Abstract
The main objective of this research was to evaluate the effect of a 47 mT static magnetic field (SMF) applied at different times of the exponential growth phase of a Chlorella microalgae-bacteria consortium. For this, growth parameters, cell division, the photochemical activity of photosystem II (PS II), and the biochemical composition of the microorganisms were studied. Biomass concentration and productivity of cultures exposed to SMF increased concerning control cultures, reaching maximum values when this physical agent was applied in the early exponential phase, 0.89 g L−1 and 0.075 g L−1 d−1, respectively. In addition, SMF application stimulated binary and multiple cell division of cultures exposed during early and late exponential phases. PS II quantum yield was significantly increased over control cultures immediately after applying SMF during early (0.70) and late (0.73) exponential phases. In addition, in cultures exposed to SMF, the quantum yield for electron transport (ϕEo) increased, and the absorption flux per reaction center (ABS/RC) decreased, which was associated with an increase in the active reaction centers of PS II. Extracellular protein, carbohydrate, and polysaccharide content varied when SMF was applied during the early exponential phase. No significant differences were observed regarding the lipid content of the control cultures and those exposed to SMF. It is concluded that SMF increases the formation of radical pairs in photosystem II due to the increase in the number of active reaction centers, which could constitute the mechanism of action of this system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae have a varied chemical composition and great metabolic flexibility, which has allowed the development of a wide range of applications (Yan et al. 2016; Gateau et al. 2017; Su et al. 2017). These applications range from simple biomass production for food and feed to valuable products for green applications and wastewater treatment (Show 2022). Microalgae under specific culture conditions are associated with bacteria, generating cooperative associations between them (Solimeno and García 2017; Yao et al. 2019; Iqbal et al. 2022). The symbiosis between microalgae-heterotrophic bacteria brings significant advantages to cultures since both microorganisms provide the culture medium with essential substances for growth, such as O2 released by microalgae and CO2 released by heterotrophic bacteria (Li et al. 2019a).
In cultures of a microalgae-bacteria consortium under photoautotrophic conditions, the microalgae obtain energy from the sun or some artificial light source and carbon from inorganic compounds (Siqueira et al. 2018). The reactions that are triggered from this type of autotrophic metabolism are part of oxygenic photosynthesis and can be expressed as a redox reaction driven by light energy (harvested by chlorophyll molecules) through photosystems I (PS I) and II (PS II) (Masojıdek et al. 2004). In general, it can be said that the main objective of the light reactions of photosynthesis is to promote the synthesis of adenosine triphosphate (ATP) from adenosine diphosphate ADP molecules and nicotinamide adenine dinucleotide phosphate (NADPH) from NADPH+ molecules (Leister 2022).
Improving the photosynthetic efficiency of microalgae is one of the critical factors in increasing production yields and decreasing the production costs of these cultures. The application of magnetic fields in microalgae cultures has been a strategy followed by several researchers, which has shown its usefulness in increasing the quantum yield of PS II (Deamici et al. 2019a), and the production of photosynthetic oxygen (Small et al. 2012), both parameters associated with the primary photochemistry of microorganisms. Several species of microalgae, such as Chlorella fusca (current name: Desmodesmus abundans), Nannochloropsis oculata and Spirulina platensis (current name: Arthrospira platensis), have been studied under the action of a static magnetic field (SMF) (Shao et al. 2018; Chu et al. 2020; Deamici et al. 2021). The results obtained from these previous studies have demonstrated the efficacy of SMF in increasing biomass productivity and the content of metabolites of industrial interest in these microalgae cultures.
Despite the advantages of SMF in microalgae cultures, there are still some issues to be resolved regarding this physical agent. One of the most critical aspects that have limited the development of this technology is that the action mechanisms proposed to explain the effects found need more scientific evidence to support them (Santos et al. 2017). On the other hand, the influence of SMF on the photosynthetic apparatus of microalgae in consortia with bacteria has been little discussed in the literature consulted. The SMF can affect the development of bacterial communities positively or negatively, noting that these effects will be associated with the particularity of each species' growth or the organisms' culture conditions (Zhao et al. 2020; Zieliński et al. 2021b). This behavior of bacteria exposed to SMF during their growth in consortia with microalgae could directly affect the development of the photosynthetic microorganisms. Therefore, this study aims to evaluate the effect of SMF on the photosynthetic apparatus and its relationship with the cell division and the biochemical composition of the biomass of a microalgae-bacteria consortium.
Materials and methods
Microalgae strain and culture conditions
A consortium of Cuban native microalgae-bacteria was used, where Chlorella sp. predominates. The strain was isolated from the aquaculture station of Maffo, Contramaestre, Cuba, in 2002 and is kept in the stock of the Ecotoxicology and Environmental Services Laboratory (LESA) of the National Center of Applied Electromagnetism (CNEA), Cuba. For the development of cultures, the modified Bristol culture medium was used, which contains (g L−1): NaNO3 (1.000), CaCl2.2H2O (0.025), MgSO4.7H2O (0.075), K2HPO4 (0.075), KH2PO4 (0.175), NaCl (0.250). In addition, 3 mL of a solution containing the following trace elements was added: EDTA (39.80%), Fe (10.20%), Zn (0.53%), Mn (0.44%), Mo (0.56%), Co (0.46%), Cu (0.49%), Thiamin (0.162%), Biotin (0.006%), Cyanocobalamin (0.008%), Excipients (0.390%) (Silveira-Font et al. 2018).
To evaluate the morphology of the microalgae-bacteria consortium, a scanning electron microscope was used, coupled to an energy dispersive X-ray spectroscopy equipment (SEM–EDX) (Phenom, The Netherlands) at acceleration voltages of 15 kV. Freeze-dried microalgae-bacteria consortium samples were mixed with gold particles to improve resolution and then they were observed and photographed under the microscope.
The cultures were grown in triplicate cylindrical glass flasks with a capacity of 1 L, a diameter of 9 cm. The temperature conditions in the culture lab were stable at 22 ± 2.3 °C. An LED light panel was used to keep the cultures illuminated under continuous light. The light intensity was adjusted daily with a Traceable Light Meter, maintaining the cultures with an illumination of 58 µmol photons m−2 s−1. Cultures were aerated by bubbling filtered air through 0.20 µm glass microfiber syringe filters at an airflow capacity of 9.2 L min−1.
Protocol for the application of SMF
The cultures were exposed to a non-homogeneous SMF (frequency 0) with a mean value of 47 mT and a variation gradient of 0.003 and 0.002 mT. The magnetic device was built with rectangular ferrite magnets and characterized at CNEA, Santiago de Cuba, Cuba. The glass bottles with samples were placed in the center of the magnetic device to expose the cultures to the SMF, guaranteeing the same intensity of light and aeration as the control cultures. The intensity of the SMF applied to the cultures was chosen following the results obtained in previous research (Silveira-Font et al. 2018). In addition, this chosen intensity of SMF coincides with the range of intensities reported in the literature, where stimulator effects of SMF on microalgae have been observed (Silveira-Font et al. 2018; Deamici et al. 2019b; Chu et al. 2020; Font et al. 2023).
