Abstract
In this study, Chlorella vulgaris treated with static magnetic field (SMF) was investigated in terms of the algal density, biomass, extracellular polysaccharide content and distribution, percentage of algal aggregation, total protein content, enzyme activity, malondialdehyde content, and nutrient removal. The algal density and biomass under 800 G SMF were highest on the 16th day and were 29.02% and 35.67% greater than the control group (0 G group), respectively. Soluble EPS decreased with an increase in magnetic field strength, while bound EPS exhibited an opposite trend. Algal aggregation occurred in all treatment groups, with the aggregation percentage in the high magnetic field-treated groups being lower than that in the control group. Total protein content of the 800 G group was the lowest at 1.13 μg (106 cells)-1 and the enzyme activity of treated algae was higher than that of the control group. SMF affected the growth and reproduction of C. vulgaris by affecting the antioxidant response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication of water can cause algal blooms, which in turn causes water quality problems such as malodour and taste issues and algal toxin formation (Chiou et al. 2010; Lee et al. 2014). However, microalgae contain a large amount of oil, which potentially can be used as a source of biofuels, providing a renewable energy source (Schenk et al. 2008; Mata et al. 2010). In addition, microalgae also have many other uses, such as raw materials for cosmetics and animal feed (Markou and Nerantzis 2013). Several studies highlighted the effect of abiotic (i.e., temperature, light, heavy metals, and nutrients concentration) and biotic (i.e., ecological competition) factors on algal growth (Guerrini et al. 2000; Granum et al. 2002; Ghasemi et al. 2009; Widjaja et al. 2009; Rugnini et al. 2018).
Aggregation has a great impact on algal and cyanobacterial growth and recycling. It has been reported that, compared with colonial Microcystis, the photosynthetic rate and activity of unicellular Microcystis are lower (Wu and Song 2008). Extracellular polymeric substances contain polysaccharides and other macromolecules such as proteins, lipids, and DNA. In this study, the quantities of extracellular polysaccharides (EPS) were analyzed. EPS can affect the surface charge, structure, flocculation, sedimentation performance, adsorption capacity, and dehydration performance of microbial aggregates (Sheng et al. 2010). In addition, EPSs are the main “cement” in the aggregation of cells and the cell products (Sutherland 2001). Therefore, the EPS content plays an important role in the formation of algal aggregates (Li et al. 2013).
Magnetic treatment is a physical treatment method which is non-toxic and harmless, with no associated secondary pollution. Magnetic fields can be classified as either static magnetic field (SMF) or variable magnetic fields. It has been reported that magnetic fields can exert different biological effects on organisms (Reiter 1998). Some studies have used magnetic fields to control the growth of algae to purify water (Newman and Watson 1999; Liang et al. 2004). It has also been reported that the growth rate of Arthrospira (Spirulina) platensis treated with an SMF of 100 G was the highest, while growth was significantly inhibited at 700 G (Hirano et al. 1998). A similar phenomenon has also been reported for Chlorella kessleri, with a SMF treatment of 100 G increasing biomass. Changes of algal biochemical composition, photosynthesis, and ultrastructure have been observed (Small et al. 2012). Other studies reported that SMF could promote the growth and oxygen production of Scenedesmus obliquus (Tu et al. 2015). However, these studies have focused on the effects of magnetic fields on algae growth, but few studies have focused on the effects on Chlorella vulgaris growth and its EPS production.
In this study, the influence of SMF on C. vulgaris was investigated. Growth rate and biomass of algae treated with different intensity magnetic fields were assessed. Furthermore, the EPS content was quantified and the distribution of several sugars and proteins on the algal surface was observed. Furthermore, the relationship between algal aggregation and other test results was explored. SMF can enhance electron transfer at the electrolysis interface, affecting the bio-electrocatalytic conversion of multiple enzyme components (Katz et al. 2005). Furthermore, SMF can affect enzyme activity (peroxidase (POD), catalase (CAT)) and malondialdehyde (MDA)) altering cell responses to the external environment (Halliwell and Gutteridge 1992). Therefore, the enzyme activity and MDA content of algae were also measured, to explore the response of C. vulgaris to different magnetic field strengths. The results of this research will further our understanding of the effects of magnetic fields on algae and provide new ideas for algal resource utilization and management.
