Abstract
Macroalgae are an economically significant and fast-growing global commodity. However, pollution significantly affects the production and quality of macroalgae. Various studies have shown copper and zinc concentrations above 20 ppb (Cu) and 30 ppb (Zn) generally inhibit macroalgae growth. However, accumulation of metals in macroalgae is affected by several different factors, substances, and processes. Currently, not all of these factors, such as salinity, are well-understood and require further research. This review provides some background information on the biology and uses of macroalgae, as well as the kinetics of metal accumulation, the role of copper and zinc as toxic substances in macroalgae, and defensive mechanisms that macroalgae use to protect against metal toxicity from those metals. Finally, it describes various ways in which copper and zinc accumulation in macroalgae could be used in multiple beneficial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Algae are an important component of the aquatic ecosphere. Macroalgae, commonly called “seaweed,” comprise the majority (> 95% by mass) of all commercially cultivated aquatic plants. Annual global production of macroalgae exceeds US$5.5 billion (FAO 2016). Worldwide, macroalgae are one of the fastest-growing sectors of aquaculture with an annual growth rate exceeding 8% (FAO 2016). Macroalgae are primarily harvested for food purposes—both for human and animal consumption, though several other uses exist, including in fertilizers, and as biofuels, pharmaceuticals, mineral extraction, environmental monitoring, and environmental remediation (Muse et al. 1999; Henriques et al. 2015; Konda et al. 2015; Tuhy et al. 2015; Cabrita et al. 2016; Racionero-Gomez et al. 2016; Paiva et al. 2017; Porse and Rudolph 2017; Moutinho et al. 2018). While overwhelmingly located in eastern Asia for food production, a greater demand for the derivatives has increased the popularity of macroalgae globally (FAO 2016).
Pollution is a major issue that limits the open-water cultivation of macroalgae. However, recent research has revealed useful applications of macroalgae to address issues associated with water pollution, both through biosorption—the physiochemical process using biomass to remove metals by surface accumulation—and as instruments of biological remediation (Fourest et al. 1994). Additionally, recent research has revealed the potency of various metallic phycotoxins, especially copper and zinc, while providing insight into the current state of pollution globally, which will be elaborated upon in this paper.
This paper will specifically focus on copper and zinc, as recent research indicates that these metals are more critical than previously thought in macroalgae cultivation and for various other potential uses. For this reason, the primary focus will be on metal accumulation in marine macroalgae for the purpose of cultivation. Where information is unavailable, information on wild-grown macroalgae is used. Additionally, microalgae and terrestrial plant species are used as references in cases where information is unavailable or limited on macroalgae. In summary, the focus here is on the biological interaction between macroalgae and metal pollutants, as well as applications of such accumulation.
Macroalgae
Macroalgae encompass a large variety of different aquatic plant species. Traditionally, they are placed in three divisions: Chlorophyta, Phaeophyta, and Rhodophyta. These groups are also called “green,” “brown,” and “red” macroalgae, respectively, based upon the presence of various defining pigments (McHugh 2003). Within the groupings of macroalgae, the biochemical composition of the species varies extensively between species, among other factors (Paiva et al. 2017). With thousands of species within each division, macroalgae represent significant diversity (Guiry 2012). Despite this diversity, relatively few species of macroalgae have been extensively studied, particularly in commercial applications. However, the recent surge in commercial popularity has led to a growing interest in macroalgae research.
Though algae research has only recently gained momentum, human use of macroalgae has existed for millennia. Archeological evidence found in modern-day Chile suggests that macroalgae have been consumed as food for more than 10,000 years (Dillehay et al. 2008). Written records of macroalgae consumption date back more than 2500 years in ancient Chinese literature, where it has remained an important regional food product since (McHugh 2003; Tseng 2004). As a whole food, macroalgae provide many key nutrients in the human diet (Madden et al. 2012; Paiva et al. 2017; Wells et al. 2017). Beyond the macronutritional favorability of macroalgae, many species also contain significant levels of beneficial bioactive compounds and inorganic micronutrients (Ruperez 2002; Cabrita et al. 2016; Liu et al. 2017).
In the past Century, macroalgae consumption has increased across the world. Much of the recent popularity can be attributed to an increase in the use of the algae-derived hydrocolloids (carrageenan, agar, alginate, etc.), which are used in a variety of food and industrial applications (Nayar and Bott 2014). This is reflected by the increase of over 400% in the production of Kappaphycus alvarezii, Eucheuma spp., and Gracilaria spp. from 2005 to 2014 (FAO 2016). Despite this growth in production, the high demand for these hydrocolloids, such as in the case of agar, has resulted in critical shortages of the product (Callaway 2015; Santos and Melo 2018).
Beyond human consumption, macroalgae have been used as a feed component for various livestock operations. Various studies within the past 20 years have shown macroalgae to be a viable substitute in animal feed (Evans and Critchley 2014; Rjiba-Ktita et al. 2017). Table 1 shows a few recent examples of effects of dietary macroalgae inclusion observed in various freshwater and marine fish. There exists a strong potential for partial meal replacement with macroalgae without compromising fish growth, though the results vary and are species-specific with respect to both fish and macroalgae (Wassef et al. 2013; Sotoudeh and Mardani 2017). Additionally, dietary macroalgae substitution has shown improved immune response in fish exposed to disease (Ali et al. 2016; Yangthong et al. 2016; Choi et al. 2017).
Macroalgae dietary inclusion has shown similar benefits in lower-trophic aquatic organisms as in finfish. Abalone (Haliotis laevigata) and sea urchins (Tripneustes gratilla) have shown improved growth with 5% macroalgae-meal inclusion (Cyrus et al. 2015; Bansemer et al. 2016). An evaluation of Ulva lactuca substitution in the diets of Pacific white shrimp (Litopenaeus vannamei) showed no significant effect on growth at a 50% inclusion, though other studies showed reduced growth rate above 6% inclusion. This change may be explained through methodology, as the latter replaced individual feed ingredients, while the former used graduated replacement of the whole feed (Pallaoro et al. 2016; Qiu et al. 2018a, 2018b). Harvest period and location may also contribute to the variable performance, as macroalgae nutritional content varies both on temporal and spatial scales (Wu et al. 2014; Makkar et al. 2016).
Macroalgae have been included in terrestrial livestock diets with some success. Writings from 46 B.C. noted that Greeks would feed algae to their cattle, indicating sufficient palatability for the animals (Caesar et al. 1962). Macroalgae have been shown to be viable alternative protein sources to conventional feed crops for terrestrial livestock, though further work is required to assess the digestibility of these proteins (Tayyab et al. 2016; Tibbetts et al. 2016). However, in sheep, dietary substitution using macroalgae appear to have no significant negative effect on animal growth performance at 5% inclusion, compared to normal feed, with the Orkney sheep having adapted to meeting most of their dietary needs from macroalgae (El-Waziry et al. 2015; Hansen et al. 2003). Macroalgae has been further investigated as a possible carbon mitigation solution, as a species of red macroalgae (Asparagopsis taxiformis) has been shown to reduce the methane—a potent greenhouse gas—generated from ruminants by 95%, at an inclusion rate of just 2% organic matter without compromising growth (Machado et al. 2016). However, care must be taken when attempting to translate these successes across agricultural livestock, as with fish, since success varies by species, both animal and algal (Machado et al. 2015; Makkar et al. 2016). Caution must also be exercised with regard to palatability, as a high rate of meal replacement with macroalgae can result in a lower feeding rate. Furthermore, since many terrestrial animals are not adapted to digest the fibrous material in macroalgae, excessive meal replacement with macroalgae can result in a lower feed conversion ratio (Rjiba-Ktita et al. 2017; Cabrita et al. 2017). Despite such concerns, macroalgae remains a cheap and viable feed substitute for most livestock at low levels of inclusion (≤ 10%).
Macroalgae used as biofuels may serve to further reduce greenhouse gas emissions. As a third-generation biofuel, macroalgae does not require terrestrial resources or intensive processing that is required by first- and second-generation biofuels, respectively (Alam et al. 2015). Furthermore, the high biomass productivity compared to terrestrial crops, coupled with a low lignin content, allows for high yields of biofuels from macroalgae (Wi et al. 2009). However, high metal content in macroalgae can limit their use in bioenergy applications. Metals can inhibit the production of biofuels from macroalgae (Nkemka and Murto 2012).
High metal content can limit many other applications of macroalgae. Exposure to numerous abiotic substances, including lead and mercury for example, limits safe consumption of macroalgae from polluted areas (Balina et al. 2016; Desideri et al. 2016). As a fertilizer, macroalgae metal content can significantly contribute to the metal content of surrounding soils and groundwater sources, resulting in metal accumulation in topsoil (Greger et al. 2007; O’Neill et al. O'Neill et al. 2014). Similarly, high metal concentrations in algal biofuel digestate limit its use in land application (Nkemka and Murto 2012). However, the high metal contents of macroalgae also may offer benefits. High internal copper content aids in the pyrolysis of alginates derived from macroalgae by destabilizing the alginate polymers (Rowbotham et al. 2013). Copper was also shown to increase the total sugar content of Cladaphora spp. potentially enhancing the value of the algae as a biofuel feedstock (Cao et al. 2015). The aforementioned examples illustrate the importance of understanding the accumulation of metals in macroalgae.
Metal accumulation kinetics
Aquatic metal bioavailability
In aquatic environments, algae are exposed to a variety of trace metals. These metals originate from a variety of sources, both natural and artificial. While natural levels of metals are a significant source of trace metals, often such concentrations (< 10 ppb) are relatively harmless to many aquatic organisms. The primary concern regarding trace metals in aquatic environments stem from human activity (Giusti 2001; Ryan et al. 2012; Gubelit et al. 2016). Anthropogenic emissions of metals contribute to greater accumulation of metals in aquatic organisms compared to non-impacted locations, often contributing to biological deterioration within the organism (Chakraborty et al. 2014; Cabral-Oliveira et al. 2016).
However, not all metals are in a form that is usable by the organism. Various water constituents and biological compounds influence which metals are available for use or accumulation in the algae. Bioavailability has a direct impact on the ability of macroalgae to accumulate pollutants (Pereira et al. 2009). Much like the direct influence of aquatic metal concentrations, the availability of the metals is impacted directly as well as indirectly by anthropogenic activity, such as the acidification of waters which may increase the availability of trace metals (Millero et al. 2009; Harrison et al. 2010; Gao et al. 2017a). Thus, pollution and environmental degradation cannot be measured solely as concentration of various metals as numerous other constituents affect the form, function, and potential threat of the metals (Xu et al. 2014). Among the many water constituents affecting the availability of metals in water, salinity and the concentration of various complexing agents are some of the most uncertain and powerful factors influencing metal availability.
Salinity is a factor that is often discussed with respect to metal accumulation in marine macroalgae. Chemically, salinity affects the solubility of individual metal species through the common ion effect, where higher concentrations of dissolved salts generally reduce the amount of dissolution possible for other salts. In aquatic environments higher salinities should result in lower dissolved metal concentrations. In many biological systems, the lower solubility often correlates with lower mineral availability for cellular use or accumulation (Riba et al. 2003). In macroalgae research this effect often results in the lower accumulation rate of metals (Turner et al. 2008; Connan and Stengel 2011; Ytreberg et al. 2011; Oh et al. 2012; Farias et al. 2017).