Two schemes of exposure of the cultures to SMF at different moments of the exponential growth phase were evaluated. The first protocol was one hour of consortium exposure to SMF during the early exponential phase (EP early, day 3). The second was one hour of exposure to SMF during the late exponential phase (EP late, day 7). A control culture was maintained in all experiments performed (no exposure to SMF). Control cultures were only exposed to the geomagnetic field under the same experimental conditions of temperature, lighting, and aeration as the study cultures. The exposure time of the microorganisms to the SMF was chosen, taking into account previous research group results and references from the literature (Bauer et al. 2017; Deamici et al. 2019b, 2021; Font et al. 2023).
Evaluation of the growth of the Chlorella microalgae-bacteria consortium
The consortium growth was monitored daily for ten days, using a calibration curve of dry weight against optical density at 750 nm. The dry weight for the curve was made by filtering 10 mL of samples on microfiber filters (Sartorius Stedim Biotech, Belgium) using the method described by Moheimani et al. (2013). The optical absorption of the samples was correlated with the concentration by dry weight (X, g L−1) measured gravimetrically. The samples under study were measured by taking 3 mL of culture and measuring the optical density at 750 nm. With the values obtained from the X calculation, the biomass productivity (P, g L−1 day−1) was calculated (Eq. 1). The specific growth rate (μ, day−1), and the doubling time (Tg, d) were calculated, following Eqs. 2 and 3. To evaluate the immediate effect of SMF on μ of microorganisms, for the calculation of this parameter, only the biomass concentration data of days 3, 4, 5, and 6 were taken for the cultures exposed to SMF during the EP early and days 6, 7, 8, and 9 for the cultures exposed during EP late.
where Xt and X0 are the biomass concentrations (CDW, g L−1) at times tt and t0, respectively, and t0 and tt are the first and last day of the cell growth.
where Xt and X0 are the biomass concentrations at times tt and t0, respectively.
where Tg is the doubling time and µ is the specific growth rate.
Cell division study of microalgae
A study of the cell division after applying SMF was developed by counting single cells, cells in the binary division, and cells in multiple divisions (Fig. 1). The cell count was performed at 0, 0.5, 1, 1.5, 24, and 48 h after the application of SMF, for the control and SMF exposed cultures. The cell count times were chosen to evaluate the evolution and permanence of the effects of the SMF on the microalgae during the first hours and days after its application. An aliquot of 1 mL of sample was used for counting with an heocytometer (Neubauer).
Analysis of the fluorescence parameters of chlorophyll a
The effect of SMF on quantum yield (QY) and transient fluorescence (OJIP test) was studied daily for control cultures and cultures exposed to this physical agent at different time points during growth. An analysis was also carried out on the cells before and immediately after the application of the SMF. An AquaPen-C fluorimeter (Photon Systems Instruments, Czech Republic) was used. Culture samples were diluted whenever necessary until reaching an optical density at 680 nm of less than 0.1. For the analysis of the quantum yield of PS II (QY) and the transient fluorescence test (OJIP), 3 mL of samples were taken and and dark adapted for 5 min. All measurements were performed in triplicate.
Quantum Yield (QY) and Transient fluorescence (OJIP test)
The QY is a parameter of Chl fluorescence, which is used to measure PS II's efficiency. QY is equivalent to Fv/Fm, where Fv corresponds to the variable maximum fluorescence and Fm corresponds to the maximum fluorescence intensity (Du et al. 2018). The typical fluorescence increase in dark-adapted oxygenic microorganisms after illumination with high-intensity actinic light shows four phases: O, J, I, and P phases (Stirbet 2011). The O (F0) phase is the initial fluorescence obtained at 30 μs. Then there are two intermediate steps, phases J and I (FJ and FI, respectively), obtained at 2 and 60 ms, respectively, before reaching the maximum recorded fluorescence, phase P (FM), between 250–400 ms.
Fluorescence transients were recorded up to 1000 ms with data acquisition every 10 μs for the first 2 ms and at 1 ms intervals after that. The terms and formulas for the OJIP test parameters are shown in Table 1 (Strasser et al. 2004). The OJIP test was performed on intact cells previously incubated in the dark for 5 min. All analyses were developed in triplicate.
Biochemical characterization of biomass
Photosynthetic pigments, proteins, carbohydrates, and lipids, were determined following the protocols described by Moheimani et al. (2013). During the ten days of culture, 10 mL of each sample was filtered under a vacuum with 0.45 µm microfiber filters,washed with Milli Q water and frozen at -20 °C. Before performing each analysis, the samples were thawed, and 2 mL of liquid nitrogen was added to facilitate cell disruption.
Photosynthetic pigments were determined by using 90% acetone as an extraction solvent. For calculations of the contents of chlorophylls a, b (µg mL−1), the equations developed by Ritchie (2008) were used. Protein determination was performed using the Lowry method, and carbohydrate determination was performed using the phenol–sulfuric colorimetric method (2013). Lipid determination was performed according to the Folch method using methanol and chloroform as extraction solvents (2013).
Data processing and statistical analysis
Fisher's least significant difference (LSD) procedure was used to perform multiple comparisons of means with a significance level of p ≤ 0.05. The t-test was also used, with a confidence level of 95%, to compare the samples before and immediately after the application of SMF.
Results and discussion
Growth kinetics of cultures exposed to SMF, at different times of exponential growth
Microscopic characterization of the culture showed that the consortium was composed of bacillary-shaped bacteria, which generally develop a chemoheterotrophic metabolism (Fig. 2). At the same time, most of the consortium was composed of unicellular green microalgae with a spherical shape, which appeared isolated or forming aggregates. From the morphological analysis of the microalga, the species was identified following the dichotomous keys of Whitford and Schumacher (Palmer 1959). The morphological identification showed that the species that predominated in the microalgae-bacteria consortium was Chlorella sp.
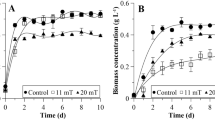
Figure 3 shows the growth kinetics of the consortium exposed to an SMF of 47 mT applied at different times during the exponential phase. The lag phase had a short duration of one day for all cultures, Indicating that they were adapted to the conditions of the experimental culture. From day 3, the exponential growth phase began until day 7. Around the eighth day of culture, the linear growth or slowdown phase began, in which cells started to shade each other, limiting access to light. In cultures exposed to SMF during EP early, an increase in biomass concentration of 0.32 to 0.82 g L−1 was observed from day 4 until day 10. In cultures exposed to SMF during EP late, biomass concentration increased from 0.59 to 0.75 g L−1 from day 8 until day 10. The exposure of microorganisms to SMF stimulated cell growth, regardless of the growth phase where it was applied. However, in this study, it was observed that the exposure of cells to SMF during EP early was more efficient than during EP late.
