Abstract
Plant-derived allelochemicals have the potential to be used as eco-friendly measures for control of Microcystis aeruginosa blooms. However, the susceptibility of M. aeruginosa to plant allelochemicals under a range of environmental factors, such as alkalinity, is not clear. This study investigated the effects of different alkalinity treatments on 1,2,3-trihydroxybenzene (pyrogallol) toxicity to a toxic strain of the cyanobacterium, M. aeruginosa (FACHB 905). Pyrogallol toxicity to M. aeruginosa cultures, both pre-adapted to a range of alkalinity levels and un-adapted, increased when alkalinity was increased from 0.09 to 1.51 meq L−1. The mean inhibition ratios calculated according to OD650, cell concentration, Chl a, and carotenoid concentrations of M. aeruginosa cultures were highest in the highest alkalinity treatment (1.51 meq L−1), i.e., up to 74%, 80%, 73%, and 87% for alkalinity-adapted cells on day 3. The lowest cell concentrations and photosynthesis pigment concentrations were found in the highest alkalinity treatment (1.51 meq L−1) for un-adapted cells in 12-day bioassays. This trend was more obvious over time. Pyrogallol and TPC (total phenolic compounds) concentrations measured immediately after pyrogallol addition into the culture medium decreased more rapidly in higher alkalinity treatments. In contrast, faster oxygen consumption and higher production of quinone end products occurred within the first 30 min after pyrogallol addition at higher alkalinity levels. Quinones and oxygen radicals have been shown previously to be more toxic to cyanobacteria than pyrogallol itself. This provides a potential explanation for the enhanced pyrogallol toxicity to M. aeruginosa under higher alkalinity. Therefore, it is important to take alkalinity into account when considering pyrogallol as a potential biocide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing frequency and magnitude of toxic cyanobacterial blooms are partially driven by accelerated eutrophication and reduced water flow. Cyanobacterial blooms are a threat to the structure and function of aquatic ecosystems (Paerl and Otten 2013). Successful control of cyanobacterial blooms has been a major challenge, despite multiple approaches including algicide treatment (Jones and Orr 1994), nutrient removal, and flow regulation/flushing (Huisman et al. 2018). Therefore, new ways are being developed to prevent and mitigate cyanobacterial blooms. Microcystis aeruginosa (Kützing) Lemmerman is one of the most ubiquitous bloom-forming species in freshwater bodies (Harke et al. 2016). It is, however, sensitive to plant-derived bioactive compounds such as pyrogallol from the submerged macrophyte Myriophyllum spicatum L. (Nakai et al. 2000), salcolin from barley straw (Xiao et al. 2014), and epigallocatechin-3-gallate from tea (Camellia sinensis (L.) Kuntze) (Lu et al. 2013). Microcystis aeruginosa has been shown to be more sensitive to allelochemicals from M. spicatum than the diatom Stephanodiscus minutulus (Kützing) Cleve & Möller, and the green alga Scenedesmus armatus (Chodat) Chodat (Körner and Nicklisch 2002). When co-cultured with M. spicatum, or exposed to allelopathic polyphenols, M. aeruginosa was also inhibited more effectively than the chlorophyte Selenastrum capricornutum Printz (Zhu et al. 2010). Another study showed that M. aeruginosa was more sensitive to the extracts of four Chinese herbal plants than Chlorella pyrenoidosa H. Chick (Ye et al. 2014), which is a green alga. Cyanobacteria also exhibited a greater susceptibility to gallic acid in mesocosms compared with green algae, brown algae, and cryptophytes (Techer et al. 2016). Therefore, it appears possible to use plant-derived allelochemicals as an eco-friendly control agent for M. aeruginosa.