While past research has attributed lower metal accumulation rates in macroalgae to increased salinity, inconsistencies in the methodology between studies allow for much uncertainty. Many studies have assessed salinity by diluting seawater with clean, freshwater to achieve lower salinities (e.g., Mamboya et al. 2009; Connan and Stengel 2011; Mantri et al. 2011; Oh et al. 2012). The lower growth rates and other algal activity observed in these studies may stem from lower concentrations of beneficial nutrients, resulting in the apparent metabolic depression. This is reinforced by studies that assessed macroalgae performance under various salinity levels, finding that the macroalgae performed better at the higher salinities when using similar dilution techniques, even in low-metal environments (Ganesan et al. 1999; Mantri et al. 2011; Nejrup and Pedersen 2012; Jie et al. 2016). As freshwater intrusion often carries nutrients and a higher metal concentration than seawater, it is difficult to translate these findings to real-world settings (Howarth et al. 2002; Hurd et al. 2014). Thus, without further research, the exact relationship between salinity and metal accumulation in macroalgae remains uncertain.
Metal complexes heavily influence the form of metals in aquatic environments. Metal-complexing ions and molecules, called ligands, interact with metal cations in water to form various large compounds. These molecules form coordination complexes with the metal ion. Such complexes can affect the way that the metal interacts with the environment and aquatic life. For instance, the presence of dissolved organic carbon molecules can attract metal ions, preventing accumulation upon or within the macroalgae (Santore et al. 2001). In brown macroalgae, for example, this occurs primarily in carboxyl, thiol, sulfonate, amine, and amide groups (Raize et al. 2004).
The nutrients used by macroalgae can affect the form and availability of toxic metals. In the macroalga, Ulva australis, growth in areas with higher nitrogen- and phosphate-based nutrient concentrations has been shown to contribute to a greater accumulation of metals, especially zinc, suggesting that the nutrients may have accelerated the uptake of the metals (Farias et al. 2017). In Ulva fasciata, nitrate-based nutrition resulted in higher accumulation of metals and associated toxic effects than ammonia (Lee and Wang 2001). The higher accumulation of these metals reveals that nutrients can potentially interact with the ability of macroalgae to regulate metal accumulation.
Chelators are ligands that form multiple bonds with a metal ion. Chelation of these metals can reduce the binding affinity of the metal to surface sites on macroalgae, effectively reducing the surface accumulation of the metal. Additionally, the large compound formed between the chelating agent and the metal ion is too big for most algae to pass through their ion transport sites directly into their cells. However, this may not always be the case, as some chelators have been shown to increase bioavailability for macroalgae, such as EDTA with respect to iron, copper, and zinc (Luoma 1983; Clabeaux et al. 2013). Macroalgae may produce such chelators, in the form of proteins, as a protective response to oxidative stress caused by an abundance of metals (Morris et al. 1999; Merrifield et al. 2004; Suresh Kumar et al. 2008; Costa et al. 2015).
Macroalgal metal accumulation
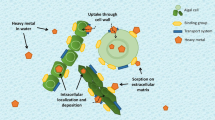
Metal accumulation in macroalgae, illustrated with copper in Fig. 1, occurs by two processes: adsorption and intracellular accumulation. Adsorption represents the accumulation of metals upon the surface of macroalgae. Intracellular metal accumulation entails all accumulation of metals that pass across the cellular membrane of the macroalgae. The two phases occur simultaneously, accumulating toward an equilibrium concentration based upon multiple water, metal, and species-specific parameters (Perez et al. 2007; Henriques et al. 2017a). For this reason, metal accumulation may vary on a spatial and temporal scale (Jung et al. 2016). Additionally, the macroalgal biochemical composition and toxicological history can also affect metal accumulation upon or within macroalgae.
Compared to intracellular accumulation, adsorption is the faster accumulation mechanism. The electronegative forces involved between the positively charged metal cation and the negatively charged cell wall induce a relatively rapid accumulation of metals on the algae surface. For this reason, metal adsorption is highly influenced by the chemical composition of the algal cell wall. The concentrations of various chemical compounds, such as carboxyl groups, affect the ability of a macroalgae to adsorb a variety of metals (Yun et al. 2001). These functional groups can be further influenced based upon a number of aquatic constituents and contaminants (Daneshvar et al. 2017). The functional groups act as biotic ligands (denoted as “Lbio” in Fig. 1), which can adsorb metals independently of live algal cell function, since dead algal cells maintain these functional groups, but are not inhibited by chemical treatment to increase adsorption capacity.
Studies suggest that polysaccharide content affects accumulation in metal adsorption, particularly the ratio of saturated-to-unsaturated polysaccharides. Possibly as the dominant ligands affecting accumulation within the cells of the macroalgae, sulfated polysaccharides are believed to intercept metal ions before the cations can harm cellular function (Garcia-Rios et al. 2007; Huang et al. 2013; Moenne et al. 2016). Additionally, differences in thallus thickness as well as seasonal growth patterns may influence the rate and concentrations of accumulation. Fast-growing annual macroalgae accumulate metals at a higher rate than the slower-growing perennials, while thallus thickness affects the concentration of surface accumulation with respect to total biomass (Stengel et al. 2004; Romera et al. 2007).
The sorptive properties of macroalgae have led researchers to explore the use of the biomass as a low-cost biosorbent material for the removal of various polluting metals. Pulverized biomass has demonstrated success as a biosorbent, often producing significant levels of removal at relatively rapid rates (Yun et al. 2001; Rangabhashiyam et al. 2016). Furthermore, the nature of the surface binding allows for potential recycling of both the metals and biosorbent with processed biomass (Kaduková and Virčíková 2005). However, while dead biomass may quickly remove free metals in an aquatic environment, the lack of an active ion transport function results in a lower overall removal of metals compared to live biomass (Henriques et al. 2015).
Intracellular accumulation occurs as an active cellular process across the cell membrane. Compared to accumulation through biosorption, intracellular accumulation is a relatively slow process. While sorption sites may saturate in a matter of hours, intracellular accumulation can continue for days (Henriques et al. 2017b). The adsorption of metals can have a direct effect on intracellular accumulation, limiting the influence of extracellular metal concentrations on metal accumulation (Romera et al. 2007). As a result of the lowered accumulation rate, macroalgae may experience a delayed toxic response, independent of the dissolved metal concentration (Andrade et al. 2006). To limit the potential toxicity of different metals, algae possess a number of defenses to limit many abiotic metals.
Intracellular metal accumulation in algae is regulated selectively by ion transport systems. Compared with other, non-essential metals at equal concentrations, biotic metals, like copper and zinc, accumulate to significantly higher concentrations within an algal cell (Jarvis and Bielmyer-Fraser 2015). Since algal cells normally take up these essential metals as part of normal biological function, the propensity to accumulate these essential metals implies that excessive zinc and copper can potentially overload the cell’s ability to regulate the metals by damaging critical internal components with the production of damaging oxidant molecules (Collén et al. 2003). This has also been observed with ammonia, nitrates, and phosphates, which allow for greater accumulation of these trace metals, especially zinc (Lee and Wang 2001; Farias et al. 2017).
Toxicological response and antioxidant defense
Many factors influence the toxicological response of macroalgae to metal pollution. In addition to algal and metal species, time of exposure is a major factor that greatly influences metal tolerance in macroalgae. Toxic effects have been observed at lower metal concentrations for relatively long periods of exposure (3 weeks) (Amado Filho et al. 1997). Under steady periods of accumulation, live macroalgae remove more dissolved metals than dead macroalgal biomass (Henriques et al. 2017a). However, after periods of metal-induced toxicity, macroalgae tend to adapt for future instances of pollution (Costa et al. 2015). These adaptations include alterations in various defense mechanisms, as compared with algae from pristine environments, including elevated baseline antioxidant levels and more efficient enzyme activity (Sáez et al. 2015).
Under mild to moderate levels of metal pollution, the concentrations of various biochemicals in macroalgae may increase to defend against future oxidative stress. When exposed to increased levels of copper, several species of Cladophora responded with an increased total sugar content (Cao et al. 2015). The increased sugar content functions by thickening the cell wall, better protecting the algae from surface damage. However, the increased thickness does not limit intracellular accumulation compared to non-exposed algae cells (Andrade et al. 2004). While the rate of accumulation in the cell does not decrease, the damaging potential of the metals does decrease, as algae develop a stronger internal detoxification mechanism following past episodes of metal toxicity (Reed and Moffat 1983).
Compared with algae from low-pollution environments, algae in severely polluted areas may experience reduced cellular metabolic activity because of past metal accumulation (Costa et al. 2015). Although the effects of chronic pollution can be partially reversed and repaired and accumulation reversed, when macroalgae are immersed in cleaner water, acute pollution has been shown to cause irreparable damage to algal cells (Wu et al. 2009; Wang et al. 2014). Acute metal pollution reduces an alga’s ability to accumulate metals as cells rupture under intense oxidative stress (Andrade et al. 2006). Some researchers point to this issue with live biomass as a reason to use pretreated dead biomass for aquatic remediation of metal pollution, such as copper, which can severely damage algae cells, even at relatively low concentrations (Kaduková and Virčíková 2005).
Copper and zinc
Copper is an important metal in both biological and industrial applications. Copper is a transition metal with two primary oxidation states: copper I (reduced) and copper II (oxidized). In many biological systems, the respective oxidation and reduction of these ions is essential for enzyme function (Koch et al. 1997). Copper is required for both algal photosynthesis and respiration (Quigg 2016). Required concentrations vary by species (Peers et al. 2005). While essential to cellular function, copper can easily become toxic to macroalgae.
Zinc, like copper, is a critical micronutrient in the biological systems of photosynthetic organisms. Zinc is an important cofactor in many enzymes that are vital to key plant processes, including protein synthesis, and cell membrane structure. In the biological functions of plants, zinc, unlike copper, does not partake in redox reactions within the cell, existing exclusively in the Zn(II) form (Broadley et al. 2012). In algae, zinc deficiency hinders carbon assimilation, indicating that the micronutrient may be a limiting factor for some algae growth (Malasarn et al. 2013). Furthermore, zinc enrichment—below a toxic threshold—has been shown to increase growth rates (Kumar et al. 2014; Cao et al. 2015). However, while it has the potential to accelerate growth in algae, often the dissolved concentration of zinc exceeds beneficial levels, thus posing a threat as a phytotoxin.
Copper and zinc as biocides
Macroalgae, like microalgae, are sensitive to fluctuations of aquatic metal concentrations. Unlike most finfish, relatively low concentrations of copper and zinc can induce a toxic effect in macroalgae (Bielmyer et al. 2012). Concentrations at which copper and zinc significantly reduced the growth rates of various macroalgae are shown in Tables 2 and 3, respectively.
In many aquatic systems, copper-based compounds are used as an algaecide (Viriyatum and Boyd 2016). Copper functions as a pesticide in multiple ways. In excess, copper can cause the formation of reactive oxygen species (ROS) within the algal cell (Ebenezer et al. 2014). These oxidants can damage critical biochemical components in the cells of the algae, resulting in protein denaturation and lipid peroxidation (Collén et al. 2003). Severe toxicological effects from the formation of free radicals can result in deterioration of the cell structure, eventually culminating in cell necrosis (Baumann et al. 2009; Contreras et al. 2009). Excessive copper may also inhibit photosynthesis. In a study observed using a variety of aquatic plants, copper free radicals replaced magnesium in chlorophyll. The resulting copper-substituted chlorophyll could not undergo photosynthesis (Kupper et al. 1996). Though other metals have demonstrated similar effects, copper is often the most potent free radical in chlorophyll due to the higher affinity for it in chlorophyll compared to other metals (Baumann et al. 2009).