Materials and methods
Experimental setting
A schematic of the SMF treatment device is shown in Fig. 1, consisting of a threaded rod, scale-plate, movable magnetic pole, fixed pole (Fig. 1(a)), and a custom-built container (Fig. 1(b)). The magnitude of the magnetic field was altered by adjusting the threaded rod, with the designed magnetic field strength ranging from 200 Gs to 2000 G. A gauss meter (Digital gauss meter HT-20, Hengtong, China) was used to measure the magnetic field strength.
One hundred fifty milliliters of algal solution with a concentration of 3 × 105cells mL−1 was placed in the three compartments of the custom-built container, allowing the analysis of three parallel samples (Fig. 1(b)). Five sets of samples were placed within the magnetic field for 2 h per day for 16 days. For the remaining time, the samples were placed in a light incubator (temperature 28 ± 1 °C, 12 h/12 h light-dark cycle at 120 μmol photons m−2 s−1).
Algal strain and growth conditions
Chlorella vulgaris (No. FACHB-8) was obtained from the Freshwater Culture Collection at the Institute of Hydrobiology (Wuhan, China). Cultivation was performed in 1000-mL conical flasks containing 400 mL BG-11 medium (Rippka et al. 1979) at 28 ± 1 °C with a 12 h/12 h light-dark cycle at a light intensity of 120 μmol photons m−2 s−1. The initial concentrations of C. vulgaris in all groups were 3 × 105 cells mL−1.
Measurement of algal density, aggregation percentage, and biomass
To assess C. vulgaris, the algal density of all groups was measured on alternate days for 16 days. The algal density and aggregation percentage of C. vulgaris were quantified using an automatic algae counter (Countstar BioMarine, ALIT Life Science, China). The aggregation percentage was measured on the 16th day, with each sample measured five times and the averaged results reported. Calculations were performed according to Eq. (1):
On the 16th day, after centrifuging at 12000×g for 20 min and removing the supernatant, the samples were dried at 105 °C for 24 h to evaluate the biomass (g L−1).
EPS extraction, analysis, and distribution
EPS fractions were divided into soluble EPS (S-EPS) and bound EPS (B-EPS), with B-EPS being tightly adhered to the algal cell membrane, while S-EPS was dissolved in the cell medium (Hsieh et al. 1994; Nielsen et al. 1997; Matsumoto et al. 2014). B-EPS can be classified as either loosely bound EPS (LB-EPS) or tightly bound EPS (TB-EPS). In the present study, S-EPS and B-EPS were both measured. On the last day, samples were centrifuged at 2500×g for 15 min with the S-EPS fraction separated in the supernatant. Then, a 0.05% NaCl solution was added to the harvested substrate and ultrasonicated (SB-3200D, Scientz, China) at 150 W for 1 min. Finally, the samples were centrifuged at 12000×g for 20 min and the supernatant was collected for B-EPS analysis (Xu et al. 2013b).
EPS content was determined via the phenol-sulfuric acid method (Dubois et al. 1956). In addition, the spatial distribution of various components in EPS was observed by confocal laser scanning microscopy (CLSM) (Leica SP8, Germany) after staining with various fluorescence probes (Staudt et al. 2004). Initially, samples were fixed with 2.5% glutaraldehyde and then multiple fluorescent staining (fluorescein-isothiocyanate (FITC) (Sigma, USA), concanavalin A (Con A) (Sigma), and calcofluor white (CW) (Sigma) was used to observe the distribution of proteins, α-D-glucopyranose and β-D-glucopyranose polysaccharides on the surface of C. vulgaris. The excitation/emission wavelengths used to observe FITC, Con A, and CW were 488/520 (green), 561/580 (red), and 400/435 nm (blue), respectively. Staining and microscopic observations were performed according to previously reported methods (Chen et al. 2007; Adav et al. 2010).