Most studies on the inhibitory effects of plant allelochemicals are typically conducted in controlled conditions that might not reflect environmental conditions (Gao et al. 2017a). In natural systems, a range of environmental factors can interact to affect cyanobacterial responses to allelochemicals. There has already been some experimental evidence testing this hypothesis. The toxicity of eight plant allelochemicals produced by M. spicatum towards M. aeruginosa was higher at lower temperatures and light intensities (Nakai et al. 2014). Hydroquinone exerted very strong inhibitory effect on the cyanobacterium Synechocystis sp. after pre-exposure at pH 7.0, but exhibited no inhibition after pre-exposure at pH 11.0 for 4 days (Bährs et al. 2013). However, the susceptibility of M. aeruginosa to plant allelochemicals under some other environmental factors, such as alkalinity, is not clear.
Alkalinity is a common measure used as a proxy for many chemical and biological processes in waterbodies (Nguyen and Rittmann 2016). Higher alkalinity reflects an increase in the acid-neutralizing ability and pH buffering capacity of freshwaters (Dunnivant 2005; Somridhivej and Boyd 2017). Increased alkalinity has been shown to affect the concentrations of free metal ions in the water column, thereby changing their toxicity to aquatic organisms (Miller and Mackay 1980). Alkalinity significantly reduced copper accumulation in macroalgae Ulva fasciata Delile (Geddie and Hall 2019). Hydroxide (OH−), bicarbonate (HC\( {\mathrm{O}}_3^{-} \)), and carbonate (C\( {\mathrm{O}}_3^{2-} \)) are the main components of alkalinity. Bicarbonate (HC\( {\mathrm{O}}_3^{-} \)) is often the dominant component of alkalinity in most surface fresh waters (Stumm and Morgan 1996). Higher bicarbonate can provide higher inorganic carbon availability for some aquatic phototrophs (Kahara and Vermaat 2003). Alkalinity varies between freshwater bodies worldwide and over time. For example, the median alkalinity value was 0.80 meq L−1 in Danish lakes (Vestergaard and Sand-Jensen 2000), 1.64 meq L−1 in summer and 1.68 meq L−1 in winter in Spanish reservoirs (Marcé et al. 2015), 1.11 meq L−1 in 134 UK lakes (Carvalho et al. 2011), and lower than 1.00 meq L−1 in Lake Taihu, China, over the period 1991–1999 (Chen et al. 2003). Cyanobacterial abundance in summer clearly increased from low alkalinity lakes (< 0.2 meq L−1) to high alkalinity lakes (> 1.0 meq L−1) based on phytoplankton composition data collected from > 1500 European lakes (Carvalho et al. 2013). However, the outcome of different alkalinity levels on allelopathic effects from aquatic plants on cyanobacteria is unclear.
Pyrogallol is a phenolic acid which can inhibit the growth of M. aeruginosa. It was isolated and identified from the submerged aquatic freshwater macrophyte M. spicatum (Nakai et al. 2000). Although there have been many studies showing the physiological and ecological mechanisms for this inhibition (Shao et al. 2009; Lu et al. 2014, 2017; Wang et al. 2016), the effect of alkalinity on its toxicity to cyanobacteria is unknown. This information is needed to optimize the use of pyrogallol for control of cyanobacterial bloom species.
In this study, a strain of M. aeruginosa was selected to test pyrogallol toxicity under three alkalinity levels. Since bioassay experiments with M. aeruginosa strains commonly use BG11 media (Shao et al. 2009; Lu et al. 2014, 2017; Wang et al. 2016), which has an alkalinity lower than the alkalinity levels of many lakes around the world (Vestergaard and Sand-Jensen 2000; Carvalho et al. 2011; Marcé et al. 2015) but higher than the alkalinity of some other lakes, e.g., Taihu Lake (Chen et al. 2003). Therefore, low alkalinity of 0.09 meq L−1, standard alkalinity in BG 11 medium of 0.38 meq L−1, and high alkalinity of 1.51 meq L−1 were chosen for the two experiments conducted in the study. In the first experiment, cells were pre-adapted to different alkalinities, simulating levels that M. aeruginosa has been observed to grow in the field (Chen et al. 2003; Maileht et al. 2013). In the second experiment, cells without pre-adaption were subjected to abrupt shifts in alkalinity. The underlying toxicity mechanisms were also examined by analyzing the autoxidation process of pyrogallol under varying alkalinities.