Chlorella is characterized by a rapid growth rate and short generation times (Ji et al. 2019). In consortia with bacteria, both microorganisms perform cooperative interactions. Microalgae supply organic compounds released during growth and with O2 required for the oxidation of organic matter (Wang et al. 2023). At the same time, the bacteria provide the microalgae the CO2 needed for photosynthesis, organic growth factors, and vitamins that significantly improve microalgae growth compared to individual cultures (Gonçalves et al. 2016). Applying the SMF in this type of culture could stimulate the development of both microorganisms. For example, by increasing the cellular growth of microalgae, it increases the O2 content expelled to the medium (Small et al. 2012), which bacteria use for their metabolism. As for bacteria, the influence of the SMF on these microorganisms has also been reported (Konopacki and Rakoczy 2019; Raouia et al. 2020; Zieliński et al. 2021a, b). The increase in the bacterial community of cultures could increase the dissolved inorganic carbon content, which the algae can use as a carbon source during photosynthesis.
The effect of SMF on the maximum biomass concentration, productivity, growth rate, and doubling time of cultures is shown in Table 2. The maximum biomass concentration and the productivity of the cultures exposed to SMF increased significantly compared to the control cultures. The application of SMF during EP early was also the best protocol regarding maximum biomass concentration and productivity, reaching values of 0.89 g L−1 and 0.075 g L−1 day−1, respectively. Similar results were obtained by various authors where an increase in biomass concentration (Xmax) and productivity (P) was observed concerning control cultures when SMF was applied (Deamici et al. 2016; Shao et al. 2018; Costa et al. 2020; Luo et al. 2020).
Regarding the SMF exposure time, various authors have already studied it widely it. It was observed that both the permanent exposure to SMF during the 15 days of culture and the exposure of 1 h during the 15 days of culture could stimulate cell growth. Deamici et al. (2016) observed in their application studies of different SMF intensities (30 mT and 60 mT) and different exposure times (1 h day−1 and 24 h day−1) that the 60 mT 24 h day−1 scheme led to an increase with Xmax reaching 1.94 g L−1, which was significantly different concerning control cultures and cultures exposed to other SMF application schemes. The application of 60 mT (24 h day−1) on Spirulina sp. also increased Xmax compared to the control cultures and the other treatments (Veiga et al. 2020). Our study demonstrated that SMF applied during EP early affected cell growth, and this effect was sustained over time.
The growth rate and the duplication time are mutually dependent parameters that characterize the cell duplication of microorganisms during the exponential growth phase. The application of SMF during EP early and EP late increased the growth rate of microorganisms, thus decreasing doubling times (Tg). These data show the stimulating effect on cell growth of the application of this physical agent, regardless of the growth phase where it is applied, being more effective during EP early. Various authors have obtained similar results. It has been observed that the application of SMF could decrease the Tg of the cultures depending on the induction of SMF applied and the exposure time (Deamici et al. 2016). Secondly, some authors have described that the changes in the counts of Chlorella pyrenoidosa could be attributed to the influence of magnetic fields on the biological functions of the microorganism due to changes in hormones and the activity of some enzymes, the membrane transport of ions or DNA transcription (Han et al. 2016).
Study of the cell division of microalgae under the effect of SMF
Microalgae display various reproductive patterns. Microalgae reproduce all cell structures through the cell cycle to produce one or several daughter cells (Zachleder et al. 2016). Organisms create a new cell through steps related to growth, DNA replication, nuclear division, and cell division (Borowitzka 2016). In this research, the effect of SMF on the cell division of microalgae was evaluated by counting single cells, cells in the binary division, and cells in multiple division.
First of all, it is essential to highlight that two patterns of cell division were observed in the consortium of microalgae-bacteria studied (Fig. 4). During the first days of culture, cell division was by binary fission and multiple fission. However, only the binary division reproductive pattern was observed from day 7. The presence of one or several reproductive patterns is related to the growth conditions or the phase of the life cycle where the organisms are found (Zachleder et al. 2016). Considering the previous consideration, the microorganisms were well adapted to the experimental culture conditions in the first stage of growth. However, around day 7, cell division could be limited due to the light limitation due to increased biomass concentration. Bišová and Zachleder (2014) explained that light is one of the main factors affecting cell division in photosynthetic microorganisms. This means that to the extent that microorganisms are efficient in capturing photosynthetic radiation, the most efficient cell division will be, developing cells where cell division is multiple.
Evaluation of the content of single cells, cells in binary division and cells in multiple division of cultures exposed to SMF during early exponential phase (EP early, Fig a) and late exponential phase (EP late, Fig b); at different times after the application of the SMF (0, 0.5, 1, 1.5, 24 and 48 h). The error bars represent the standard deviation (n = 3). Different letters represent significant differences (LSD Test, P ≤ 0.05) between control cultures and SMF-treated cultures in the same time period
In our research with the application of SMF during EP early, the concentration of cells in binary division (13.5 × 104 cells mL−1) and in multiple division (19.5 × 104 cells mL−1) increased with significant differences (P ≤ 0.05) compared to control cultures. This increase in mitotic cells was observed from 0.5 h after the application of SMF. It was sustained over time, which contributed to the increase in the concentration of single cells from 24 h after the application of the SMF. However, in cultures exposed to SMF during EP late, the increased concentration of binary dividing cells was observed starting 1 h after SMF exposure. In general, it is known that the formation of cells in binary or multiple fission is directly proportional to the increase in DNA replication-division sequences (Bišová and Zachleder 2014). In addition, the number of daughter cells formed from the same mother cell will increase with increasing light intensity and lengthening of the light period (Zachleder et al. 2021).
Evaluation of the effect of SMF on photosystem II of the photosynthetic organisms present in the microalgae-bacteria consortium
Based on the results obtained related to the cell division and its direct relationship with the light phase of photosynthesis, it was decided to evaluate the effect of SMF on photosystem II of microorganisms, which has a close relationship with the efficiency of the processes dependent on the light during photosynthesis.
We evaluated the effect of an SMF on QY before and immediately after SMF application and the kinetics of QY during ten days of culture (Fig. 5). The QY increased significantly (p ≤ 0.05) immediately after SMF application, for the cultures exposed during EP early (day 3) and the cultures exposed during EP late (day 7). It was also observed that under control conditions, QY tends to increase daily until day 8 and then stabilizing or decreasing, clearly highlighting the adaptation of microorganisms to culture conditions. However, in the cultures exposed to SMF, the stimulus immediately increased the QY, an effect that was maintained over time. Similar results were obtained by Luo et al. (2021) who applied a 40 mT SMF on a Chlorella microalgae-bacteria consortium. These researchers observed that from day 4 of the culture, the quantum yield of PS II increased concerning the control cultures, which coincided with the increase in biomass concentration.
When the fluorescence of chlorophyll is plotted against the logarithm of time, its kinetics is obtained, which presents four inflections named OJIP. The letter O corresponds to the minimum value of fluorescence (Fo) and is when all the reaction centers are oxidized or “open.” J is related to the partial reduction of QA (quinone A). In contrast, I is related to the partial reduction of QA and QB (quinone A and B), and P is the maximum value of fluorescence (Fm), where all the reaction centers are reduced or “closed” (Stirbet 2011). These inflections provide information on the photochemical activity of PSII, and reflect the different reduction processes of the electron transport chain (Li et al. 2019b).