Water quality analysis
Samples were filtered through a polysulfone membrane filter (0.45 μm) and the pH value, total organic carbon (TOC), total phosphorus (TP), nitrate (NO3−-N), and nitrite (NO2−-N) concentration was measured. TOC was determined using a total organic carbon analyzer (TOC-L, Shimadzu, Japan). TP, NO3−-N and NO2−-N concentrations were measured via the standard method (Mallmann et al. 1945). Based on the results of preliminary experiments, the water quality of 0 and 800 G groups was measured.
Measurements of total protein, malondialdehyde, and enzyme activity
Twenty milliliter of algal samples was collected on the 16th day and centrifuged at 2500×g for 15 min. Then, the supernatant was carefully removed, with the remaining sediment containing the required algal cells. Then, 10 mL of 0.1 M phosphate buffer-sterile (PBS, pH 7.4) was mixed with the sediment. The mixture was centrifuged at 12000×g for 20 min and the substrate retained. Then, the substrate was mixed with 8 mL PBS and algal cells were lysed at 12000 rpm for 2 min using a high speed homogenizer (FSH-2A, Jintan Xinrui Instrument, China). Finally, the cell lysis fluid was centrifuged at 4000×g for 15 min at 4 °C and the resulting supernatant was used to measure the total protein (T-pro) content, MDA content, POD, and CAT enzymatic activity. These parameters were determined using assay kits (Nanjing Jiancheng Bioengineering Institute, China).
Statistical analysis
GraphPad Prism and SPSS v.22 software were used for data processing. The results are expressed as mean values ± standard deviation (SD). Comparisons between different treatments were performed by one-way analysis of variance (ANOVA) and significant differences were determined by LSD t test. Before performing the one-way ANOVA, the Shapiro-Wilk test was used to evaluate the assumption of normality, and the homogeneity of variance was tested by Levene’s test. For all tests, p values of < 0.05 were considered to indicate significant differences.
Results
Changes in algal density and biomass under SMF treatment
The algal density was measured on alternate days for 16 days (Fig. 2) indicating that the algal density of different groups increased rapidly. In contrast, the density of algae that had not been treated with SMF was consistently at lower than the comparative treated groups, while algae treated with 800 G SMF grew the fastest. At SMF strengths below 800 G, the growth of algae increased in accordance with the increase in magnetic field strength. The algal density of groups treated with 800 G SMF on the last day was 4.09 × 107 cells mL−1, which was 29.02% higher than that of the control group.
The trends in change of biomass and algal density on the last day of experiments generally remained the same. As the strength of SMF increased, the biomass increased initially and then decreased, reaching the highest level of 1.483 ± 0.460 g L−1 at a magnetic field strength of 800 G (Fig. 3). The biomass of the group treated with 800 G SMF was 35.67% higher than the control group. However, the biomass of algal cells treated with 1500 G SMF was lowest (0.570 ± 0.4531 g·L−1), at 47.87% less than the control group.
Changes in EPS under SMF treatment
The EPS content showed significant differences among different groups. As the magnetic field strength increased, the S-EPS content decreased gradually, with the content of the group treated with an SMF strength of 800 G being the lowest. The S-EPS results in groups treated with 800 G and 1000 G were significantly different from the other three groups (p < 0.05). However, a different trend was observed for the B-EPS content. The B-EPS content increased with increasing magnetic field strengths and the algae treated with 1500 G SMF exhibited the highest level of B-EPS secretion. S-EPS values ranged between 1.49 and 2.02 pg·cell−1 while B-EPS values ranged between 0.15 and 0.23 pg·cell−1, with the S-EPS content consistently being much larger than the B-EPS content (Figs. 4 and 5).
Aggregation percentage of C. vulgaris treated by SMF
On the16th day of treatment, the control group exhibited the highest aggregation percentage (17.89%), while the group treated with 800 G SMF exhibited the lowest aggregation percentage (13.18%). The aggregation percentage of the control group was 29.74% higher than that of the 800 G group. Furthermore, the aggregation percentage of groups treated with 800 G and 1000 G SMF was significantly different from the other three groups (Fig. 6).