In marine environments, copper is used in the paints on the exterior of various ships and structures as an antifouling agent (Girling et al. 2015). While often intended to curtail microalgal proliferation, the addition of copper-based biocides to aquatic environments can greatly inhibit macroalgal growth as well (Wendt et al. 2013). These biocidal paints represent a significant source of aquatic copper, which can pose a threat to nearby macroalgae (Turner et al. 2009). In the case of copper, biosorption represents a strong potential indicator of acute toxic effect, with greater toxic effects linked to increased copper sorption (Ma et al. 2003). Additionally, copper supplements often as copper sulfate are used to reduce the disease prevalence in aquaculture operations, such as Columnaris disease in channel catfish (Farmer et al. 2017). These antifouling paints and pesticides have contributed to significant levels of copper contamination in aquatic environments (Boxall et al. 2000; Boyle et al. 2016).
Much like copper, zinc is a major component of many commercial algaecides. Common zinc compounds include zinc sulfate and pyrithione zinc (Boxall et al. 2000; Girling et al. 2015). These compounds affect the processes of the algae cell in a similar manner to copper by inducing oxidative stress. The oxidation of cellular lipids such as those within the cell membrane affect the algal cell’s ability to regulate the exchange of ions, which can lead to a higher degree of accumulation and subsequent oxidative stress (Zhang et al. 2016). However, such periods of unregulated accumulation are brief, as algae increase intracellular antioxidant levels in response to zinc toxicity (Hamed et al. 2017). Additionally, zinc is a less-potent phycotoxin to many macroalgae than other major metals (e.g., copper, cadmium, and lead), though it ranks among the most potentially toxic essential metals (Mendes et al. 2013).
In addition to purposeful dosing as an algaecide, aquatic copper and zinc contamination can result from a number of different sources, both natural and anthropogenic. Natural sources, such as from weathered rock or volcanic activity, contribute to a relatively low concentration of metal—commonly referred to as the “background concentration.” While natural sources can account for some concentrations of copper, the concern in many aquatic systems is pollution from human activity (Dai et al. 2007; Gao et al. 2017b).
Environmental copper and zinc contamination
Copper and zinc pollution is a widespread issue. The US EPA-recommended limits of these metals for aquatic life in saltwater, as well as a few contaminated sites from around the world, are summarized in Table 4. Figure 2 represents the scope of the issue for copper in context of recorded instances of marine pollution. Compared to copper and other potent phycotoxic metals, there is less data regarding zinc toxicity to macroalgae. From limited data, zinc contamination has resulted in decreased growth rates in macroalgae subjected to concentrations at or below 100 ppb (Amado Filho et al. 1997; Girling et al. 2015). These algae would experience inhibited growth in a number of locations around the world (Beiras et al. 2003; Wan et al. 2007; Achary et al. 2017; Okogbue et al. 2017).
Macroalgae copper tolerance and recorded pollution levels. Limiting concentrations (bars) that fall below a line, except US EPA limit, (USEPA 2000) indicate that toxic effects would likely be observed in that macroalgae if it were grown in that location
Among anthropogenic sources of copper and zinc pollution, mining has demonstrated some of the longest-lasting effects of contamination. Chile, a major producer of copper, has experienced lasting effects of aquatic copper contamination. In an area of northern Chile, mine tailings have been shown to contribute to a high concentration of copper in nearby coastal waters (Correa et al. 1999). More than two decades later, the same site maintained high concentrations of the metal. Though still contaminated, the site has experienced a resurgence in biotic diversity, though primarily from opportunistic species (Medina et al. 2005). Similarly, zinc contamination may originate from mining activities, suggesting that zinc mining sites may also compromise nearby coastal waters, just like copper (Sağlam and Akçay 2016).
In addition to ore extraction, other industrial processes can cause significant metal pollution in aquatic environments. Industrial wastes, both solid and liquid, contribute to copper and zinc pollution in waterways. Because copper is used in a variety of processes, industrial waste effluents consistently contribute to copper pollution (Srinivasa Gowd and Govil 2008). Refinement and recycling processes have been identified as major polluters. Metal processing and leakage from oil refinery operations have also been shown to significantly contribute to aquatic copper pollution (Beg et al. 2001; Jones et al. 2003; Nikolic et al. 2011; Chae et al. 2014). Recycling processes, particularly those involving discarded electronic devices, have also been shown to significantly contribute to copper pollution (Leung et al. 2006; Tang et al. 2010; Damrongsiri et al. 2016).
Although industrial activities may contribute the majority of the copper pollution, anthropogenic copper pollution can result from a variety of domestic activities. Municipal runoff can significantly and negatively influence the metal concentrations in an aquatic environment (Padhi et al. 2013). Sources of this metal contamination include automobile brake dust, building roofing and siding, and atmospheric deposition (Davis et al. 2001; Gunawardena et al. 2013; Charters et al. 2016). Thus, in high-density urban areas, particularly those affected by infrequent rainfall, copper pollution from municipal activity can severely affect aquatic biology. However, in such an environment, copper is not the only metal of concern.
As a pollutant in the aquatic environment, zinc contamination is derived many of the same applications as copper. This includes protective coatings, paints, biocides, and metal alloys (Boxall et al. 2000; Girling et al. 2015; Feng et al. 2017; Ren et al. 2017). Other major sources of aquatic zinc pollution include mine drainage, atmospheric deposition, and municipal runoff (Riba et al. 2003; Turner and Rice 2010; Fongmoon et al. 2014). Additionally, as with copper, anthropogenic pollution has significantly increased the concentration of zinc in estuarine and marine waters (Jones et al. 2003).
Interactive metal toxicity
Metals interact in aquatic environments with different effects. Three general relationships exist between metals that influence their relative toxicity: additive, antagonistic, and synergistic. Relationships vary by metal and plant species. For example, zinc and calcium displayed an additive toxicity in the red macroalgae Gracilaria domingensis (Mendes et al. 2014). This effect demonstrates a difference from the expected result observed in an assortment of Sargassum species, where calcium accumulation has been shown to reduce the accumulation potential of zinc (de França et al. 2002).
In an additive toxic relationship, each metal exerts their toxic potential independent of the concentration of the other metals present. In such a relationship the detrimental effects caused by one toxic constituent neither exacerbates nor detracts from the potential toxicity of another metal. An example of additive toxicity occurs between copper (II) and cadmium (II) (Mendes et al. 2014). While both are damaging to essential components of algae cells through the formation of reactive oxygen species (ROS), the processes are different, as copper participates in redox reactions unlike cadmium (Babu et al. 2014). Since the metals generate ROS independently, the concentration of copper does not affect the toxicity of cadmium.
Some metals compete for binding and transport sites. These metals interact antagonistically, reducing the overall toxic effect experienced by the algae. Compared with the toxic responses generated by individual metals, metal mixtures often produce a lower toxic response due to the increased competition between the metals in solution (Jarvis and Bielmyer-Fraser 2015). A similar relationship explains the reduced toxicity usually experienced in hard water environments, compared to soft waters with the same individual metal concentrations (Santore et al. 2001). Antagonistic metal interactions can help to protect algae. Such is the case between cadmium (II) and zinc (II), where zinc accumulation in algae reduces accumulation of cadmium, as zinc exhibits a stronger affinity than cadmium for a variety of biotic ligands (Lee and Wang 2001; Sánchez-Thomas et al. 2016). Though zinc accumulation can be toxic to the cell, the toxic potency of zinc is much less than cadmium at equal concentrations.
Synergistic behaviors between metals cause an increased potency of the total mixture damaging similar regulation sites. The increased toxicity largely depends upon the target organism. For instance, copper and zinc behave synergistically in the marine red alga Gracilaria domingensis (Mendes et al. 2014). By comparison, the same binary mixture given to several species of the freshwater diatom Navicula presents the opposite case, instead exhibiting an antagonistic relationship (Nagai and De Schamphelaere 2016). A possible explanation stems from the combination of metals and biochemicals within the algae. Metals interact with photosynthetic pigment in a variety of manners, which may explain the variations between algae (Fargašová 1999). However, the interactions between metals and biological compounds occur differently than metal-metal relationships.
Toxic metal interactions do not always follow the same trend, though. While copper and calcium demonstrated a synergistic toxicity in Gracilaria domingensis, the opposite was observed in Cystoseira barbata, with calcium reducing the toxicity of the copper (Pellegrini et al. 1993; Mendes et al. 2014). The differences in toxicity may be attributed to the differences between species, though differences in metal-metal concentrations may explain the dissimilar findings, as Pellegrini et al. (1993) found changes in metal-metal interactions as concentrations varied. However, very little data exist on the matter, suggesting the need for further research.
Interactive effects may occur via substitution of metals in various biological compounds and enzymes. Compared with other transition metals, zinc has a relatively flexible function in various proteins. Substitution of zinc with another metal, such as copper, often reduces or inhibits the function of the protein. As copper possesses a higher affinity for binding sites in these coordination complexes, the presence of free copper in an aquatic environment can be detrimental to cellular function (Bertini and Luchinat 1994). Metallosubstitution can affect the toxicity of metal compounds, too. For instance, pyrithione zinc is added as an algaecide booster, increasing the toxic potential of copper. Copper ions replace the zinc ions, forming copper pyrithione (CuPT). The combination of the free zinc ions and the CuPT inflicts more biocidal damage than the independent constituents (Bao et al. 2014). The sensitivity of algae to various metals, combined with algal accumulation properties, has led researchers to investigate various species of macroalgae for a range of environmental purposes.
Biomonitoring and remediation applications
Macroalgae have been extensively studied as aquatic ecosystem health indicators (Chaudhuri et al. 2007; Morrison et al. 2008; Rajfur and Klos 2014). Some widespread species of opportunistic macroalgae, such as those of the Ulva genus, have demonstrated strong potential as biomonitors in pollution applications, in part due to a high ratio of intracellular-to-extracellular metal accumulation (Muse et al. 1999; Baumann et al. 2009; Farias et al. 2017; Ozyigit et al. 2017; Valdés et al. 2018). The diverse biological composition between species allows researchers to evaluate changes in different pollutants using a range of macroalgae (Chakraborty et al. 2014). Similarly, macroalgae have been used to assess the environmental impact of nuclear activity (Nonova and Tosheva 2016). In combination with sediment samples, macroalgae can provide a comprehensive assessment of long-term metal pollution in different bodies of water (Fostier et al. 2016; Gubelit et al. 2016).
In addition to biomonitoring, macroalgae have also been investigated as tools of bioremediation. As a sorbent material, macroalgal biomass offers cheap, rapid pollution mitigation with minimal negative environmental side effects (Murphy et al. 2009; Dittert et al. 2013; Kidgell et al. 2014). When used in conjunction with a chemical desorbent, the macroalgae biomass can be used multiple times to remediate polluted waters (Senthilkumar et al. 2006; Herrero et al. 2008). Given the higher accumulation capacity compared to dead biomass, live macroalgae may be used for remediation purposes, too (Henriques et al. 2017a, 2017b). In terrestrial applications, phytoremediation has shown promise as a low-cost, effective metal remediation technique (Vangronsveld et al. 2009). Recent research shows promise with microalgae for simultaneous removal of metal pollutants and energy production (Arora et al. 2017; Palma et al. 2017). Given the possible biological similarities between micro- and macroalgae, this suggests a strong possibility for macroalgae, though further in situ tests are required for further evaluation. Thus, macroalgae may provide an effective low-cost, low-maintenance option for metal removal in aquatic environments.