Figure 6 shows the OJIP curves obtained for the cultures exposed to SMF on day 3 and day 7, before and immediately after treatment. The variation of the inflections of the OJIP curves is generally associated with effects on the photosynthetic apparatus due to the stress induced by high light (Solovchenko et al. 2013; Kula et al. 2017), stress generated by high concentrations of potentially toxic substances such as lead and sodium hypochlorite (Dao and Beardall 2016; Li et al. 2022), or by the limitation of some nutrients such as nitrogen (Plyusnina et al. 2020).
The results did not show changes in inflections of the OJIP curves, so the application of the SMF, in addition to improving photosynthetic efficiency, did not cause any stress on the reduction process of PSII. However, SMF treatment increased the fluorescence emitted by Chl a in all the phases of the OJIP assay studied, being more significant in those cultures where the SMF was applied for 1 h on day 3 (Fig. 3.2 A). This could be related to the fact that during EP early (day 3), the stimulus generated by the SMF is more efficiently used by the cells compared to the cells in EP late, probably due to the greater light availability of these microorganisms because of the low biomass concentration at that time.
To complete this research stage, the parameters related to the quantum efficiencies and the specific energy flows per reaction center were estimated. These parameters allowed for characterizing the photochemical activity of PSII under exposure to SMF (Table 3).
Mo characterizes the net rate of RC closure during illumination and is associated with variations in the electron transport chain (ETO/RC) or the capture flux per RC (TRO/RC). As shown in Table 3, no variations were observed for Mo. Neither were changes observed with significant differences for the parameter that relates the probability that a trapped exciton moves an electron to the electronic chain beyond QA (ψo). However, the maximum quantum yield for primary photochemistry (ϕPo) increased significantly (p ≤ 0.05), from 0.67 to 0.70 for cultures exposed to SMF during EP early and from 0.70 to 0.73 for cultures exposed to SMF during EP late. At the same time, the electron transport quantum yield (ϕEo) and energy dissipation quantum yield (ϕDo) increased significantly compared to cultures before SMF application. Specifically, ϕEo refers to the probability that an absorbed photon will move an electron into the electron transport chain. Considering the above, we could say that SMF increases the efficiency of electron transport towards the RC, for cultures exposed during EP early and those exposed during EP late.
The absorption flux per reaction center (ABS/RC) characterizes the size of the light-gathering antenna of the photosynthetic apparatus and an increase is generally associated with damage to the photosynthetic apparatus due to external stress factors (Markou et al. 2017). These stressors of the photosynthetic apparatus decrease the number of active RCs capable of absorbing photons through Chl molecules (Singh et al. 2022). The ABS/RC ratio of cultures exposed to SMF during EP early decreased significantly from 3.44 to 3.30 for cultures before SMF application and after 1 h of SMF exposure, respectively. These results suggest that early application of SMF (EP early, Day 3) increases the amount of active RC capable of absorbing photons, which could be associated with increased RC synthesis. However, although a decreasing trend of ABS/CR was observed in cultures exposed during EP late, it was not statistically significant. According to these results, applying SMF during EP early is more efficient than that used during EP late. This behavior could be because, during EP early, cells are in better metabolic conditions to carry out light-dependent reactions during photosynthesis. In contrast, the exposure of cultures to SMF in EP late (Day 7), cells become light-limited due to increased microalgae-bacteria biomass concentration, leading to the decreased photosynthetic capacity of microorganisms.
The flow of energy trapped by CR (TRO/RC) and the flow of electron transport by CR (ETO/RC) characterize the flow of energy generated towards the RC (Markou et al. 2017). No changes were observed, with significant differences in these parameters between the cultures exposed to SMF and the control cultures. However, the dissipated energy flux per reaction center (DIO/RC) decreased significantly after applying SMF, both for the cultures exposed during EP early and late. During the first stage of photosynthesis, the chlorophylls that make up the antenna complexes are excited. Then their excited electrons are transferred to the reaction centers, which leads to photochemistry (Stirbet 2011). However, another part of the absorbed light energy is dissipated through heat and emits fluorescence. The (DIO/RC) is closely related to this dissipation process of the energy absorbed by the chlorophyll. The results obtained showed that the application of SMF increases the efficiency of the energy flow absorbed by the reaction center, thus decreasing the (DIO/RC).
Similar results were reported by Deamici et al. (2019a). They observed that applying 1 h and 24 h per day of a 30 mT SMF increased the maximum quantum yield for primary photochemistry (ϕPo) of the cyanobacterium A. platensis. In addition, this increase in the photosynthetic efficiency of microorganisms was also associated with an improvement in electron transfer to the RCs and a decrease in ABS/RC. However, in our research, in contrast to the study of Deamici et al. (2019a), it was observed that applying only 1 h during the EP early improves the photosynthetic efficiency of the cultures in a sustained manner over time. This result is vital since it facilitates the introduction of magnetic technology in industrial microalgae culture systems. On the other hand, although the stimulator effects of SMF on bacterial growth are known (Zhao et al. 2020; Zieliński et al. 2021b), the results obtained related to the increase in the quantum yield of PS II of microalgae are not directly related to the bacterial presence in the consortium, since these microorganisms, according to the characterization carried out, are heterotrophic, so they do not carry out photosynthesis (Eiler et al. 2006).
Biochemical characterization of the consortium under the effect of SMF
In microalgae, the cell composition will depend on the growth phase of the cultures and any stimulus or stress generated by variations in culture conditions (Ho et al. 2003). One of the main biomolecules in photosynthetic organisms are chlorophylls. Chl a is considered one of the most important due to its role as a light-harvesting pigment or as a redox participant in the capture events of electronic excitation and electron transport in the PSII and I in RCs of the oxygenic organisms (Björn et al. 2009). On the other hand, Chl b is an accessory pigment that absorbs radiation at different wavelengths than Chl a, so its primary function is to increase the radiation absorption capacity of Chl a (Pareek et al. 2017).
In cultures exposed to SMF and control cultures, Chl a concentration increased with the increase of the day of culture (Fig. 7). It was also shown that when the SMF was applied during EP early, the Chl a concentration increased with significant differences from day 7; while in cultures exposed to SMF during the EP late, this increase in Chl a concentration was only observed on day 10. This increase in chlorophyll content was expected since cultures increased their biomass concentration under SMF treatment and hence require a more chlorophyll molecules for capturing light energy to carry out photosynthesis. On the other hand, the concentration of Chl b in the cultures also increased with the increase in the days in the control cultures and the cultures exposed to SMF. No differences were observed between the Chl b concentration of the control cultures and the cultures exposed to SMF, until day 10, where the concentration increased significantly (P ≤ 0.05) between the cultures treated with the magnetic field and the control cultures.