T-pro, MDA, and enzyme activity in C. vulgaris treated with SMF
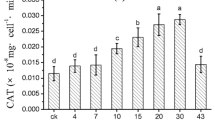
As shown in Fig. 7 (a), the differences in T-pro per 106 algal cells were observed following each treatment. The highest T-pro content was observed in the control group (1.93 μg (106 cells)-1), while the lowest content was observed in the 800 Gs SMF-treated group (1.13 μg (106 cells)-1). The overall trend showed that as the magnetic field strength increased, the content of T-pro decreased initially and then increased. When the magnetic field strength was 400 G, the MDA content was the highest, while the MDA content of the 1500 G group was the lowest. However, there was no significant difference observed in the content of MDA between groups (p > 0.05) (Fig. 7(b)). The CAT activity of the control group was lower than other magnetic field-treated groups. The group treated with a magnetic field strength of 400 G exhibited the highest CAT activity (101.37 U mg−1 T-pro), which was significantly higher than the control group (60.70 U mg−1 T-pro) (p < 0.05). The overall trend in CAT activity showed an initial increase and then a decrease as the SMF strength increased further (Fig. 7(c)). In addition, the control group exhibited the lowest POD activity at 44.63 U mg−1 T-pro, while algae treated with a magnetic field strength of 1500 G exhibited the highest POD activity of 60.99 U mg−1 T-pro. These results indicate that higher SMF strengths resulted in higher POD activity in algae (Fig. 7(d)).
Water quality
The measured water quality of the group treated with 800 G SMF was similar to that of the control group (Fig. 8). The TOC concentration in the 800 G group and the control group both increased rapidly with time. The TOC concentrations of the control group and the group treated with 800 G SMF reached a final level of 42.80 and 42.72 mg L−1, respectively (Fig. 8(a)). The trend in NO2−-N concentration exhibited a similar pattern to TOC concentrations (Fig. 8(c)), with the concentration of the control group being higher than that of the 800 G group. In addition, the NO3−-N concentration did not change significantly with time, although the NO3−-N concentration in the control group was smaller than the 800 G group (Fig. 8(b)). However, the concentration of TP reduced rapidly with time and the TP removal of the 0 and 800 G groups reached 85.15% and 84.54%, respectively.
Discussion
The effect of SMF on the growth and reproduction of C. vulgaris was evaluated by measuring algal density and biomass. The algal density results indicate that suitable SMF treatments can promote C. vulgaris growth. Previous research has shown that the chlorophyll-a content of Chlorella pyrenoidosa treated with a magnetic field was higher than the untreated group (Han et al. 2016). Some previous research has also found that A. platensis exhibited a similar response to magnetic field treatment (Li et al. 2007). This may be because magnetic fields can promote the release of oxygen from algae, enhancing photosynthesis and promoting metabolism and growth (Tu et al. 2015). However, when the SMF strength was 1500 G, a promotive effect was observed in the first 4 days, although the promotion was not observed with continued SMF exposure. The reason for this phenomenon may be that nutrients were limited, reducing the ability of cells to continually grow at a fast rate. Another possible reason is that long-duration magnetic field treatments could result in the inhibition of algal growth (Liu 2006). The magnetic field physically affects paramagnetic and diamagnetic substances in cells, which in turn affect the rate of biochemical reactions and change the growth rate of cells (Schenck 1996).
The result of biomass analysis shows that treatment with SMF at a suitable strength promotes the growth of algal biomass, while high-intensity magnetic fields inhibit the growth of algal biomass. This finding was also consistent with the results of Han et al. (2016) who confirmed that the biomass productivity of C. pyrenoidosa under 500 G magnetic field treatment (0.229 ± 0.004 g L−1 day−1), was higher than the control group (0.203 ± 0.007 g L−1 day−1).