In addition to the environmental benefits that they offer, remediating macroalgae may also provide a source of valuable metals. For high-value metals, such as rhenium, the concentration in macroalgae may provide a viable source metal (Racionero-Gomez et al. 2016). Additionally, live macroalgae provide for the remediation of various non-metal pollutants, such as nitrogen, phosphorus, and organic carbon (Suutari et al. 2016; Nwoba et al. 2017). However, research focused on using live algae for metal pollution remediation is lacking when compared to the extensive research performed on dead biomass.
Nutritional supplementation and dietary enrichment
The metals sequestered by macroalgae may have value in a number of other beneficial applications. Beyond phytomining, the high trace metal content in some samples of macroalgae can be used for nutrient enrichment purposes. Many of the metals that accumulate in macroalgae—zinc, copper, iron, etc.—are essential nutrients to humans and livestock (Ruperez 2002; García-Casal et al. 2007; Cabrita et al. 2016). As viable sources of macro- and micronutrients, alike, macroalgae also may serve as dietary supplements.
Compared to terrestrial plants, macroalgae possess a stronger potential for nutrient supplementation. Macroalgae contain lower concentrations of the antinutrient, phytic acid, than many common crops, including beans, wheat, corn, and soybean (Oliveira et al. 2009). Phytic acid functions as a chelator, binding a free metal ion to be unusable by an organism. Though potentially a metal detoxifier, phytic acid mostly restricts intake of essential metals, such as calcium, potassium, and iron (Kumar et al. 2010). Phytic acid is particularly problematic concerning zinc consumption, because the human body does not store this mineral, necessitating daily consumption by humans (Rink and Gabriel 2000).
In many developing countries zinc deficiency has resulted in severe developmental issues. This represents more than one-sixth of the entire global human population. High dietary phytic acid is a significant contributing factor to global zinc deficiency (Wessells and Brown 2012). Supplementation of zinc using macroalgae may help to alleviate this nutrient inadequacy. The low phytic acid content in macroalgae allows for higher utilization of micronutrients by humans. Furthermore, while macroalgae may contain elevated concentrations of various metals, the health risk posed by macroalgae is minimal, especially when grown in a more deliberate and controlled manner (Yong et al. 2015; Desideri et al. 2016; Rubio et al. 2017). With zinc concentrations potentially greater than that of many staple foods, macroalgae may be useful to reduce the prevalence of zinc deficiency (Malea and Haritonidis 1999; Stengel et al. 2004; Charney 2012).
The results from various studies support the possibility of copper and zinc supplementation using macroalgae in human diets. According to the Institute of Medicine, the recommended daily intake of copper and zinc for an adult is approximately 2 and 10 mg, respectively (Trumbo et al. 2001). Macroalgae of the genus Ulva are useful reference organisms when assessing the nutritional benefits of macroalgae. With high growth potential and a range of habitats, many Ulva species can be used to assess both water quality and macroalgae nutrition potential in coastal communities (Patarra et al. 2011; Tabarsa et al. 2012; Paiva et al. 2016). Studies have shown that various Ulva species, when exposed to aquatic concentrations of 10 ppb each of copper and zinc, concentrate the micronutrients well enough to meet human dietary needs with less than 100 g of dry biomass. Higher dissolved concentrations of metals in other macroalgae could meet such a target in less than 5 g (Amado Filho et al. 1997; Jarvis and Bielmyer-Fraser 2015).
Macroalgae also represent a suitable source of micronutrients for livestock. Evans and Critchley (2014) identified macroalgae as potential sources for agricultural settings, with the many benefits outweighing potential issues. Since then supplementation and dietary enrichment have shown promise using in vitro livestock trials. Macroalgae contain the necessary minerals for livestock diets (Cabrita et al. 2016). Traditionally, many livestock operations supplement minerals with enriched salt mixtures. When compared to an enriched salt mixture, mineral supplementation via macroalgae provides more bioavailable metals for growing pigs, while still meeting dietary salt requirements (Michalak et al. 2015). Similar benefits were shown in laying hens, where macroalgae-sourced minerals improved egg quality, compared to traditional supplements (Michalak et al. 2011). Macroalgae improved the mineral levels of dairy cows, leading to higher mineral concentrations in the milk, too (Rey-Crespo et al. 2014).
Indirect enrichment of foods may be achieved via the land application of macroalgae biomass. As a source of nitrogen, phosphorus, and an array of micronutrients, macroalgae provide many of the necessary components of terrestrial plant fertilizer. Beach-cast macroalgae from shorelines increase biomass in plants, compared to plants grown in compost void of algae (Greger et al. 2007). Zinc-enriched macroalgae were shown to improve both growth performance, as well as zinc concentration in soil and plant biomass (Tuhy et al. 2015; Bădescu et al. 2017). This enrichment could be achieved using macroalgae where the accumulated content is too great for direct consumption, such as macroalgae used for bioremediation (Farias et al. 2017). Even biochar from remediating macroalgae provides the necessary micronutrients for vegetable growth (Roberts et al. 2015). However, salinity remains a critical challenge in macroalgae land application treatments, as the high salinity content of marine macroalgae can contribute to a soil salinity level beyond tolerable by many crops (Greger et al. 2007). Thus, macroalgae below food quality may still be used to fortify the diets of populations in low-zinc areas, so long as the accompanying elevated salt content can be properly managed.
Though macroalgae may be enriched with essential micronutrients, the accumulation potential of toxic abiotic metals raises concern. The non-homogenous nature of pollution from anthropogenic activities can result in widespread increases in aquatic pollutants, as the contaminants display a positive relationship with each other (Chae et al. 2014; Okogbue et al. 2017). These pollutants may subsequently accumulate in nearby macroalgae, resulting in elevated levels of multiple metals in the organisms, possibly rendering the macroalgae unsafe for human consumption (El Din and El-Sherif 2012; Ozyigit et al. 2017). However, bioremediation for non-consumptive purposes still remains a viable option in these cases.
Conclusions and future research
Pollution has been a pervasive issue in macroalgae cultivation and remains a major obstacle for market growth. Copper and zinc—potent algaecides—are among the most critical metals to consider for future macroalgae production. However, through a stronger understanding of the relationship between metals and macroalgae, and methods of manipulating this interaction, the issue may be mitigated. The increase in market share and utility of algae warrant further research concerning metal accumulation. Both low-metal and high-metal algae clearly demonstrate utility, but the dearth of live algae models represents a significant data gap.
There are serious reasons why copper and zinc compounds are likely to continue to be used in aquatic applications but finding ways to minimize and remediate will likely be even more important in the future. For example, the Aquaculture Stewardship Council (2012) acknowledged challenges with fouling of net pens and noted that various compounds (often organic toxins or copper alloy or copper coating materials) were used to reduce fouling, which is itself a multibillion dollar issue in the industry. The Stewardship Council acknowledged that the addition of these toxins is an issue and is searching for more sustainable methods to reduce net fouling (among other issues). Braithwaite and McEvoy (2005) noted that if neither copper alloy or biocide treatments were applied to nets, then aggressive net cleaning or changing was needed. Various other technologies are being considered now, but cleaning of copper alloy nets would still release some amount of copper into the environment.
A better understanding of zinc tolerance and toxicity is necessary in macroalgae. Though itself a potential phycotoxin, zinc offers a protective function to prevent the accumulation of other, more damaging metals. This approach has been explored in the case of oysters, where treatment of oysters with zinc has been suggested as an approach to managing cadmium accumulation in the bivalves (Munksgaard et al. 2017). Widespread adoption of this practice may elevate aquatic zinc concentrations, requiring a stronger understanding of the impact on the local macroalgae populations. Further research may also help to determine the suitability of this zinc for human consumption.
In parallel, the importance of dietary zinc, and the lack of dietary zinc experienced in many areas around the world warrants further research in zinc-rich food products. The success of macroalgae in livestock applications suggests a strong possibility for enriched macroalgae as a nutrient supplement for human use. While other dissolved contaminants must first be addressed, the viability of macroalgae as a dietary zinc source lends the possibility of macroalgal biomass used for the phytoremediation of zinc and other essential micronutrients as enriched food sources. However, to explore this potential, the relationship between dissolved zinc and accumulated zinc must be researched further, as well as the interaction between zinc and other metals.
Through further research, environmental engineers, aquaculturists, and agriculturists can determine optimal times and environments appropriate for macroalgae cultivation, depending upon application. Though numerous factors affect accumulation, salinity is one of the easiest to manipulate through inland tank culture, allowing aquaculturists possibilities to enrich their algal product. Inland tank cultivation, such as in integrated multi-trophic aquaculture systems, also provides an alternative to effectively manage metals in macroalgae cultivation (Ratcliff et al. 2016). However, open-water cultivation of macroalgae represents a much bigger portion of total production.
Some of the challenges associated with pollution of open-water cultivation sites may be addressed through several major engineering projects. Remote sensing technologies, long established in agriculture, may soon allow farmers to evaluate coastal environments to identify potential cultivation sites for open-water cultivation by assessing various water parameters (Zhou et al. 2017). River diversions and dams may provide a more direct means of manipulating salinity in coastal environments, though potential ecological impacts warrant further investigation into the feasibility of these engineered flow control measures (Day et al. 2016). However, these means are not within consideration for many small operations due to both cost and scale. Thus, identifying natural processes and mechanisms to prevent or reduce the toxic effects of pollution is critical.
Though several studies have demonstrated the apparent relationship between salinity and the bioavailability of a range of metals, including copper and zinc, the connection has yet to be completely established using macroalgae as the model organism. A stronger understanding of the relationship between salinity and metal accumulation will improve the viability of macroalgae as a biomonitor, aquatic remediator, and a functional food source. Furthermore, a greater understanding of accumulation capacity will help remediation specialists to investigate polluted areas. The information will further assist in future designs of biological remediation systems in marine settings.
Macroalgae represent a solution to problems associated with environmental change. Macroalgae represent a significantly large potential carbon sink (Sondak et al. 2017). Additionally, the low carbon emissions associated with macroalgae biofuel production, as well as the methane reduction observed in ruminants could help to curb the effects of the global warming. However, the consequences stemming from atmospheric change are present and currently affecting the environment (Hughes et al. 2017; Yi et al. 2017). Due to sea level rise and an increase in the global population, land will become a scarcer resource (Godfray et al. 2010; Kemp et al. 2011). Thus, it is critical that food production shift to areas previously thought unsuitable for farming, now representing underutilized readily available farmland.
Cultivation of macroalgae through aquaculture will be critical to this economic transformation. Even now, with oceans covering most of the world, saltwater remains one of the most underutilized resources. However, sustainable, proactive measures must be adopted to ensure long-term success of the industry, managing challenges such as metal pollution while looking for ways to add value. With a growing presence in the world economy, macroalgae will play a critical role in the growing world economy and changing environment.