Chlorophyll a concentration (a) and chlorophyll b concentration (b) of cultures exposed to SMF or not. Chlorophyll a content per weight of biomass (c) and chlorophyll b content per weight of biomass (d). (EP early) were the cultures exposed to SMF during the early exponential phase, and (EP late) were the cultures exposed to SMF during the late exponential phase, and (control) were the cultures without any SMF treatment. The error bars represent the standard deviation (n = 3)
When evaluating the concentration of Chl a and b concerning the biomass concentration of the cultures, no significant differences were observed between the control cultures and the cultures exposed to SMF indicating that the concentration of chlorophyll a and b is directly proportional to the biomass concentration and is not directly influenced by the SMF. This confirms that the stimulator effects of SMF is not directly correlated with the concentration of photosynthetic pigments in cells. These effects of the SMF will be given by the efficiency in which the Chl molecules absorb light, increasing the maximum quantum yield for primary photochemistry (ϕPo), due to the increase in the efficiency of electron transport towards the RC, increasing the number of active RCs and decreasing the flow of energy dissipated by RC (DIO/RC).
Figure 8a compares the protein content of control cultures and those exposed to SMF during EP early and EP late at different times during growth. In general, the protein content increased with increasing culture days. When SMF was applied during EP early, an significant increase (P ≤ 0.05) in protein content was observed in cultures immediately after SMF application (26.44% w/w) compared with the same culture before SMF application (17.41% w/w) (Fig. 8b). This increase in protein content in cultures exposed to SMF during EP early was maintained until around day 8 of culture. This behavior could be considered evidence of the permanence of the effects of SMF on microorganisms after exposure to this physical agent. On the other hand, the increase in the protein content of the samples could be associated with a metabolic response of the microorganisms when exposed to the magnetic field. Khorshidi et al. (2022), in their study of the microalgae Haematococcus lacustris, observed that the application of SMF of 2 and 4 mT increased the content of hydrogen peroxide (H2O2), inducing the cells to oxidative stress. It also increased the content of astaxanthin (pigment with strong antioxidant activity) and the activity of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), as a possible response to stress induced by the magnetic field.
Evaluation of the protein content per weight of biomass at different moments of cell growth (a), where (EP early) were the cultures exposed to SMF during the early exponential phase, and (EP late) were the cultures exposed to SMF during the late exponential phase, and (control) were the cultures without any SMF treatment. Evaluation of the protein content per weight of biomass of cultures before (BMT) and immediately after (AMT) the application of the SMF (b). The error bars represent the standard deviation (n = 3). Different letters represent significant differences (LSD Test, P ≤ 0.05) between control cultures and SMF-treated cultures in the same time period
On the other hand, no statistically significant changes were observed immediately after the application of SMF during EP late. However, around day 10, there was a statistically significant increase (P ≤ 0.05) in protein content (59.57% w/w) compared to control cultures (53.77% w/w). Although SMF stimulates protein biosynthesis in cells, applying this physical agent during EP early evidently has a more marked effect than when applied during EP late.
Proteins in cells are part of almost all life processes fulfilling specific functions such as maintaining cell integrity, defense against external stress, damage repair, and regulation of metabolic processes (Khorshidi et al. 2022; Gautam et al. 2023). Although we did not evaluate the protein profile at each stage of growth, the increase in protein could be related to the metabolic processes triggered by the improvement in the photosynthetic efficiency of photosynthetic organisms, or as mentioned before, a response to the oxidative stress generated by the SMF in microorganisms (Khorshidi et al. 2022). However, the possible variation in the protein content of the bacterial community present in the consortium is not ruled out, considering that these microorganisms are also influenced by exposure to SMF (Luo et al. 2021).
Carbohydrates are synthesized intracellularly and constitute one of the most significant compounds formed from photosynthesis (Levasseur et al. 2020). Through complex metabolic pathways, microalgae and cyanobacteria can synthesize intracellular monosaccharides, storage polymeric glucans, and structurally complex extracellular polysaccharides (EPS) (Rossi and De Philippis 2016). Carbohydrates various functions, such as energy reserves, cell wall structure, and cell communication. We evaluated the carbohydrate content in cells and the EPS content in the culture medium. In general, it was observed that the carbohydrate content in cells decreased with the increase in culture days (Figs. 9a and 10a). On day 7, a statistically significant decrease (P ≤ 0.05) was observed during EP early (10.37% w/w) compared to control cultures (24.28% w/w). In addition, on day 7 a statistically significant increase (P ≤ 0.05) was also observed in the EPS content in those cultures where the SMF was applied during EP early (0.034 mg mL−1) compared to control cultures (0.025 mg mL−1). It is known that light intensity is positively correlated with carbohydrate synthesis (Rossi and De Philippis 2016) and therefore, the decrease in carbohydrates in cells during culture may be due to increased biomass concentration since cells begin to darken, being more noticeable during EP early. At the same time, the increase in the EPS concentration could be a response of the cells to the limiting light conditions, as it is known that one of the functions of EPS is the production of a physical barrier against harmful agents or environmental restrictions (Suresh Kumar et al. 2007). These results are in agreement with Luo et al. (2021) in their study with a Chlorella microalga-bacteria consortium. They observed that a 40 mT SMF increased the EPS content of the cultures, which was associated with a possible response of microalgae to nutrient limitation due to cell growth. On the other hand, no significant changes were observed by us in the carbohydrate content per biomass weight and the EPS content in solution (Figs. 9b and 10b, respectively) immediately after the application of the SMF. This suggests that the changes observed during days 7 and 8 of cultures could be associated with a metabolic response of microorganisms to the change in culture conditions due to the increase in biomass concentration and not directly to the application of SMF.
Evaluation of carbohydrate content per weight of biomass at different moments of cell growth (Figure a), where (EP early) were the cultures exposed to SMF during the early exponential phase, and (EP late) were the cultures exposed to SMF during the late exponential phase, and (control) were the cultures without any SMF treatment. Evaluation of the carbohydrate per weight of biomass of cultures before (BMT) and immediately after (AMT) the application of the SMF (Figure b). The error bars represent the standard deviation (n = 3). Different letters represent significant differences (LSD Test, P ≤ 0.05) between control cultures and SMF-treated cultures in the same time period
Evaluation carbohydrate concentration at different moments of cell growth (a), where (EP early) were the cultures exposed to SMF during the early exponential phase, and (EP late) were the cultures exposed to SMF during the late exponential phase, and (control) were the cultures without any SMF treatment. Evaluation of the carbohydrate concentration of cultures before (BMT) and immediately after (AMT) the application of the SMF (b). The error bars represent the standard deviation (n = 3). Different letters represent significant differences (LSD Test, P ≤ 0.05) between control cultures and SMF-treated cultures in the same time period
Microalgae can accumulate significant amounts of lipids, which is why they are considered promising candidates for the industrial production of biodiesel (Hess et al. 2018; Sajjadi et al. 2018). Increased lipid content is due to changes in culture conditions such as temperature, irradiance, and nutrient availability (Bellou et al. 2014). Figure 11a, compares the lipid content of control cultures and those exposed to SMF during EP early and EP late at different times during growth. In general, the lipid content increased over time. No significant differences were observed immediately after the application of the SMF in the cultures exposed during EP early nor EP late compared to the same culture before the application of the SMF (Fig. 11b). The maximum lipid concentration was reached on day 10 in both control cultures (23.31% w/w) and cultures exposed to SMF during EP early (23.91% w/w) and EP late (22.67% w /w). From the physiological point of view, the increase in lipid content in all culture conditions could be due to a response of the cells to the deterioration of the culture conditions over time.