Previous research has shown that EPS is mainly produced by secretion, adsorption, excretion, and cell lysis (Sheng et al. 2010; Xu et al. 2013a). Furthermore, EPS plays an important role in algal growth, due to functions such as water storage and serving as a carbon source (Lancelot et al. 1986; Li and Gao 2004). Therefore, in the later stages of exposure, due to the low nutrient content and high algal density in the high magnetic field strength treated groups, EPS could be utilized as a carbon and energy source (Sutherland 2001; Zhang and Bishop 2003), which may explain why the high SMF strength groups exhibited a lower S-EPS content. In addition, CLSM images exhibited the distribution of green, red, and blue areas on the surface of algal cells, which represent a visualization of protein, α-D-glucopyranose, and β-D-glucopyranose polysaccharides (Fig. 5). As the strength of the SMF increased, the distribution area of all three substances increased around algal cells. This phenomenon was consistent with the observed trend in B-EPS, indicating that more EPS tightly adhered to the surface of algal cells was more conducive to algal absorption of nutrients for growth and reproduction.
The cause of algal agglomeration appears to be highly complex. It has previously been reported that the formation of Microcystis colonies is achieved by the cohesion of individual cells within an unstructured viscous layer called mucilage (Kessel and Eloff 1975). The viscous layer is mainly composed of an anthrone-reacting polysaccharide component of EPS (Plude et al. 1991; Li et al. 2013). Larger aggregation allows algae to rapidly move up and down within the water column, thus gaining more nutrients and energy (Reynolds et al. 1987; Ibelings et al. 1991). Furthermore, the aggregation of algae into large groups is conducive to nutrients storage, preventing animals from feeding and resisting environmental stress (Reynolds 2007). Yang et al. 2010 found that the formation of aggregates coincides with an increase in polysaccharides, with polysaccharides shown to play an important role in the formation of aggregates of C. pyrenoidosa. EPS could affect the viscosity of algal surfaces, thereby promoting cell aggregation (Plude et al. 1991; De Philippis and Vincenzini 1998; Thornton 2002; Yang et al. 2008).
In the present study, the B-EPS content of the five assessed groups ranged between 0.15 and 0.23 pg cell−1 with C. vulgaris colonies appearing in all groups (Fig. 4 and Fig. 5). A previous study reported that the B-EPS content of colonial Microcystis cells was 0.34 pg cell−1(Wu and Song 2008). This indicates that the B-EPS content of different algae capable of colony formation varies significantly. In addition, EPS is negatively charged (Esparza-Soto and Westerhoff 2003) and therefore B-EPS adhered to the surface of algal cells, results in a negative surface charge on algal cells. During the process of algal cell floatation in the water column, a Lorentz force is generated under the magnetic field, which may account for the low percentage of aggregation in groups with high B-EPS contents. In general, the effects of SMF on algal aggregation and the mechanism of algal aggregation require further study.
The trend in T-pro content was opposite to the trend in algal density. The reason for this may be that the concentration of algae was high and the nutrient levels were limited, resulting in algae having to consume nutrients within their own cells to maintain growth. In general, the component diffusion coefficient of water is generally higher than that of EPS, so an increase in B-EPS can affect both the input of nutrients and the output of metabolites (Sheng et al. 2010). In addition, the form of nitrogen in BG11 medium was nitrate nitrogen, with the algal absorption of nitrate nitrogen occurring via a specific permease and reduction NH4+ via a series of reductases, finally allowing synthesizing amino acids and proteins (Vílchez et al. 1997). The magnetic field could have an effect on these enzymes (Wang et al. 2006), which would affect the protein content of algae. Moreover, the results of fluorescence imaging show that in the control group, the green area representing protein was much larger than the group treated with SMF (Fig. 5), indicating that SMF can inhibit protein production in C. vulgaris.