References
Achary MS, Satpathy KK, Panigrahi S, Mohanty AK, Padhi RK, Biswas S, Prabhu RK, Vijayalakshmi S, Panigrahy RC (2017) Concentration of heavy metals in the food chain components of the nearshore coastal waters of Kalpakkam, southeast coast of India. Food Control 72(Part B):232–243
Alam F, Mobin S, Chowdhury H (2015) Third generation biofuel from algae. Procedia Engineer 105:763–768
Ali SS, Shaaban MT, Abomohra AE, El-Safity K (2016) Macroalgal activity against multiple drug resistant Aeromonas hydrophila: a novel treatment study towards enhancement of fish growth performance. Microb Pathogenesis 101:89–95
Amado Filho GM, Karez CS, Andrade LR, Yoneshigue-Valentin Y, Pfeiffer WC (1997) Effects on growth and accumulation of zinc in six seaweed species. Ecotoxicol Environ Saf 37:223–228
Andrade LR, Farina M, Amado Filho GM (2004) Effects of copper on Enteromorpha flexuosa (Chlorophyta) in vitro. Ecotoxicol Environ Saf 58:117–125
Andrade S, Contreras L, Moffett JW, Correa JA (2006) Kinetics of copper accumulation in Lessonia nigrescens (Phaeophyceae) under conditions of environmental oxidative stress. Aquat Toxicol 78:398–401
Aquaculture Stewardship Council (2012) Final salmon aquaculture dialogue standards for aquaculture stewardship, 35–37
Arora N, Gulati K, Patel A, Pruthi PA, Poluri KM, Pruthi V (2017) A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res 24:29–39
Babu MY, Palanikumar L, Nagarani N, Devi VJ, Kumar SR, Ramakritinan CM, Kumaraguru AK (2014) Cadmium and copper toxicity in three marine macroalgae: evaluation of the biochemical responses and DNA damage. Environ Sci Pollut Res 21:9604–9616
Bădescu IS, Bulgariu D, Bulgariu L (2017) Alternative utilization of algal biomass (Ulva sp.) loaded with Zn(II) ions for improving of soil quality. J Appl Phycol 29:1069–1079
Balina K, Romagnoli F, Blumberga D (2016) Chemical composition and potential use of Fucus vesiculosus from Gulf of Riga. Energy Procedia 95:43–49
Bansemer MS, Qin JG, Harris JO, Duong DN, Currie K, Howarth GS, Stone DAJ (2016) Dietary inclusions of dried macroalgae meal in formulated diets improve the growth of greenlip abalone (Haliotis laevigata). J Appl Phycol 28:3645–3658
Bao VWW, Lui GCS, Leung KMY (2014) Acute and chronic toxicities of zinc pyrithione alone and in combination with copper to the marine copepod Tigriopus japonicus. Aquat Toxicol 157:81–93
Baumann HA, Morrison L, Stengel DB (2009) Metal accumulation and toxicity measured by PAM—chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicol Environ Saf 72:1063–1075
Beg MU, Al-Muzaini S, Saeed T, Jacob PG, Beg KR, Al-Bahloul M, Al-Matrouk K, Al-Obaid T, Kurian A (2001) Chemical contamination and toxicity of sediment from a coastal area receiving industrial effluents in Kuwait. Arch Environ Contam Toxicol 41:289–297
Beiras R, Bellas J, Fernández N, Lorenzo JI, Cobelo-Garcı́a A (2003) Assessment of coastal marine pollution in Galicia (NW Iberian Peninsula); metal concentrations in seawater, sediments and mussels (Mytilus galloprovincialis) versus embryo–larval bioassays using Paracentrotus lividus and Ciona intestinalis. Mar Environ Res 56:531–553
Bertini I, Luchinat C (1994) The reaction pathways of zinc enzymes and related biological catalysts. In: Bertini I, Gray H, Lippard S, Valentine J (eds) Bioinorganic Chemistry. University Science Books, Mill Valley, pp 37–106
Bielmyer GK, Bullington JB, DeCarlo CA, Chalk SJ, Smith K (2012) The effects of salinity on acute toxicity of zinc to two euryhaline species of fish, Fundulus heteroclitus and Kryptolebias marmoratus. Integr Comp Biol 52:753–760
Boxall ABA, Comber SD, Conrad AU, Howcroft J, Zaman N (2000) Inputs, monitoring and fate modelling of antifouling biocides in UK estuaries. Mar Pollut Bull 40:898–905
Boyle J, Sayer C, Hoare D, Bennion H, Heppel K, Lambert S, Appleby P, Rose N, Davy A (2016) Toxic metal enrichment and boating intensity: sediment records of antifoulant copper in shallow lakes of eastern England. J Paleolimnol 55:195–208
Braithwaite RA, McEvoy LA (2005) Marine biofouling on fish farms and its remediation. Adv Mar Biol 47:215–252
Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F (2012) Functions of mineral nutrients: micronutrients. In: Marschner H (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, pp. 313–404
Brown M, Newman J, Han T (2012) Inter-population comparisons of copper resistance and accumulation in the red seaweed, Gracilariopsis longissima. Ecotoxicology 21:591–600
Cabral-Oliveira J, Coelho H, Pratas J, Mendes S, Pardal M (2016) Arsenic accumulation in intertidal macroalgae exposed to sewage discharges. J Appl Phycol 28:3697–3703
Cabrita ARJ, Maia MRG, Oliveira HM, Sousa-Pinto I, Almeida AA, Pinto E, Fonseca AJM (2016) Tracing seaweeds as mineral sources for farm-animals. J Appl Phycol 28:3135–3150
Cabrita ARJ, Correia A, Rodrigues AR, Cortez PP, Vilanova M, Fonseca AJM (2017) Assessing in vivo digestibility and effects on immune system of sheep fed alfalfa hay supplemented with a fixed amount of Ulva rigida and Gracilaria vermiculophylla. J Appl Phycol 29:1057–1067
Caesar J, Hiritius A, Schneider R (1962) Bellum Africanum. Weidmann, Berlin
Callaway E (2015) Lab staple agar hit by seaweed shortage. Nature 528:171–172
Cao D, Xie P, Deng J, Zhang H, Ma R, Liu C, Liu R, Liang Y, Li H, Shi X (2015) Effects of Cu2+ and Zn2+ on growth and physiological characteristics of green algae, Cladophora. Environ Sci Pollut Res 22:16535–16541
Chae JS, Choi MS, Song YH, Um IK, Kim JG (2014) Source identification of heavy metal contamination using metal association and Pb isotopes in Ulsan Bay sediments, East Sea, Korea. Mar Poll Bull 88:373–382
Chakraborty S, Bhattacharya T, Singh G, Maity JP (2014) Benthic macroalgae as biological indicators of heavy metal pollution in the marine environments: a biomonitoring approach for pollution assessment. Ecotoxicol Environ Saf 100:61–68
Charney M (2012) USDA national nutrient database for standard reference, release 24 and dietary supplement ingredient database, release 2. J Agr Food Inform 13(4):358
Charters FJ, Cochrane TA, O'Sullivan AD (2016) Untreated runoff quality from roof and road surfaces in a low intensity rainfall climate. Sci Total Environ 550:265–272
Chaudhuri A, Mitra M, Havrillia C, Waguespack Y, Schwarz J (2007) Heavy metal biomonitoring by seaweeds on the Delmarva Peninsula, east coast of the USA. Bot Mar 50:151–158
Choi Y, Kim K, Kim D, Nam T (2017) Evaluation of different Pyropia yezoensis extracts as feed additives for growth and immunity of japanese flounder Paralichthys olivaceus. Fisheries Sci 83:819–826
Clabeaux BL, Navarro DA, Aga DS, Bisson MA (2013) Combined effects of cadmium and zinc on growth, tolerance, and metal accumulation in Chara australis and enhanced phytoextraction using EDTA. Ecotoxicol Environ Saf 98:236–243
Collén J, Pinto E, Pedersén M, Colepicolo P (2003) Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals. Arch Environ Contam Toxicol 45:337–342
Connan S, Stengel DB (2011) Impacts of ambient salinity and copper on brown algae: 1. Interactive effects on photosynthesis, growth, and copper accumulation. Aquat Toxicol 104:94–107
Contreras L, Mella D, Moenne A, Correa JA (2009) Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae). Aquat Toxicol 94:94–102
Correa JA, Castilla JC, Ramírez M, Varas M, Lagos N, Vergara S, Moenne A, Román D, Brown MT (1999) Copper, copper mine tailings and their effect on marine algae in Northern Chile. J Appl Phycol 11:57–67
Costa GB, Felix Marthiellen RL, de Simioni C, Ramlov F, Oliveira ER, Pereira DT, Maraschin M, Chow F, Horta PA, Lalau CM, da Costa CH, Matias WG, Bouzon ZL, Schmidt ÉC (2015) Effects of copper and lead exposure on the ecophysiology of the brown seaweed Sargassum cymosum. Protoplasma 253:111–125
Cyrus MD, Bolton JJ, Scholtz R, Macey BM (2015) The advantages of Ulva (Chlorophyta) as an additive in sea urchin formulated feeds: effects on palatability, consumption and digestibility. Aquac Nutr 21:578–591
Dai J, Song J, Li X, Yuan H, Li N, Zheng G (2007) Environmental changes reflected by sedimentary geochemistry in recent hundred years of Jiaozhou Bay, North China. Environ Pollut 145:656–667
Damrongsiri S, Vassanadumrongdee S, Tanwattana P (2016) Heavy metal contamination characteristic of soil in WEEE (waste electrical and electronic equipment) dismantling community: a case study of Bangkok, Thailand. Environ Sci Pollut Res 23:17026–17034
Daneshvar E, Vazirzadeh A, Niazi A, Sillanpää M, Bhatnagar A (2017) A comparative study of methylene blue biosorption using different modified brown, red and green macroalgae—effect of pretreatment. Chem Eng J 307:435–446
Davis AP, Shokouhian M, Ni S (2001) Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 44:997–1009
Day JW, Lane RR, D’Elia CF, Wiegman ARH, Rutherford JS, Shaffer GP, Brantley CG, Kemp GP (2016) Large infrequently operated river diversions for Mississippi Delta restoration. Estuar Coast Shelf Sci 183:292–303
Desideri D, Cantaluppi C, Ceccotto F, Meli MA, Roselli C, Feduzi L (2016) Essential and toxic elements in seaweeds for human consumption. J Toxicol Environ Health A 79:112–122
Dillehay TD, Ramírez C, Pino M, Collins MB, Rossen J, Pino-Navarro JD (2008) Monte Verde: seaweed, food, medicine, and the peopling of South America. Science 320:784–786
Dittert I, Vilar V, da Silva E, de Souza S, de Souza A, Botelho C, Boaventura R (2013) Turning Laminaria digitata seaweed into a resource for sustainable and ecological removal of trivalent chromium ions from aqueous solutions. Clean Technol Environ 15:955–965
Ebenezer V, Lim W, Ki J (2014) Effects of the algicides CuSO4 and NaOCl on various physiological parameters in the harmful dinoflagellate Cochlodinium polykrikoides. J Appl Phycol 26:2357–2365
El Din NGS, El-Sherif ZM (2012) Nutritional value of some algae from the north-western Mediterranean coast of Egypt. J Appl Phycol 24:613–626
El-Waziry A, Al-Haidary A, Okab A, Samara E, Abdoun K (2015) Effect of dietary seaweed (Ulva lactuca) supplementation on growth performance of sheep and on in vitro gas production kinetics. Turk J Vet Anim Sci 39:81–86
