discoid chloroplast. They were 14.43 ± 5.91 μm (range, 7–36 μm) in diameter. True protoplast percentages were 99–100% with calcofluor white staining. The viability of freshly isolated protoplasts was 98–100% (Fig. 1c).
Protoplast culture and regeneration
Staining with calcofluor white revealed that protoplasts started regenerating their cell walls as soon as 12–24 h in culture (Fig. 1d). Some protoplasts showed complete re-synthesis of their cell walls after 4 days in culture (Fig. 1e). However, complete cell wall regeneration was extensively observed during first two weeks in culture. A week later, protoplasts underwent first asymmetric cell division and progressed into a 3-celled stage (Fig. 1f).
The addition of antibiotic mixture at the beginning of protoplast culture showed significant effect on cell division after 3 weeks in culture (p = 0.002, PRE = 0.01). Also, it presented a significant interaction with temperature (p = 0.002, PRE = 0.05). The highest value of dividing cells was found at 10°C with antibiotics (11.66 ± 1.91%). After 4 weeks in culture, four main morphologies were observed: (1) prostrate thallus; (2) discoid thallus; (3) mixed thallus; and (4) cell clumps (Fig. 1g–j). Antibiotic mixture and temperature did not have significant effect on the formation of these morphological forms (p > 0.01). Prostrate thallus was the predominant form in all the cultures and blades arose almost exclusively from this one (Fig. 1m). The highest regeneration rate was found at 20°C without antibiotics (8.45 ± 7.35%). However, the regeneration values were not significantly affected by the antibiotic mixture and temperature (p > 0.01; Table 2). In one culture, we could observe successive cell divisions within some spherical protoplast-cells, and the subsequent formation of “spores” that were later released (Fig. 1k). This “spores” were 4.85 ± 0.97 μm (range, 3–6 μm), 3 times smaller than protoplasts (Fig. 1l). They were able to germinate but their further development was not followed.
Discussion
The present study reports the isolation of high amounts of protoplasts (107 protoplasts g−1 FW) from Petalonia fascia using commercial enzymes. Also, this work is the first presenting the successful regeneration of protoplasts from P. fascia.
A simple mix of commercial cellulase “Onozuka” RS and alginate lyase could completely digest P. fascia explants releasing high yields of protoplasts. The inclusion of driselase showed a positive effect on protoplast production; however, this was not statistically significant. Driselase is a natural mixture of enzymes (e.g., cellulase, hemicellulase, and pectinase) that can cleave mixed-linked glucan (MLG), also known in fungi as lichenan (Thibault and Rouau 1990). Although the presence of MLG in brown algal cell walls has been reported (Salmeán et al. 2017), our results suggest that driselase might not be crucial for improving protoplast yields.
Increased osmolarity of the enzymatic solution favored the protoplast release in P. fascia. Possible reasons for this effect are (1) stimulation of alginate lyase activity by increasing salt concentrations (Huang et al. 2013) or (2) protection of the protoplast membrane (Xiaoke et al. 2003). Similar results were found on protoplast isolation from Undaria pinnatifida gametophytes and Sphacelaria fusca (Avila-Peltroche and Won 2020; Avila-Peltroche et al. 2020).
In our experiments, chelation pre-treatment improved the protoplast yield of P. fascia. This positive effect has also been reported in protoplast isolation from Ectocarpales (Coelho et al. 2012) and Laminariales (Butler et al. 1989; Kloareg et al. 1989). However, in a previous report, this pre-treatment did not enhance protoplast release from P. fascia (Chen and Shyu 1994a). It is known that the pH of the EGTA solution used for pre-treatment is a critical factor. Butler et al. (1989) indicated that values over pH 5.5 caused extensive tissue damage in the brown alga Laminaria. The pH for the pre-treatment used in our work and the previous report was 5.5 and 6.5, respectively. This could explain the difference in the effectiveness of the pre-treatment in P. fascia. Our results also showed an optimal incubation time of 4 h, which is in the range of values reported for Petalonia and other blade forms (2–5 h; Chen and Shyu 1994a; Matsumura 1998).
Our new protocol (1% cellulase “Onozuka” RS and 4 U mL−1 alginate lyase with chelation) produced 15 and 100 times higher than the ones obtained following Chen and Shyu’s (1994a) protocol and Kevekordes’ (1993) method under the same laboratory conditions, respectively.
Also, we obtained 106 protoplasts g−1 FW from cultured samples of P. fascia using Chen and Shyu’s protocol, while Chen and Shyu (1994a) reported 108 protoplasts g−1 FW from field material of this species. Also, we obtained 106 protoplasts g−1 FW from P. fascia using the non-enzymatic method, while Kevekordes et al. (1993) reported 107 protoplasts g−1 FW from kelps (Laminariales).