In order to investigate the oxidative damage of C. vulgaris by magnetic field, MDA content and enzyme activity were measured. Oxygen free radicals produced by the organism through non-enzymatic systems can attack polyunsaturated fatty acids (PUFA) in biofilms, triggering lipid peroxidation, and thus forming lipid peroxides such as MDA (Kaur et al. 2006). The results indicate that SMF had no significant effect on the MDA content. Therefore, the magnetic field might not promote or inhibit the production of oxygen free radicals by non-enzymatic systems in algae. In addition, the increase in CAT and POD activities could be caused by the increase in reactive oxygen species production, potentially promoting antioxidant reactions to prevent cell damage (Wang et al. 2008, 2017). Considering the results of MDA content, CAT, and POD activity analysis, it appears that SMF affects the antioxidant response of algae, although the effects on different enzymes are inconsistent. Some previous studies have reported that high magnetic field strengths lead to changes in the conformation of enzymes, which in turn affect cellular biochemical reactions (Wang et al. 2006). Moreover, under the action of SMF, the transition metal ions in some enzymes become paramagnetic, resulting in an increase in enzyme activity (Katz et al. 2005). These results indicated that the response of C. vulgaris to SMF is affected by a certain stress range. The enzyme activity and MDA content were highest in the 400 G group, and therefore, 400 G SMF can be considered to negatively affect C. vulgaris. In contrast, the 800 G SMF exhibited improved resource utilization by C. vulgaris, with the algal density also the highest in this group.
Chlorella vulgaris cells grow and reproduce by utilizing the inorganic components from the BG11 medium. After lysis and decay, algae release organic matter back into the water (Sun et al. 2007), accounting for the increase in TOC concentration in surrounding water. Organic substances can be absorbed by EPS (Hinson and Kocher 1996). During processing of samples, the organic matter adsorbed by B-EPS was filtered out with the algae, resulting in the TOC concentration in water being reduced. The concentration of NO3−-N in the water slightly decreased due to algal growth, while the NO2−-N concentration was higher after the 8th day due to algal cells releasing intracellular NO2− into the water. The TP removal rates of the control group and the 800 G group were both high (85.15% and 84.54%, respectively), as these groups were able to absorb sufficient TP to support growth and reproduction.
Conclusions
In this study, algae treated by 800 G SMF grew at the fastest rate and SMF exerted a significant effect on C. vulgaris biomass. These results show that treatment with a suitable SMF strength could promote C. vulgaris proliferation and biomass formation, although excessive SMF strengths for a long duration inhibit the growth. SMF could affect enzyme activity in the algae, as well as inhibiting EPS secretion and the synthesis of total proteins within cells. According to the change of B-EPS content and the results of sample staining, it may be speculated that SMF can cause EPS to adhere more closely to the surface of algal cells. The B-EPS content of the five groups ranged between 0.15 and 0.23 pg cell−1 and C. vulgaris colonies were formed in all cases. Overall, these results confirm that SMF affects the growth and reproduction of C. vulgaris by affecting the antioxidant response of algal cells.