Evaluation of the lipid content per weight of biomass at different moments of cell growth (a), where (EP early) were the cultures exposed to SMF during the early exponential phase, and (EP late) were the cultures exposed to SMF during the late exponential phase, and (control) were the cultures without any SMF treatment. Evaluation of the lipid content per weight of biomass of cultures before (BMT) and immediately after (AMT) the application of the SMF (b). The error bars represent the standard deviation (n = 3). Different letters represent significant differences (LSD Test, P ≤ 0.05) between control cultures and SMF-treated cultures in the same time period
General discussion: The formation of radical pairs
The evaluation of SMF effects in microalgae cultures has been extensively studied (Brailo et al. 2018; Shao et al. 2018; Deamici et al. 2021). However, until now, no consensus has been reached regarding the mechanisms through which SMFs act on microalgae. The main proposed mechanisms are related to magnetic induction, the magneto-mechanical effect, and the formation of radical pairs (Santos et al. 2017; Font et al. 2023). Specifically, the formation of radical pairs is directly related to chlorophyll molecules in plant cells, which are candidate molecules considered magnetoreceptors (Hore and Mouritsen 2016). In this mechanism, the external magnetic field could drive the electrons from the singlet state to the triplet state, leading to changes in the products of metabolic reactions generated in cells (Wong et al. 2021).
Our study demonstrated that the SMF acts directly on the photosynthetic apparatus of microorganisms, specifically on PS II. In Fig. 12, the primary photochemistry of microalgae under the effect of SMF is illustrated in a simplified diagram. For a better understanding of this figure, the main photochemical processes triggered during the light phase of photosynthesis are detailed below.
Simplified diagram of the primary photochemistry of microalgae under the effect of SMF. Photochemical parameters evaluated: Quantum yield for electron transport (ϕEo), Absorption flux per reaction center (ABS/RC), and quantum yield of the PS II (ϕPo).The parameters marked in red are positively influenced by the SMF
During oxygenic photosynthesis, the chloroplasts of the cells capture light through the light-harvesting complex (LHC) present in PS I and PSII embedded in the thylakoid membranes (Junge 2019). These LHCs comprise chlorophylls a, b, bound to some proteins and carotenoids (Croce and van Amerongen 2020). Light collected at the LHC is transferred by inductive resonance to RC (Melkozernov et al. 1998). Each photosystem has one or several RCs with a special Chl a molecule inside. The RC core is defined as the minimal set of subunits required to oxidize water (Vinyard et al. 2013). PS I has a Chl molecule that absorbs at wavelengths of 700 nm (P700), while PS II has a Chl molecule that absorbs at wavelengths of 680 nm. During the first stage of photosynthesis, PS II is excited by the absorption of a photon of light energy by the special chlorophyll P 680 (Mamedov et al. 2015). This process releases an electron to the photosynthetic electron transport chain. At the same time, the oxidized form of chlorophyll (P 680+) is temporarily generated from the RC. SMF applied to the cultures during this stage of photosynthesis increases the quantum yield for electron transport (ϕEo), a photochemical parameter associated with the probability that an absorbed photon will move an electron into the electron transport chain (Table 3). With this increase in electron transport performance, cells are forced to divert their normal metabolism towards producing new RCs, to allow them to cope with the improvement in electron transport efficiency. This behavior leads to decreased absorption and flux per reaction center (ABS/RC), a parameter inversely proportional to the number of active reaction centers in the cells. This process could be accompanied by increased protein concentration in the cells immediately after applying SMF.
The special chlorophyll in the reaction center generated after light excitation (P 680+) reduces in a short period (picoseconds) the pheophytin molecule (Phe), forming the radical pair P680•+ and Phe•− (Barber 2003; Mamedov et al. 2015). The radical Phe•− reduces a plastoquinone molecule bound to the protein quinone A (QA), beginning an electron transfer process between photosystem I and II (PS I and PS II) where quinone (QB) also intervenes through the electron transport chain (Mamedov et al. 2015; Shen 2015). The energy released is then used to synthesize ATP. At this point, by increasing the flow of electron transport in the RC, the SMF makes the cells synthesize new RCs due to the stimulus received. These new RCs promote an increase in the concentration of P680•+ and Phe•− radical pairs since each new RC formed will contribute new pairs of radicals, which will later be used to reduce QA in the electron transport chain. These radical pairs increase the overall efficiency of the primary photochemistry of PS II (ϕPo) and photosynthesis, improving cell division through increasing cells in binary and multiple division (Fig. 3). These results constitute a novelty of this research since it was possible to relate the relationship between the photochemical parameters studied with one of the previously explained mechanisms related to forming radical pairs.
With the results obtained in this research related to improving the photosynthetic efficiency of microalgae, the permanence over time of the effects observed in microorganisms after exposure to SMF could also be explained. The presence of new RC and radical pairs in the cells generated by exposure to SMF allows the increase in the quantum yield of the organisms and the biomass concentration to be maintained over time until culture conditions change due to nutrient limitation or light. With the magnetic treatment of the inoculum on a laboratory scale, the increase in the photosynthetic efficiency of the microorganisms could be guaranteed to be later inoculated in the industrial photobioreactors without incurring the expenses associated with the scaling of the technology.