Protoplast regeneration of P. fascia involved the formation of prostrate and discoid and mixed thalli, as well as cell clumps. Among the four forms, the first one was the predominant in cultures. Erect thalli (blades) arose usually from the prostrate thalli after 4 weeks of culture. Discoid thalli and cell clumps have been also found in protoplast cultures from Petalonia binghamiae. In this species, blades only emerged from discoid thalli formed by protoplasts from immature blades and young plantlets (Chen and Shyu 1994b). Prostrate and discoid thalli have been reported in the life cycle of P. fascia (Hsiao 1969; Kogame 1997; Lee et al. 2003). The occurrence of “spores” from group of cells formed within the spherical protoplast-cell has not been reported in protoplast regeneration of brown algae. Chen and Shyu (1994b) indicated that outer cells in clumps detached in later stages of protoplast cultures from P. binghamiae; however, this does not seem to be the case in our cultures, as these “spores” were smaller than the other cells in the group. Protoplast-derived sporangia have been only reported in the green seaweed Ulva lactuca (Gupta et al. 2018). A detailed examination of this process is needed for understanding the nature of these “spores”.
Temperature did not have a significant effect on the development of the morphological forms and blades. A similar trend was found by Hsiao (1970), who reported that prostrate and discoid thalli, as well as blades, were present in cultures from 6 to 20 °C. The addition of antibiotics at the beginning of the culture did not enhance the regeneration in P. fascia. This suggests that protoplasts from this species are not very sensitive to microbial contamination, which differs from those ones of kelps (Benet et al. 1997).
In conclusion, high amounts of protoplasts could be obtained from P. fascia using a simple mix of commercial enzymes (cellulase “Onozuka” RS + alginate lyase), short incubation time (4 h), chelation pre-treatment, and increased osmolarity. Protoplasts regenerated into blades through the formation of prostrate thalli after 4 weeks in culture. Other forms were also observed but in less frequency. Antibiotics were not needed for improving regeneration and temperatures from 10 °C to 20 °C were suitable for protoplast culture.
Data availability
The data that support the findings of this study are available from the corresponding author, Tae Oh Cho, upon reasonable request.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Avila-Peltroche J, Won BY (2020) Protoplast production from Sphacelaria fusca (Sphacelariales, Phaeophyceae) using commercial enzymes. J Mar Biosci Biotechnol 12:50–58
Avila-Peltroche J, Won BY, Cho TO (2020) Optimization of protoplast isolation from the gametophytes of brown alga Undaria pinnatifida using response surface methodology. J Appl Phycol 32:2233–2244
Avila-Peltroche J, Won BY, Cho TO (2019) Protoplast isolation and regeneration from Hecatonema terminale (Ectocarpales, Phaeophyceae) using a simple mixture of commercial enzymes. J Appl Phycol 31:1873–1881
Benet H, Gall AE, Asensi A, Kloareg B (1997) Protoplast regeneration from gametophytes and sporophytes of some species in the order Laminariales (Phaeophyceae). Protoplasma 199:39–48
Boo SM (2010) Scytosiphonaceae, Petrospongiaceae. In: Kim HS, Boo SM (eds) Algal flora of Korea, volume 2, number 1. Heterokontophyta: Phaeophyceae: Ectocarpales. Marine brown algae I. National Institute of Biological Resources, Incheon
Burris KP, Dlugosz EM, Collins AG, Stewart CN, Lenaghan SC (2016) Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.). Plant Cell Rep 35:693–704
Bustamante DE, Won BY, Cho TO (2016) The conspecificity of Pterosiphonia spinifera and P. arenosa (Rhodomelaceae, Ceramiales) inferred from morphological and molecular analyses. Algae 31:105–115
Butler DM, Ostgaard K, Boyen C, Evans LV, Jensen A, Kloareg B (1989) Isolation conditions for high yields of protoptasts from Laminaria saccharina and L. digitata (Phaeophyceae). J Exp Bot 40:1237–1246
Chen C-S, Shyu J-F (1994a) Isolation of protoplasts from four species of brown algae. Bot Bull Acad Sin 35:95–104
Chen C-S, Shyu J-F (1994b) Regeneration of the protoplasts from the brown alga, Endarachne binghamiae (Phaeophyta; Punctariales, Scytosiphonaceae). Bot Bull Acad Sin 35:189–193
Coelho SM, Scornet D, Rousvoal S, Peters N, Dartevelle L, Peters AF, Cock JM (2012) Isolation and regeneration of protoplast from Ectocarpus. Cold Spring Harbor Protoc. https://doi.org/10.1101/pdb.prot067959
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Softw 34:1–24
Davey MR, Anthony P, Power JB, Lowe KC (2005) Plant protoplasts: status and biotechnological perspectives. Biotechnol Adv 23:131–171
Elhai JD, Calhoun PS, Ford JD (2008) Statistical procedures for analysing mental health services data. Psychiatry Res 160:129–136
Ferrari SLP, Cribari-Neto F (2004) Beta regression for modelling rates and proportions. J Appl Stat 31:799–815
Field A (2009) Discovering statistics using SPSS, 3rd edn. Sage, Los Angeles
Fisher DD, Gibor A (1987) Production of protoplasts from the brown alga, Sargassum muticum (Yendo) Fensholt (Phaeophyta). Phycologia 26:488–495
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Los Angeles https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Gupta V, Trivedi N, Simoni S, Reddy CRK (2018) Marine macroalgal nursery: a model for sustainable production of seedlings for large scale farming. Algal Res 31:463–468
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Hsiao SIC (1969) Life history and iodine nutrition of the marine brown alga, Petalonia fascia (O.F. Müll.) Kuntze. Can J Bot 47:1611–1616
Hsiao SIC (1970) Light and temperature effects on the growth, morphology, and reproduction of Petalonia fascia. Can J Bot 48:1359–1361
Huang L, Zhou J, Li X, Peng Q, Lu H, Du Y (2013) Characterization of a new alginate lyase from newly isolated Flavobacterium sp. S20. J Ind Microbiol Biotechnol 40:113–122
Jackman S (2015) pscl: classes and methods for R developed in the Political Science Computational Laboratory, Department of Political Science. Stanford University, Stanford, California. R package version 1.4.9. URL http://pscl.stanford.edu/.