Evans FD, Critchley AT (2014) Seaweeds for animal production use. J Appl Phycol 26:891–899
FAO (2016) The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. FAO, Rome, p 200
Fargašová A (1999) Toxicity of Cd2+ in mixture with Cu2+, Zn2+, Pb2+ and Fe2+ on growth and chlorophyll content of alga Scenedesmus quadricauda. Biologia 54:661–666
Farias DR, Hurd CL, Eriksen RS, Simioni C, Schmidt E, Bouzon ZL, Macleod CK (2017) In situ assessment of Ulva australis as a monitoring and management tool for metal pollution. J Appl Phycol 29:2489–2502
Farmer BD, Beck BH, Mitchell AJ, Rawles SD, Straus DL (2017) Dietary copper effects survival of channel catfish challenged with Flavobacterium columnare. Aquac Res 48:1751–1758
Feng J, Chen J, Chen M, Su X, Shi Q (2017) Effects of biocide treatments on durability of wood and bamboo/high density polyethylene composites against algal and fungal decay. J Appl Polym Sci 134:1996–2003
Fongmoon D, Pongnikorn S, Chaisena A, Iamsaard S (2014) Particulate matters collected from ceramic factories in Lampang Province affecting rat lungs. J Zhejiang Univ Sci B 15(1):75–83
Fostier AH, Costa FN, Korn MSGA (2016) Assessment of mercury contamination based on mercury distribution in sediment, macroalgae, and seagrass in the Todos os Santos Bay, Bahia, Brazil. Environ Sci Pollut Res 23:19686–19695
Fourest E, Canal C, Roux J (1994) Improvement of heavy metal biosorption by mycelial dead biomasses (Rhizopus arrhizus, Mucor miehei and Penicillium chrysogenum): pH control and cationic activation. FEMS Microbiol Rev 14:325–332
de França FP, Tavares APM, da Costa ACA (2002) Calcium interference with continuous biosorption of zinc by Sargassum sp. (Phaeophyceae) in tubular laboratory reactors. Bioresour Technol 83:159–163
Gaete Olivares H, Moyano Lagos N, Jara Gutierrez C, Carrasco Kittelsen R, Lobos Valenzuela G, Hidalgo Lillo ME (2016) Assessment oxidative stress biomarkers and metal bioaccumulation in macroalgae from coastal areas with mining activities in Chile. Environ Monit Assess 188(25)
Ganesan M, Mairh OP, Eswaran K, Subba Rao PV (1999) Effect of salinity, light intensity and nitrogen source on growth and composition of UIlva fasciata Delile (Chlorophyta, Ulvales). Indian J Mar Sci 28:70–73
Gao G, Liu Y, Li X, Feng Z, Xu Z, Wu H, Xu J (2017a) Expected CO2-induced ocean acidification modulates copper toxicity in the green tide alga Ulva prolifera. Environ Exp Bot 135:63–72
Gao L, Wang Z, Shan J, Chen J, Tang C, Yi M (2017b) Aquatic environmental changes and anthropogenic activities reflected by the sedimentary records of the Shima River, Southern China. Environ Pollut 224:70–81
García-Casal MN, Pereira AC, Leets I, Ramírez J, Quiroga MF (2007) High iron content and bioavailability in humans from four species of marine algae. J Nutr 137:2691–2695
Garcia-Rios V, Freile-Pelegrin Y, Robledo D, Mendoza-Cozatl D, Moreno-Sanchez R, Gold-Bouchot G (2007) Cell wall composition affects Cd2+ accumulation and intracellular thiol peptides in marine red algae. Aquat Toxicol 81:65–72
Girling JA, Thomas KV, Brooks SJ, Smith DJ, Shahsavari E, Ball AS (2015) A macroalgal germling bioassay to assess biocide concentrations in marine waters. Mar Poll Bull 91:82–86
Giusti L (2001) Heavy metal contamination of brown seaweed and sediments from the UK coastline between the Wear River and the Tees River. Environ Int 26:275–286
Gledhill M, Nimmo M, Hill SJ, Brown MT (1997) The toxicity of copper(II) species to marine algae, with particular reference to macroalgae. J Phycol 33:2–11
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Greger M, Malm T, Kautsky L (2007) Heavy metal transfer from composted macroalgae to crops. Eur J Agron 26:257–265
Gubelit Y, Polyak Y, Dembska G, Pazikowska-Sapota G, Zegarowski L, Kochura D, Krivorotov D, Podgornaya E, Burova O, Maazouzi C (2016) Nutrient and metal pollution of the eastern Gulf of Finland coastline: sediments, macroalgae, microbiota. Sci Total Environ 550:806–819
Guiry MD (2012) How many species of algae are there? J Phycol 48:1057–1063
Gumgum B, Unlu E, Tez Z, Gulsun Z (1994) Heavy-metal pollution in water, sediment and fish from the Tigris River. Chemosphere 29:111–116
Gunawardena J, Egodawatta P, Ayoko GA, Goonetilleke A (2013) Atmospheric deposition as a source of heavy metals in urban stormwater. Atmos Environ 68:235–242
Hagemann M (2016) Coping with high and variable salinity: molecular aspects of compatible solute accumulation. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 359–372
Hamed SM, Zinta G, Klöck G, Asard H, Selim S, AbdElgawad H (2017) Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol Environ Saf 140:256–263
Hansen HR, Hector BL, Feldmann J (2003) A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim Feed Sci Technol 105:21–28
Harrison JA, Bouwman AF, Mayorga E, Seitzinger S (2010) Magnitudes and sources of dissolved inorganic phosphorus inputs to surface fresh waters and the coastal zone: a new global model. Glob Biogeochem Cycles 24:1–16
Henriques B, Rocha LS, Lopes CB, Figueira P, Monteiro RJR, Duarte AC, Pardal MA, Pereira E (2015) Study on bioaccumulation and biosorption of mercury by living marine macroalgae: prospecting for a new remediation biotechnology applied to saline waters. Chem Eng J 281:759–770
Henriques B, Lopes CB, Figueira P, Rocha LS, Duarte AC, Vale C, Pardal MA, Pereira E (2017a) Bioaccumulation of Hg, Cd and Pb by Fucus vesiculosus in single and multi-metal contamination scenarios and its effect on growth rate. Chemosphere 171:208–222
Henriques B, Rocha LS, Lopes CB, Figueira P, Duarte AC, Vale C, Pardal MA, Pereira E (2017b) A macroalgae-based biotechnology for water remediation: simultaneous removal of Cd, Pb and Hg by living Ulva lactuca. J Environ Manag 191:275–289
Herrero R, Lodeiro P, Rojo R, Ciorba A, Rodríguez P, Sastre de Vicente ME (2008) The efficiency of the red alga Mastocarpus stellatus for remediation of cadmium pollution. Bioresour Technol 99:4138–4146
Howarth RW, Sharpley A, Walker D (2002) Sources of nutrient pollution to coastal waters in the United States: implications for achieving coastal water quality goals. Estuaries 25:656–676
Huang H, Liang J, Wu X, Zhang H, Li Q, Zhang Q (2013) Comparison in copper accumulation and physiological responses of Gracilaria lemaneiformis and G. lichenoides (Rhodophyceae). Chin J Oceanol Limnol 31:803–812
Hughes TP, Kerry JT, Álvarez-Noriega M, et al (2017). Global warming and recurrent mass bleaching of corals. Nature, 543 :373–377
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, 2nd edn. Cambridge University Press, Cambridge
Jarvis TA, Bielmyer-Fraser GK (2015) Accumulation and effects of metals in two seaweed species. Comp Biochem Physiol C 171:28–33
Jie X, Xiaohong Z, Chunlei G, Meijie J, Ruixiang L, Zongling W, Yan L, Shiliang F, Xuelei Z (2016) Effect of temperature, salinity and irradiance on growth and photosynthesis of Ulva prolifera. Acta Oceanol Sinica 35:114–121
Jones BG, Chenhall BE, Debretsion F, Hutton AC (2003) Geochemical comparisons between estuaries with non-industrialised and industrialised catchments: the Huon and Derwent River estuaries, Tasmania. Aust J Earth Sci 50:653–667
Jung SM, Kang SG, Son JS, Jeon JH, Lee HJ, Shin HW (2016) Temporal and spatial variations in the proximate composition, amino acid, and mineral content of Pyropia yezoensis. J Appl Phycol 28:3459–3467
Kaduková J, Virčíková E (2005) Comparison of differences between copper bioaccumulation and biosorption. Environ Int 31:227–232
Kemp A, Horton B, Donnelly J, Mann M, Vermeer M, Rahmstorf S (2011) Climate related sea-level variations over the past two millennia. Proc Nat Acad Sci U S A 108:11017–11022
Kidgell JT, de Nys R, Paul NA, Roberts DA (2014) The sequential application of macroalgal biosorbents for the bioremediation of a complex industrial effluent. PLoS One 9(7):e101309
Koch KA, Pena MMO, Thiele DJ (1997) Copper-binding motifs in catalysis, transport, detoxification and signaling. Chem Biol 4:549–560
Konda N, Singh S, Simmons BA, Klein-Marcuschamer D (2015) An investigation on the economic feasibility of macroalgae as a potential feedstock for biorefineries. Bioenerg Res 8:1046–1056
Kumar V, Sinha AK, Makkar HPS, Becker K (2010) Dietary roles of phytate and phytase in human nutrition: a review. Food Chem 120:945–959
Kumar SD, Santhanam P, Selvaraju A, Devi S, Kumar N, Prasath B, Selvakumaran J, Thillainayagam S, Ananthi P (2014) Effect of different dosages of zinc on the growth and biomass in five marine microalgae. Int J Fish Aquat 6:1–8
Kupper H, Kupper FC, Spiller M (1996) Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J Exp Bot 47:259–266
Lee W, Wang W (2001) Metal accumulation in the green macroalga Ulva fasciata: effects of nitrate, ammonium and phosphate. Sci Total Environ 278:11–22
Leung A, Cai ZW, Wong MH (2006) Environmental contamination from electronic waste recycling at Guiyu, Southeast China. J Mater Cycles Waste 8:21–33
Liu X, Yuan W, Meng X (2017) Extraction and quantification of phlorotannins from edible brown algae. Trans ASABE 60:265–271
Luoma S (1983) Bioavailability of trace-metals to aquatic organisms - a review. Sci Total Environ 28:1–22
Ma M, Zhu W, Wang Z, Witkamp GJ (2003) Accumulation, assimilation and growth inhibition of copper on freshwater alga (Scenedesmus subspicatus 86.81 SAG) in the presence of EDTA and fulvic acid. Aquat Toxicol 63:221–228
Machado L, Kinley RD, Magnusson M, de Nys R, Tomkins NW (2015) The potential of macroalgae for beef production systems in Northern Australia. J Appl Phycol 27:2001–2005
Machado L, Magnusson M, Paul NA, Kinley R, Nys RD, Tomkins N (2016) Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J Appl Phycol 28:3117–3126
Madden M, Mitra M, Ruby D, Schwarz J (2012) Seasonality of selected nutritional constituents of edible Delmarva seaweeds. J Phycol 48:1289–1298
Makkar HPS, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P (2016) Seaweeds for livestock diets: a review. Anim Feed Sci Technol 212:1–17