Conclusions
The application of SMF of 47 mT increased the biomass concentration and the productivity of the Chlorella microalgae-bacteria consortium, being more significant and sustained when applied during EP early. SMF improved the cell division of microorganisms, increasing the concentration of cells in the binary division and multiple division for cultures exposed during EP early; and cells in the binary division for cultures exposed during EP late. The characterization of the photochemical activity of PSII showed that SMF increases the maximum quantum yield by primary photochemistry (ϕPo), the quantum yield by electron transport (ϕEo), and the quantum yield by energy dissipation (ϕDo) of the cultures exposed during EP early and EP late. Applying SMF stimulated the protein synthesis of the cultures exposed during EP early. Furthermore, the carbohydrate content in the cells decreased with a consequent increase in EPS, of the cultures exposed during EP early. No variations were observed in terms of lipid concentration between the cultures exposed to SMF and the control cultures. The formation of pair radicals P680•+ and Phe•− radicals is proposed as a mechanism of action of SMF in microalgae cultures due to the increase of the RC of PS II, generating an increase in the photosynthetic efficiency of the microorganisms.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Barber J (2003) Photosystem II: The engine of life. Quart Rev Biophys 36:71–89
Bauer LM, Costa JAV, da Rosa APC, Santos LO (2017) Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour Technol 244:1425–1432
Bellou S, Baeshen MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G (2014) Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol Adv 32:1476–1493
Bišová K, Zachleder V (2014) Cell-cycle regulation in green algae dividing by multiple fission. J Exp Bot 65:2585–2602
Björn LO, Papageorgiou GC, Blankenship RE (2009) A viewpoint: why chlorophyll a? Photosynth Res 99:85–98
Borowitzka MA (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 601–652
Brailo M, Pećarević M, Grilec D, Mišković M, Lale D, Jurjević M, Čalić M, Mikuš J, BratošCetinić A (2018) The influence of electromagnetic field on viability of marine microalgae Tetraselmis suecica and bacteria Escherichia coli and Enterococcus faecalis. Naše More 65:71–77
Chu F-J, Wan T-J, Pai T-Y, Lin H-W, Liu S-H, Huang C-F (2020) Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J Environ Manage 253:109680
Costa SS, Peres BP, Machado BR, Costa JAV, Santos LO (2020) Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour Technol 315:123880
Croce R, van Amerongen H (2020) Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science 369:eaay2058
Dao LH, Beardall J (2016) Effects of lead on two green microalgae Chlorella and Scenedesmus: Photosystem II activity and heterogeneity. Algal Res 16:150–159
Deamici KM, Cardias BB, Costa JAV, Santos LO (2016) Static magnetic fields in culture of Chlorella fusca: Bioeffects on growth and biomass composition. Process Biochem 51:912–916
Deamici KM, Cuellar-Bermudez SP, Muylaert K, Santos LO, Costa JAV (2019a) Quantum yield alterations due to the static magnetic fields action on Arthrospira platensis SAG 21.99: Evaluation of photosystem activity. Bioresour Technol 292:121945
Deamici KM, Santos LO, Costa JAV (2019b) Use of static magnetic fields to increase CO2 biofixation by the microalga Chlorella fusca. Bioresour Technol 276:103–109
Deamici KM, Santos LO, Costa JAV (2021) Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst Eng 44:1453–1460
Du N, Gholami P, Kline DI, DuPont CL, Dickson AG, Mendola D, Martz T, Allen AE, Mitchell BG (2018) Simultaneous quantum yield measurements of carbon uptake and oxygen evolution in microalgal cultures. PloS One 13:e0199125
Eiler A, Olsson JA, Bertilsson S (2006) Diurnal variations in the auto-and heterotrophic activity of cyanobacterial phycospheres (Gloeotrichia echinulata) and the identity of attached bacteria. Freshw Biol 51:298–311
Font YS, Díaz YO, Cuypers A, Alemán EI, Vandamme D (2023) The effect of magnetic field treatment on the cultivation of microalgae: An overview of involved mechanisms. J Appl Phycol 35:1525–1536
Gateau H, Solymosi K, Marchand J, Schoefs B (2017) Carotenoids of microalgae used in food industry and medicine. Mini Rev Med Chem 17:1140–1172
Gautam H, Sharma A, Trivedi PK (2023) Plant microProteins and miPEPs: Small molecules with much bigger roles. Plant Sci 326:111519
Gonçalves AL, Pires JC, Simões M (2016) Wastewater polishing by consortia of Chlorella vulgaris and activated sludge native bacteria. J Clean Prod 133:348–357
Han S, Jin W, Chen Y, Tu R, Abomohra AE-F (2016) Enhancement of lipid production of Chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl Biochem Biotech 180:1043–1055
Hess SK, Lepetit B, Kroth PG, Mecking S (2018) Production of chemicals from microalgae lipids–status and perspectives. Eur J Lipid Sci Technol 120:1700152
Ho TY, Quigg A, Finkel ZV, Milligan AJ, Wyman K, Falkowski PG, Morel FM (2003) The elemental composition of some marine phytoplankton. J Phycol 39:1145–1159
Hore PJ, Mouritsen H (2016) The radical-pair mechanism of magnetoreception. Annu Rev Biophys 45:299–344
Iqbal K, Saxena A, Pande P, Tiwari A, Joshi NC, Varma A, Mishra A (2022) Microalgae-bacterial granular consortium: Striding towards sustainable production of biohydrogen coupled with wastewater treatment. Bioresour Technol 354:127203
Ji X, Li H, Zhang J, Saiyin H, Zheng Z (2019) The collaborative effect of Chlorella vulgaris-Bacillus licheniformis consortia on the treatment of municipal water. J Hazard Mater 365:483–493
Junge W (2019) Oxygenic photosynthesis: history, status and perspective. Quart Rev Biophys 52:e1
Khorshidi N, Hassanpour H, Ziyadi H (2022) Static magnetic field improved growth and astaxanthin production in Haematococcus lacustris via the regulation of carbohydrate accumulation, H2O2 level, and antioxidant defense system. J Appl Phycol 34:2283–2295
Konopacki M, Rakoczy R (2019) The analysis of rotating magnetic field as a trigger of gram-positive and gram-negative bacteria growth. Biochem Eng J 141:259–267
Kula M, Kalaji H, Skoczowski A (2017) Culture density influence on the photosynthetic efficiency of microalgae growing under different spectral compositions of light. J Photochem Photobiol B 167:290–298
Leister D (2022) Enhancing the light reactions of photosynthesis: Strategies, controversies and perspectives. Mol Plant 16:4–22
Levasseur W, Perré P, Pozzobon V (2020) A review of high value-added molecules production by microalgae in light of the classification. Biotechnol Adv 41:107545
Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W, Huo S, Cheng P, Liu J, Addy M (2019a) Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour Technol 291:121934
Li N, Liu Z, Wang P, Suman K, Zhang J, Song Y (2022) Effects of sodium hypochlorite treatment on the chlorophyll fluorescence in photosystem II of microalgae. Sci Total Environ 833:155192
Li X, Li W, Zhai J, Wei H, Wang Q (2019b) Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour Technol 273:368–376
Luo X, Zhang H, Li Q, Zhang J (2020) Effects of static magnetic field on Chlorella vulgaris: growth and extracellular polysaccharide (EPS) production. J Appl Phycol 32:2819–2828