References
Adav S, Lin J, Yang Z, Whiteley C, Lee D, Peng X, Zhang Z (2010) Stereological assessment of extracellular polymeric substances, exo-enzymes, and specific bacterial strains in bioaggregates using fluorescence experiments. Biotechnol Adv 28:255–280
Chen M, Lee D, Tay J, Show K (2007) Staining extracellular polymeric substances and cells in bioaggregates. Appl Microbiol Biotechnol 75:467–474
Chiou Y, Hsieh M, Yeh H (2010) Effect of algal extracellular polymer substances on UF membrane fouling. Desalination 250:648–652
De Philippis R, Vincenzini M (1998) Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev 22:151–175
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Esparza-Soto M, Westerhoff P (2003) Biosorption of humic and fulvic acids to live activated sludge biomass. Water Res 37:2301–2310
Ghasemi R, Ghaderian SM, Kramer U (2009) Accumulation of nickel in trichomes of a nickel hyperaccumulator plant, alyssum inflatum. Northeast Nat 16:81–92
Granum E, Kirkvold S, Myklestad SM (2002) Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: diel variations and effects of N depletion. Mar Ecol Prog Ser 242:83–94
Guerrini F, Cangini M, Boni L, Trost P, Pistocchi R (2000) Metabolic responses of the diatom Achnanthes brevipes (Bacillariophyceae) to nutrient limitation. J Phycol 36:882–890
Halliwell B, Gutteridge JMC (1992) Comments on review of free-radicals in biology and medicine. Free Radic Biol Med 12:93–95
Han S, Jin W, Chen Y, Tu R, Abomohra AE (2016) Enhancement of lipid production of Chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl Biochem Biotechnol 180:1043–1055
Hinson RK, Kocher WM (1996) Model for effective diffusivities in aerobic biofilms. J Environ Eng 122:1023–1030
Hirano M, Ohta A, Abe K (1998) Magnetic field effects on photosynthesis and growth of the cyanobacterium Spirulina platensis. J Ferment Bioeng 86:313–316
Hsieh K, Murgel G, Lion L, Shuler M (1994) Interactions of microbial biofilms with toxic trace metals: 1. Observation and modeling of cell growth, attachment, and production of extracellular polymer. Biotechnol Bioeng 44:219–231
Ibelings B, Mur L, Walsby A (1991) Diurnal changes in buoyancy and vertical distribution in populations of Microcystis in two shallow lakes. J Plankton Res 13:419–436
Katz E, Lioubashevski O, Willner I (2005) Magnetic field effects on bioelectrocatalytic reactions of surface-confined enzyme systems: enhanced performance of biofuel cells. J Am Chem Soc 127:3979–3988
Kaur G, Alam S, Jabbar Z, Javed K, Athar M (2006) Evaluation of antioxidant activity of Cassia siamea flowers. J Ethnopharmacol 108:340–348
Kessel M, Eloff J (1975) The ultrastructure and development of the colonial sheath of Microcystis marginata. Arch Microbiol 106:209–214
Lancelot C, Mathot S, Owens NJP (1986) Modeling protein synthesis, a step to an accurate estimate of net primary production: Phaeocystis pouchetii colonies in Belgian coastal waters. Mar Ecol Prog Ser 32:193–202
Lee S, Kim S, Kim M, Lim K, Jung Y (2014) The effect of hydraulic characteristics on algal bloom in an artificial seawater canal: a case study in Songdo City, South Korea. Water 6:399–413
Li YG, Gao KS (2004) Photosynthetic physiology and growth as a function of colony size in the cyanobacterium Nostoc sphaeroides. Eur J Phycol 39:9–15
Li ZY, Guo SY, Li L, Cai MY (2007) Effects of electromagnetic field on the batch cultivation and nutritional composition of Spirulina platensis in an air-lift photobioreactor. Bioresour Technol 98:700–705
Li M, Zhu W, Gao L, Lu L (2013) Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates. J Appl Phycol 25:1023–1030
Liang W, Qu J, Chen L (2004) Algal inactivation and removal by pulsed magnetic field with varying frequency. Environ Sci 25:38–42
Liu W (2006) Research on the effect of magnetic field on the growth of algae. Water Purif Technol 25:19–21
Mallmann W, Buswell A, Gilcreas F, McCrady M, Nichols M, Olson T, Parr L, Tripp J (1945) Report of the standard methods committee on examination of water and sewage. Am J Public Health Nations Health 35:957–958
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Matsumoto T, Yamamura H, Hayakawa J, Watanabe Y, Harayama S (2014) Influence of extracellular polysaccharides (EPS) produced by two different green unicellular algae on membrane filtration in an algae-based biofuel production process. Water Sci Technol 69:1919–1925