Kevekordes K, Beardall J, Clayton MN (1993) A novel method for extracting protoplasts from large brown algae. J Exp Bot 44:1587–1593
Kim T-H, Jung W-K (2019) R&D Trends of brown algae as potential candidates in biomedical application. J Mar Biosci Biotechnol 11:1–13
Kloareg B, Polne-Fuller M, Gibor A (1989) Mass production of viable protoplasts from Macrocystis pyrifera. Plant Sci 62:105–112
Kogame K (1997) Sexual reproduction and life history of Petalonia fascia. Phycologia 36:389–394
Kogame K, Horiguchi T, Masuda M (1999) Phylogeny of the order Scytosiphonales (Phaeophyceae) based on DNA sequences of rbcL, partial rbcS, and partial LSU nrDNA. Phycologia 38:496–502
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVA. Front Psychol 4:863
Lee K-W, Cho J-H, Shin J-A (2003) A study on cultivation of Petalonia fascia (Scytosiphonales, Phaeophyta) by vegetative regeneration. Algae 18:333–339
Matsumoto K, Ichihara K, Shimada S (2014) Taxonomic reinvestigation of Petalonia (Phaeophyceae, Ectocarpales) in southeast of Honshu, Japan, with a description of Petalonia tenuis sp. nov. Phycologia 53:127–136
Matsumura W (1998) Efficient isolation and culture of viable protoplasts from Laminaria longissima Miyabe (Phaeophyceae). Bull Fish Sci Hokkaido Univ 49:85–90
Mejjad M, Loiseaux-de-Goër S, Ducreux G (1992) Protoplast isolation, development, and regeneration in different strains of Pilayella littoralis (L.) Kjellm. (Phaeophyceae). Protoplasma 169:42–48
Peters G (2018) userfriendlyscience: quantitative analysis made accessible. https://doi.org/10.17605/osf.io/txequ. R package version 0.7.2: https://userfriendlyscience.com
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collections of algae. Proceedings of the U.S.–Japan Conference, Hakone, pp 63–75
R Core Team (2016). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 3 Mar 2021
Reddy CRK, Gupta MK, Mantri VA, Bhavanath J (2008) Seaweed protoplast: status, biotechnological perspectives and needs. J Appl Phycol 20:619–632
Salmeán AA, Duffieux D, Harholt J, Qin F, Michel G, Czjzek M, Willats WGT, Hervé C (2017) Insoluble (1→3), (1→4)-β-D-glucan is a component of cell walls in brown algae (Phaeophyceae) and is masked by alginates in tissues. Sci Rep 7:2880
Sellke T, Bayarri MJ, Berger JO (2001) Calibration of p values for testing precise null hypotheses. Am Stat 55:62–71
Smithson M, Verkuilen J (2006) A better lemon squeezer? Maximum-likelihood regression with Beta-distributed dependent variables. Psychol Methods 11:54–71
Sullivan GM, Feinn R (2012) Using effect size-or why the P value is not enough. J Grad Med Educ 4:279–282
Thibault J-F, Rouau X (1990) Studies on enzymic hydrolysis of polysaccharides in Sugar beet pulp. Carbohydr Polym 13:1–16
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Xiaoke H, Xiaolu J, Huashi G (2003) Isolation of protoplast from Undaria pinnatifida by alginate lyase digestion. J Ocean Univ China 2:58–61
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1F1A1060346 and NRF-2021R1I1A2059577), by the Ministry of Ocean and Fisheris (MarineBiotics Project, 20210469), and by the National Marine Biodiversity Institute of Korea (2021M01100) to Tae Oh Cho. This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01051909) to Boo Yeon Won.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jose Avila-Peltroche. The first draft of the manuscript was written by Jose Avila-Peltroche and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avila-Peltroche, J., Won, B.Y. & Cho, T.O. Protoplast isolation and regeneration from the potential economic brown alga Petalonia fascia (Ectocarpales, Phaeophyceae). J Appl Phycol 34, 543–550 (2022). https://doi.org/10.1007/s10811-021-02648-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02648-0