Malasarn D, Kropat J, Hsieh SI, Finazzi G, Casero D, Loo JA, Pelligrini M, Wollman F-A, Merchant SS (2013) Zinc deficiency impacts CO2 assimilation and disrupts copper homeostasis in Chlamydomonas reinhardtii. J Biol Chem 288:10672–10683
Malea P, Haritonidis S (1999) Seasonal accumulation of metals by red alga Gracilaria verrucosa (Huds.) Papens. from Thermaikos Gulf, Greece. J Appl Phycol 11:503–509
Mamboya F, Lyimo TJ, Landberg T, Björk M (2009) Influence of combined changes in salinity and copper modulation on growth and copper uptake in the tropical green macroalga Ulva reticulata. Estuar Coast Shelf Sci 84:326–330
Mantri VA, Singh RP, Bijo AJ, Kumari P, Reddy CRK, Jha B (2011) Differential response of varying salinity and temperature on zoospore induction, regeneration and daily growth rate in Ulva fasciata (Chlorophyta, Ulvales). J Appl Phycol 23:243–250
McHugh DJ (2003) A guide to the seaweed industry. FAO Fisheries Technical Paper 441, Rome. 105 pp
Medina M, Andrade S, Faugeron S, Lagos N, Mella D, Correa JA (2005) Biodiversity of rocky intertidal benthic communities associated with copper mine tailing discharges in northern Chile. Mar Poll Bull 50:396–409
Mendes LF, Zambotti-Villela L, Colepicolo P, Marinho-Soriano E, Stevani CV, Yokoya NS (2013) Metal cation toxicity in the alga Gracilaria domingensis as evaluated by the daily growth rates in synthetic seawater. J Appl Phycol 25:1939–1947
Mendes L, Stevani C, Zambotti-Villela L, Yokoya N, Colepicolo P (2014) Toxic effect of metal cation binary mixtures to the seaweed Gracilaria domingensis (Gracilariales, Rhodophyta). Environ Sci Pollut Res 21:8216–8223
Merrifield ME, Ngu T, Stillman MJ (2004) Arsenic binding to Fucus vesiculosus metallothionein. Biochem Biophys Res Commun 324:127–132
Michalak I, Chojnacka K, Dobrzański Z, Górecki H, Zielińska A, Korczyński M, Opaliński S (2011) Effect of macroalgae enriched with microelements on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings. J Anim Physiol Anim Nutr 95:374–387
Michalak I, Chojnacka K, Korniewicz D (2015) New feed supplement from macroalgae as the dietary source of microelements for pigs. Open Chem 13:1341–1352
Millero FJ, Woosley R, Ditrolio B, Waters J (2009) Effect of ocean acidification on the speciation of metals in seawater. Oceanography 22:72–85
Oliveira MN, Freitas ALP, Carvalho AFU, Sampaio TMT, Farias DF, Alves Teixeira DI, Gouveia ST, Pereira JG, Sena MMCC (2009) Nutritive and non-nutritive attributes of washed-up seaweeds from the coast of Ceará, Brazil. Food Chem 115:254–259
Moenne A, González A, Sáez CA (2016) Mechanisms of metal tolerance in marine macroalgae, with emphasis on copper tolerance in Chlorophyta and Rhodophyta. Aquat Toxicol 176:30–37
Morris CA, Nicolaus B, Sampson V, Harwood JL, Kille P (1999) Identification and characterization of a recombinant metallothionein protein from a marine alga, Fucus vesiculosus. Biochem J 338:553–560
Morrison L, Baumann HA, Stengel DB (2008) An assessment of metal contamination along the Irish coast using the seaweed Ascophyllum nodosum (Fucales, Phaeophyceae). Environ Pollut 152:293–303
Morshedi V, Nafisi Bahabadi M, Sotoudeh E, Azodi M, Hafezieh M (2018) Nutritional evaluation of Gracilaria pulvinata as partial substitute with fish meal in practical diets of barramundi (Lates calcarifer). J Appl Phycol 30:619–628
Moutinho S, Linares F, Rodríguez JL, Sousa V, Valente LMP (2018) Inclusion of 10% seaweed meal in diets for juvenile and on-growing life stages of Senegalese sole (Solea senegalensis). J Appl Phycol. https://doi.org/10.1007/s10811-018-1482-6
Munksgaard NC, Burchert S, Kaestli M, Nowland SJ, O’Connor W, Gibb KS (2017) Cadmium uptake and zinc-cadmium antagonism in Australian tropical rock oysters: potential solutions for oyster aquaculture enterprises. Mar Poll Bull 123:47–56
Murphy V, Hughes H, McLoughlin P (2009) Enhancement strategies for Cu(II), Cr(III) and Cr(VI) remediation by a variety of seaweed species. J Hazard Mater 166:318–326
Muse JO, Stripeikis JD, Fernandez FM, d'Huicque L, Tudino MB, Carducci CN, Troccoli OE (1999) Seaweeds in the assessment of heavy metal pollution in the Gulf San Jorge, Argentina. Environ Pollut 104:315–322
Nagai T, De Schamphelaere KAC (2016) The effect of binary mixtures of zinc, copper, cadmium, and nickel on the growth of the freshwater diatom Navicula pelliculosa and comparison with mixture toxicity model predictions. Environ Toxicol Chem 35:2765–2773
Nayar S, Bott K (2014) Current status of global cultivated seaweed production and markets. World Aquacult 45:32–37
Nejrup LB, Pedersen MF (2012) The effect of temporal variability in salinity on the invasive red alga Gracilaria vermiculophylla. Eur J Phycol 47:254–263
Nikolic D, Milosevic N, Zivkovic Z, Mihajlovic I, Kovacevic R, Petrovic N (2011) Multi-criteria analysis of soil pollution by heavy metals in the vicinity of the copper smelting plant in Bor (Serbia). J Serb Chem Soc 76(4):625–641
Nkemka VN, Murto M (2012) Exploring strategies for seaweed hydrolysis: effect on methane potential and heavy metal mobilisation. Process Biochem 47:2523–2526
Nonova T, Tosheva Z (2016) Sr-90, pb-210, po-210 and Ra isotopes in marine macroalgae and mussel Mytilus galloprovincialis from the Bulgarian Black Sea zone. J Radioanal Nucl Chem 307:1183–1194
Oh J, Choi EM, Han YS, Yoon J, Park A, Jin K, Lee J-W, Choi H, Kim S, Brown MT, Han T (2012) Influence of salinity on metal toxicity to Ulva pertusa. Toxicol Environ Health Sci 4:9–13
Okogbue CO, Oyesanya OU, Anyiam OA, Omonona VO (2017) Assessment of pollution from produced water discharges in seawater and sediments in offshore, Niger Delta. Environ Earth Sci 76(10):359
O'Neill A, Gupta BS, Phillips DH (2014) Distribution of arsenic and risk assessment of activities on a golf course fertilised with arsenic-containing Ascophyllum nodosum seaweed. Sci Total Environ 482:252–259
Ozyigit II, Uyanik OL, Sahin NR, Yalcin IE, Demir G (2017) Monitoring the pollution level in Istanbul coast of the Sea of Marmara using algal species Ulva lactuca L. Pol J Environ Stud 26(2):773–778
Padhi RK, Biswas S, Mohanty AK, Prabhu RK, Satpathy KK, Nayak L (2013) Temporal distribution of dissolved trace metal in the coastal waters of southwestern Bay of Bengal, India. Water Environ Res 85:696–705
Paiva L, Lima E, Neto AI, Marcone M, Baptista J (2017) Health-promoting ingredients from four selected Azorean macroalgae. Food Res Int 89(1):432–438
Pallaoro MF, Vieira FN, Hayashi L (2016) Ulva lactuca (Chlorophyta Ulvales) as co-feed for pacific white shrimp. J Appl Phycol 28:3659–3665
Palma H, Killoran E, Sheehan M, Berner F, Heimann K (2017) Assessment of microalga biofilms for simultaneous remediation and biofuel generation in mine tailings water. Bioresour Technol 234:327–335
Patarra R, Paiva L, Neto A, Lima E, Baptista J (2011) Nutritional value of selected macroalgae. J Appl Phycol 23:205–208
Peers G, Quesnel SA, Price NM (2005) Copper requirements for iron acquisition and growth of coastal and oceanic diatoms. Limnol Oceanogr 50:1149–1158
Peixoto M, Svendsen J, Malte H, Pereira L, Carvalho P, Pereira R, Gonçalves J, Ozório R (2016) Diets supplemented with seaweed affect metabolic rate, innate immune, and antioxidant responses, but not individual growth rate in European seabass (Dicentrarchus labrax). J Appl Phycol 28:2061–2071
Pellegrini M, Laugier A, Sergent M, Phan-Tan-Luu R, Valls R, Pellegrini L (1993) Interactions between the toxicity of the heavy metals cadmium, copper, zinc in combinations and the detoxifying role of calcium in the brown alga Cystoseira barbata. J Appl Phycol 5:351–336
Pereira P, de Pablo H, Rosa-Santos F, Pacheco M, Vale C (2009) Metal accumulation and oxidative stress in Ulva sp. substantiated by response integration into a general stress index. Aquat Toxicol 91:336–345
Perez AA, Farias SS, Strobl AM, Perez LB, Lopez CM, Pineiro A, Roses O, Fajardo MA (2007) Levels of essential and toxic elements in Porphyra columbina and Ulva sp from San Jorge Gulf, Patagonia Argentina. Sci Total Environ 376:51–59
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Qiu X, Neori A, Kim JK, Yarish C, Shpigel M, Guttman L, Ben Ezra D, Odintsov V, Davis DA (2018a) Evaluation of green seaweed Ulva sp. as a replacement of fish meal in plant-based practical diets for pacific white shrimp, Litopenaeus vannamei. J Appl Phycol 30:1305–1316
Qiu X, Neori A, Kim JK, Yarish C, Shpigel M, Guttman L, Ben Ezra D, Odintsov V, Davis DA (2018b) Green seaweed Ulva sp. as an alternative ingredient in plant-based practical diets for Pacific white shrimp, Litopenaeus vannamei. J Appl Phycol 30:1317–1333
Quigg A (2016) Micronutrients. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 359–372
Racionero-Gomez B, Sproson AD, Selby D, Grocke DR, Redden H, Greenwell HC (2016) Rhenium uptake and distribution in Phaeophyceae macroalgae, Fucus vesiculosus. R Soc Open Sci 3(5):1–18
Raize O, Argaman Y, Yannai S (2004) Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol Bioeng 87:451–458
Rajfur M, Klos A (2014) Use of algae in active biomonitoring of surface waters. Ecol Chem Eng Sci 21:561–576
Rangabhashiyam S, Suganya E, Lity AV, Selvaraju N (2016) Equilibrium and kinetics studies of hexavalent chromium biosorption on a novel green macroalgae Enteromorpha sp. Res Chem Intermed 42:1275–1294
Ratcliff JJ, Wan AHL, Edwards MD, Soler-Vila A, Johnson MP, Abreu MH, Morrison L (2016) Metal content of kelp (Laminaria digitate) co-cultivated with Atlantic salmon in an integrated multi-trophic aquaculture system. Aquaculture 459:234–243
Reed R, Moffat L (1983) Copper toxicity and copper tolerance in Enteromorpha compressa (L.) Grev. J Exp Mar Biol Ecol 69:85–103
Ren T, Fu G, Liu T, Hu K, Li H, Fang W, Yang X (2017) Toxicity and accumulation of zinc pyrithione in the liver and kidneys of Carassius auratus Gibelio: association with P-glycoprotein expression. Fish Physiol Biochem 43:1–9
Rey-Crespo F, Lopez-Alonso M, Miranda M (2014) The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Animal 8:580–586
Riba I, Garcia-Luque E, Blasco J, Delvalls TA (2003) Bioavailability of heavy metals bound to estuarine sediments as a function of pH and salinity values. Chem Speciat Bioavailab 15(4):101–114