Newman J, Watson R (1999) Preliminary observations on the control of algal growth by magnetic treatment of water. Hydrobiologia 415:319–322
Nielsen P, Jahn A, Palmgren R (1997) Conceptual model for production and composition of exopolymers in biofilms. Water Sci Technol 36:11–19
Plude J, Parker D, Schommer O, Timmerman R, Hagstrom S, Joers J, Hnasko R (1991) Chemical characterization of polysaccharide from the slime layer of the cyanobacterium Microcystis flos-aquae C3-40. Appl Environ Microbiol 57:1696–1700
Reiter RJ (1998) Melatonin in the context of the reported bioeffects of environmental electromagnetic fields. Bioelectrochem Bioenerg 47:135–142
Reynolds CS (2007) Variability in the provision and function of mucilage in phytoplankton: facultative responses to the environment. Hydrobiologia 578:37–45
Reynolds CS, Oliver RL, Walsby AE (1987) Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. N Z J Mar Freshw Res 21:379–390
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rugnini L, Costa G, Congestri R, Antonaroli S, Sanita di Toppi L, Bruno L (2018) Phosphorus and metal removal combined with lipid production by the green microalga Desmodesmus sp.: an integrated approach. Plant Physiol Biochem 125:45–51
Schenck J (1996) The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys 23:815–850
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1:20–43
Sheng GP, Yu HQ, Li XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv 28:882–894
Small DP, Huner NP, Wan W (2012) Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 33:298–308
Staudt C, Horn H, Hempel D, Neu T (2004) Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol Bioeng 88:585–592
Sun XJ, Qin BQ, Zhu GW (2007) Release of colloidal phosphorus, nitrogen and organic carbon in the course of dying and decomposing of cyanobacteria. Environ Sci 27:341–345
Sutherland IW (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology-UK 147:3–9
Thornton D (2002) Diatom aggregation in the sea: mechanisms and ecological implications. Eur J Phycol 37:149–161
Tu R, Jin W, Xi T, Yang Q, Han SF, Abomohra Ael F (2015) Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res 86:132–138
Vílchez C, Garbayo I, Lobato MV, Vega J (1997) Microalgae-mediated chemicals production and wastes removal. Enzym Microb Technol 20:562–572
Wang HY, Zeng XB, Guo SY (2006) Growth of Chlorella vulgaris under different magnetic treatments. Progr Mod Biomed 6:106–109
Wang HY, Zeng XB, Guo SY, Li ZT (2008) Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagnetics 29:39–46
Wang Z, Zhang J, Li E, Zhang L, Wang X, Song L (2017) Combined toxic effects and mechanisms of microsystin-LR and copper on Vallisneria natans (Lour.) Hara seedlings. J Hazard Mater 328:108–116
Widjaja A, Chien C, Ju Y (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40:13–20
Wu ZX, Song LR (2008) Physiological comparison between colonial and unicellular forms of Microcystis aeruginosa Kutz. (Cyanobacteria). Phycologia 47:98–104
Xu H, Yan Z, Cai H, Yu G, Yang L, Jiang H (2013a) Heterogeneity in metal binding by individual fluorescent components in a eutrophic algae-rich lake. Ecotoxicol Environ Saf 98:266–272
Xu H, Yu G, Jiang H (2013b) Investigation on extracellular polymeric substances from mucilaginous cyanobacterial blooms in eutrophic freshwater lakes. Chemosphere 93:75–81
Yang Z, Kong F, Shi X, Min Z, Cao H (2008) Changes in the morphology and polysaccharide content of Microcystis aeruginosa (cyanobacteria) during flagellate grazing. J Phycol 44:716–720
Yang Z, Liu Y, Ge J, Wang W, Chen Y, Montagnes D (2010) Aggregate formation and polysaccharide content of Chlorella pyrenoidosa Chick (Chlorophyta) in response to simulated nutrient stress. BioresourTechnol 101:8336–8341
Zhang X, Bishop PL (2003) Biodegradability of biofilm extracellular polymeric substances. Chemosphere 50:63–69
Funding
The study was supported by the National Science Foundation of China (51308127) and the Major Science and Technology Program for Water Pollution Control and Treatment (2012ZX07103-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, X., Zhang, H., Li, Q. et al. Effects of static magnetic field on Chlorella vulgaris: growth and extracellular polysaccharide (EPS) production. J Appl Phycol 32, 2819–2828 (2020). https://doi.org/10.1007/s10811-020-02164-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02164-7