Luo X, Zhang H, Zhang J (2021) The influence of a static magnetic field on a Chlorella vulgaris-Bacillus licheniformis consortium and its sewage treatment effect. J Environ Manage 295:112969
Mamedov M, Nadtochenko V, Semenov A (2015) Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms. Photosynth Res 125:51–63
Markou G, Dao LH, Muylaert K, Beardall J (2017) Influence of different degrees of N limitation on photosystem II performance and heterogeneity of Chlorella vulgaris. Algal Res 26:84–92
Masojıdek J, Koblızek M, Torzillo G (2004) Photosynthesis in microalgae. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp 20–39
Melkozernov AN, Schmid VH, Schmidt GW, Blankenship RE (1998) Energy redistribution in heterodimeric light-harvesting complex LHCI-730 of photosystem I. J Phys Chem B 102:8183–8189
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 265–284
Palmer CM (1959) Algae in water supplies: an illustrated manual on the identification, significance, and control of algae in water supplies, vol 2. US Department of Health, Education and Welfare, Public Health Service, Cincinnati
Pareek S, Sagar NA, Sharma S, Kumar V, Agarwal T, González‐Aguilar GA, Yahia EM (2017) Chlorophylls: Chemistry and biological functions. In: Yahia EM (ed) Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd Edn. Wiley-Blackwell, Hoboken pp 269–284
Plyusnina TY, Khruschev S, Degtereva N, Konyukhov I, Solovchenko A, Kouzmanova M, Goltsev V, Riznichenko G, Rubin A (2020) Gradual changes in the photosynthetic apparatus triggered by nitrogen depletion during microalgae cultivation in photobioreactor. Photosynthetica 58:443–451
Raouia H, Hamida B, Khadidja A, Ahmed L, Abdelwaheb C (2020) Effect of static magnetic field (200 mT) on biofilm formation in Pseudomonas aeruginosa. Arch Microbiol 202:77–83
Ritchie RJ (2008) Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46:115–126
Rossi F, De Philippis R (2016) Exocellular polysaccharides in microalgae and cyanobacteria: Chemical features, role and enzymes and genes involved in their biosynthesis. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 565–590
Sajjadi B, Chen W-Y, Raman AAA, Ibrahim S (2018) Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew Sustain Energy Rev 97:200–232
Santos LO, Deamici KM, Menestrino BC, Garda-Buffon J, Costa JAV (2017) Magnetic treatment of microalgae for enhanced product formation. World J Microbiol Biotechnol 33:169
Shao W, Ebaid R, Abomohra AE-F, Shahen M (2018) Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour Technol 265:163–169
Shen J-R (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48
Show PL (2022) Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour Technol 357:127329
Silveira-Font Y, Gómez-Luna L, Kufundala-Wemba MD, Salazar-Hernández D, Ortega-Díaz Y (2018) Variación de la composición de pigmentos de Chlorella vulgaris Beijerinck, con la aplicación del campo magnético estático. Rev Cubana Quím 30:55–67
Singh H, Kumar D, Soni V (2022) Performance of chlorophyll a fluorescence parameters in Lemna minor under heavy metal stress induced by various concentration of copper. Sci Rep 12:10620
Siqueira SF, Queiroz MI, Zepka LQ, Jacob-Lopes E (2018) Introductory chapter: Microalgae biotechnology. A brief introduction. In: Jacob-Lopes E, Zepka LQ, Queiroz MI (eds) Microalgal Biotechnology. IntechOpen, Riejeka pp 1–11
Small DP, Hüner NP, Wan W (2012) Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 33:298–308
Solimeno A, García J (2017) Microalgae-bacteria models evolution: From microalgae steady-state to integrated microalgae-bacteria wastewater treatment models–a comparative review. Sci Total Environ 607:1136–1150
Solovchenko A, Solovchenko O, Khozin-Goldberg I, Didi-Cohen S, Pal D, Cohen Z, Boussiba S (2013) Probing the effects of high-light stress on pigment and lipid metabolism in nitrogen-starving microalgae by measuring chlorophyll fluorescence transients: Studies with a Δ5 desaturase mutant of Parietochloris incisa (Chlorophyta, Trebouxiophyceae). Algal Res 2:175–182
Stirbet A (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence. Springer, Dordrecht, pp 321–362
Su Y, Song K, Zhang P, Su Y, Cheng J, Chen X (2017) Progress of microalgae biofuel’s commercialization. Renew Sustain Energy Rev 74:402–411
Suresh Kumar A, Mody K, Jha B (2007) Bacterial exopolysaccharides–a perception. J Basic Microbiol 47:103–117
Veiga MC, Fontoura MM, de Oliveira MG, Costa JAV, Santos LO (2020) Magnetic fields: Biomass potential of Spirulina sp. for food supplement. Bioprocess Biosyst Eng 43:1231–1240
Vinyard DJ, Ananyev GM, Charles Dismukes G (2013) Photosystem II: The reaction center of oxygenic photosynthesis. Annu Rev Biochem 82:577–606
Wang Y, Ning W, Han M, Gao C, Guo W, Chang J-S, Ho S-H (2023) Algae-mediated bioremediation of ciprofloxacin through a symbiotic microalgae-bacteria consortium. Algal Res 71:103062
Wong SY, Wei Y, Mouritsen H, Solov’yov IA, Hore P (2021) Cryptochrome magnetoreception: Four tryptophans could be better than three. J R Soc Interface 18:20210601
Yan N, Fan C, Chen Y, Hu Z (2016) The potential for microalgae as bioreactors to produce pharmaceuticals. Int J Mol Sci 17:962
Yao S, Lyu S, An Y, Lu J, Gjermansen C, Schramm A (2019) Microalgae–bacteria symbiosis in microalgal growth and biofuel production: a review. J Appl Microbiol 126:359–368
Zachleder V, Bišová K, Vítová M (2016) The cell cycle of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 3–46
Zachleder V, Ivanov IN, Kselíková V, Bialevich V, Vítová M, Ota S, Takeshita T, Kawano S, Bišová K (2021) Characterization of growth and cell cycle events affected by light intensity in the green alga Parachlorella kessleri: A new model for cell cycle research. Biomolecules 11:891
Zhao B, Sha H, Li J, Cao S, Wang G, Yang Y (2020) Static magnetic field enhanced methane production via stimulating the growth and composition of microbial community. J Clean Prod 271:122664
Zieliński M, Dębowski M, Kazimierowicz J (2021a) The effect of static magnetic field on methanogenesis in the anaerobic digestion of municipal sewage sludge. Energies 14:590
Zieliński M, Zielińska M, Cydzik-Kwiatkowska A, Rusanowska P, Dębowski M (2021b) Effect of static magnetic field on microbial community during anaerobic digestion. Bioresour Technol 323:124600
Funding
The authors are thankful for the financial support from Project 1 and Project 5 of the Programme VLIR-IUC-UOS – Universidad de Oriente CU2019IUC030A105-77143 and the UHasselt BOF-BILA fellowship R-9903.
Author information
Authors and Affiliations
Contributions
Y.S. Font: Conceptualization, Investigation, validation, visualization, writing-original Draft Y.O. Díaz: Conceptualization, writing – Review and editing A. Cuypers: Conceptualization, Resources, Writing – Review and Editing, funding acquisition, E.I Alemán: writing – review and editing, validation, funding acquisition, supervision; D. Vandamme: conceptualization, resources, writing – Review and editing – supervision – funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silveira Font, Y., Ortega Díaz, Y., Cuypers, A. et al. Influence of a static magnetic field on the photosynthetic apparatus, cell division, and biomass composition of a Chlorella microalgae-bacteria consortium. J Appl Phycol 36, 41–56 (2024). https://doi.org/10.1007/s10811-023-03137-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03137-2