Rink L, Gabriel P (2000) Zinc and the immune system. Proc Nutr Soc 59:541–552
Rjiba-Ktita S, Chermiti A, Bodas R, France J, López S (2017) Aquatic plants and macroalgae as potential feed ingredients in ruminant diets. J Appl Phycol 29:449–458
Roberts DA, Paul NA, Cole AJ, de Nys R (2015) From waste water treatment to land management: conversion of aquatic biomass to biochar for soil amelioration and the fortification of crops with essential trace elements. J Environ Manag 157:60–68
Romera E, Gonzalez F, Ballester A, Blazquez ML, Munoz JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol 98:3344–3353
Rowbotham JS, Dyer PW, Greenwell HC, Selby D, Theodorou MK (2013) Copper(II)-mediated thermolysis of alginates: a model kinetic study on the influence of metal ions in the thermochemical processing of macroalgae. Interface Focus 3:1–16
Rubio C, Napoleone G, Luis-González G, Gutiérrez AJ, González-Weller D, Hardisson A, Revert C (2017) Metals in edible seaweed. Chemosphere 173:572–579
Ruperez P (2002) Mineral content of edible marine seaweeds. Food Chem 79:23–26
Ryan S, McLoughlin P, O'Donovan O (2012) A comprehensive study of metal distribution in three main classes of seaweed. Environ Pollut 167:171–177
Sáez CA, Roncarati F, Moenne A, Moody AJ, Brown MT (2015) Copper-induced intra-specific oxidative damage and antioxidant responses in strains of the brown alga Ectocarpus siliculosus with different pollution histories. Aquat Toxicol 159:81–89
Sağlam E, Akçay M (2016) Chemical and mineralogical changes of waste and tailings from the murgul Cu deposit (Artvin, NE Turkey): implications for occurrence of acid mine drainage. Environ Sci Pollut Res 23:6584–6607
Sánchez-Thomas R, Moreno-Sánchez R, García-García JD (2016) Accumulation of zinc protects against cadmium stress in photosynthetic Euglena gracilis. Environ Exp Bot 131:19–31
Santore RC, Di Toro DM, Paquin PR, Allen HE, Meyer JS (2001) Biotic ligand model of the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish and Daphnia. Environ Toxicol Chem 20:2397–2402
Santos R, Melo RA (2018) Global shortage of technical agars: back to basics (resource management). J Appl Phycol. https://doi.org/10.1007/s10811-018-1425-2
Senthilkumar R, Vijayaraghavan K, Thilakavathi M, Iyer PVR, Velan M (2006) Seaweeds for the remediation of wastewaters contaminated with zinc(II) ions. J Hazard Mater 136:791–799
Silva DM, Valente LMP, Sousa-Pinto I, Pereira R, Pires MA, Seixas F, Rema P (2014) Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L. juveniles. Effects on growth performance and gut histology. J Appl Phycol 27:1671–1680
Sondak CFA, Ang PO, Beardall J, Bellgrove A, Boo SM, Gerung GS, Hepburn CD, Hong DD, Hu Z, Kawai H, Largo D, Lee JA, Lim P-E, Mayakun J, Nelson WA, Oak JH, Phang S-M, Sahoo D, Peerapornpis Y, Yang Y, Chung IK (2017) Carbon dioxide mitigation potential of seaweed aquaculture beds (SABs). J Appl Phycol 29:2363–2373
Sotoudeh E, Mardani F (2017) Antioxidant-related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquac Nutr 24:777–785
Srinivasa Gowd S, Govil P (2008) Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu. India Environ Monit Assess 136:197–207
Stengel DB, Macken A, Morrison L, Morley N (2004) Zinc concentrations in marine macroalgae and a lichen from western Ireland in relation to phylogenetic grouping, habitat and morphology. Mar Poll Bull 48:902–909
Suresh Kumar K, Ganesan K, Subba Rao PV (2008) Heavy metal chelation by non-living biomass of three color forms of Kappaphycus alvarezii (Doty) Doty. J Appl Phycol 20:63–66
Suutari M, Leskinen E, Spilling K, Kostamo K, Seppälä J (2016) Nutrient removal by biomass accumulation on artificial substrata in the northern Baltic Sea. J Appl Phycol 29:1707–1720
Tabarsa M, Rezaei M, Ramezanpour Z, Waaland JR (2012) Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J Sci Food Agr 92:2500–2506
Tang X, Shen C, Shi D, Cheema SA, Khan MI, Zhang C, Chen Y (2010) Heavy metal and persistent organic compound contamination in soil from Wenling: an emerging e-waste recycling city in Taizhou area, China. J Hazard Mater 173:653–660
Tayyab U, Novoa-Garrido M, Roleda MY, Lind V, Weisbjerg MR (2016) Ruminal and intestinal protein degradability of various seaweed species measured in situ in dairy cows. Anim Feed Sci Technol 213:44–54
Tibbetts SM, Milley JE, Lall SP (2016) Nutritional quality of some wild and cultivated seaweeds: nutrient composition, total phenolic content and in vitro digestibility. J Appl Phycol 28:3575–3585
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101:294–301
Tseng CK (2004) The past, present and future of phycology in China. Hydrobiologia 512:11–20
Tuhy L, Samoraj M, Basladynska S, Chojnacka K (2015) New micronutrient fertilizer biocomponents based on seaweed biomass. Pol J Environ Stud 24:2213–2221
Turner A, Rice L (2010) Toxicity of tire wear particle leachate to the marine macroalga, Ulva lactuca. Environ Pollut 158:3650–3654
Turner A, Pedroso SS, Brown MT (2008) Influence of salinity and humic substances on the uptake of trace metals by the marine macroalga, Ulva lactuca: experimental observations and modelling using WHAM. Mar Chem 110:176–184
Turner A, Pollock H, Brown MT (2009) Accumulation of Cu and Zn from antifouling paint particles by the marine macroalga, Ulva lactuca. Environ Pollut 157:2314–2319
U.S. Environmental Protection Agency (USEPA) (2000) National Recommended Water Quality Criteria; 65 FR 31682. USEPA Office of Water, Washington, DC, USA, pp 31682–31719
Valdés FA, Gabriela Lobos M, Díaz P, Sáez CA (2018) Metal assessment and cellular accumulation dynamics in the green macroalga Ulva lactuca. J Appl Phycol 30:663–671
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res 16:765–794
Varol M, Şen B (2012) Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena 92:1–10
Viriyatum R, Boyd CE (2016) Slow-release coated copper sulfate as an algicide for aquaculture. J World Aquacult Soc 47:667–675
Wan L, Wang N, Li Q, Sun B, Zhou Z, Xue K, Ma Z, Tian J, Song L (2007) Distribution of dissolved metals in seawater of Jinzhou Bay, China. Environ Toxicol Chem 27:43–48
Wan A, Soler-Vila A, O’Keeffe D, Casburn P, Fitzgerald R, Johnson M (2016) The inclusion of Palmaria palmata macroalgae in Atlantic salmon (Salmo salar) diets: effects on growth, haematology, immunity and liver function. J Appl Phycol 28:3091–3100
Wang Z, Wang X, Ke C (2014) Bioaccumulation of trace metals by the live macroalga Gracilaria lemaneiformis. J Appl Phycol 26:1889–1897
Wassef EA, El-Sayed A-FM, Sakr EM (2013) Pterocladia (Rhodophyta) and Ulva (Chlorophyta) as feed supplements for European seabass, Dicentrarchus labrax L. fry. J Appl Phycol 25:1369–1376
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Wendt I, Arrhenius Å, Backhaus T, Hilvarsson A, Holm K, Langford K, Tunovic T, Blanck H (2013) Effects of five antifouling biocides on settlement and growth of zoospores from the marine macroalga Ulva lactuca L. Bull Environ Contam Toxicol 91:426–432
Wessells KR, Brown KH (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 7(11):e50568
Wi SG, Kim HJ, Mahadevan SA, Yang D, Bae H (2009) The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol 100:6658–6660
Wu T, Hsu Y, Sung M, Hsu Y, Lee T (2009) Expression of genes involved in redox homeostasis and antioxidant defense in a marine macroalga Ulva fasciata by excess copper. Aquat Toxicol 94:275–285
Wu X, Wang G, Fu X (2014) Variations in the chemical composition of Costaria costata during harvest. J Appl Phycol 26:2389–2396
Xu L, Wang T, Ni K, Liu S, Wang P, Xie S, Meng J, Zheng X, Lu Y (2014) Ecological risk assessment of arsenic and metals in surface sediments from estuarine and coastal areas of the southern Bohai Sea, China. Human Ecol Risk Assess 20:388–401
Yangthong M, Hutadilok-Towatana N, Thawonsuwan J, Phromkunthong W (2016) An aqueous extract from Sargassum sp enhances the immune response and resistance against Streptococcus iniae in the asian sea bass (Lates calcarifer bloch). J Appl Phycol 28(6):3587–3598
Yi S, Heki K, Qian A (2017) Acceleration in the global mean sea level rise: 2005–2015. Geophys Res Lett 44:11905–11913
Yong YS, Yong WTL, Ng SE, Anton A, Yassir S (2015) Chemical composition of farmed and micropropagated Kappaphycus alvarezii (Rhodophyta, Gigartinales), a commercially important seaweed in Malaysia. J Appl Phycol 27:1271–1275
Ytreberg E, Karlsson J, Ndungu K, Hassellov M, Breitbarth E, Eklund B (2011) Influence of salinity and organic matter on the toxicity of Cu to a brackish water and marine clone of the red macroalga Ceramium tenuicorne. Ecotoxicol Environ Saf 74:636–642
Yun YS, Park D, Park JM, Volesky B (2001) Biosorption of trivalent chromium on the brown seaweed biomass. Environ Sci Technol 35:4353–4358
Yunus SM, Hamzah Z, Wood AK, Ahmad (2015) Assessment of heavy metals in seawater and fish tissues at Pulau Indah, Selangor, Malaysia. AIP Conference Proceedings, 1659 (1):050007
Zhang C, Wang J, Tan L, Chen X (2016) Toxic effects of nano-ZnO on marine microalgae Skeletonema costatum: attention to the accumulation of intracellular Zn. Aquat Toxicol 178:158–164
Zhou Y, Lang RH, Dinnat EP, Le Vine DM (2017) L-band model function of the dielectric constant of seawater. IEEE Trans Geosci Remote Sens 55:6964–6974
Zhu D, Wen X, Xuan X, Li S, Li Y (2016) The green alga Ulva lactuca as a potential ingredient in diets for juvenile white spotted snapper Lutjanus stellatus Akazaki. J Appl Phycol 28:703–711
Funding
The work is supported through funding from the North Carolina State University Department of Biological and Agricultural Engineering and the William White Endowment of the NC Agriculture Foundation and the North Carolina State University College of Agriculture and Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geddie, A.W., Hall, S.G. An introduction to copper and zinc pollution in macroalgae: for use in remediation and nutritional applications. J Appl Phycol 31, 691–708 (2019). https://doi.org/10.1007/s10811-018-1580-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1580-5