Materials and methods
Cyanobacterial culturing
Microcystis aeruginosa (strain FACHB 905) with single cell morphology was obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (FACHB), the Chinese Academy of Sciences. Cultures were grown in autoclaved BG 11 medium (Rippka et al. 1979) under controlled conditions (12:12 h light: dark cycle with light intensity of 25 μmol photons (PAR) m−2 s−1 at 25 ± 1 °C). Cultures were manually shaken twice each day, and cells in exponential growth phase were prepared for the experiments.
Assessment of pyrogallol toxicity to M. aeruginosa under varying alkalinities
The effect of alkalinity on the toxicity of pyrogallol to M. aeruginosa was tested based on the effects on growth with and without pre-adaptation (Table 1). Three alkalinity treatment levels were chosen, i.e., low alkalinity of 0.09 meq L−1, standard alkalinity in BG 11 medium of 0.38 meq L−1, and high alkalinity of 1.51 meq L−1. Alkalinity was adjusted up or down by addition or removal of Na2CO3 from standard BG11 medium. The initial cell concentration was about 1.5 × 106 cells mL−1. The concentration of pyrogallol added to the cultures was 5.0 mg L−1. This was a concentration with an inhibition ratio higher than 30% on M. aeruginosa growth in our preliminary experiment, while 10 mg L−1 pyrogallol killed all M. aeruginosa cells in the preliminary experiment. Pyrogallol was purchased from Sigma-Aldrich (USA) and dissolved in Milli-Q water. An equivalent volume of pyrogallol-free Milli-Q water was added to the controls. All flasks were sealed with a breathable sealing membrane and cultured under the conditions described above. Experiments were conducted in triplicate. The total culture volume was 100 mL for the first experiment and 150 mL for the second experiment.
The first experiment (expt. I) was designed to investigate the effects of pyrogallol on M. aeruginosa pre-adapted to three alkalinities before subjecting them to the 3-day bioassays. This experiment was designed to compare short-term responses of M. aeruginosa to pyrogallol exposure in water with different ambient alkalinities. Cultures were pre-adapted over three cell cycles to different alkalinities by sub-culturing into BG 11 medium containing different concentrations of Na2CO3. The effects of alkalinity on OD650 of M. aeruginosa after alkalinity adaption over two and three cycles showed the same trends. The pre-adapted cultures were then exposed to pyrogallol for 3 days. The controls under each alkalinity level were set up simultaneously. Subsamples were collected at the start (day 0) and end (day 3) of the experiment for OD650 measurement, cell counts, and measurement of photosynthetic pigment concentrations.
The second experiment (expt. II) was used to test the effect of pyrogallol on M. aeruginosa cells without pre-adaptation to different alkalinities, in 12-day bioassays. The rationale for this experiment was to test how M. aeruginosa responded to pyrogallol toxicity while changing alkalinity simultaneously. The changes over time were also tested in this experiment. M. aeruginosa cells in exponential growth phase were cultured in original BG 11 medium and inoculated into new growth media containing different levels of alkalinity. Treatment cultures also had pyrogallol added. The control for each of the three treatments was the same, i.e., M. aeruginosa cultured in the original BG 11 medium, where alkalinity was retained at 0.38 meq L−1. Subsamples were collected every other day to determine OD650 and photosynthetic pigment concentrations of M. aeruginosa cells.
Measurement of growth parameters of M. aeruginosa
Five milliliters of subsamples was sampled from the cultures and measured spectrophotometrically at 650 nm. Subsamples were preserved with 1% Lugol’s iodine solution until counted by an optical microscope (BA210, MOTIC, China) at × 400 magnification with a hemocytometer as previously described (Gao et al. 2017b).
Measurement of chl a and carotenoid concentrations of M. aeruginosa
A 5-mL subsample of each culture was centrifuged at 7100×g for 10 min and the pellets were harvested and extracted with 95% ethanol for 24 h in the dark. The extracts were centrifuged again at 7100×g for a further 10 min. The absorbance of the supernatant was measured at 665, 649, and 470 nm. The concentrations of chl a and carotenoids in the extracts were calculated as described previously (Lichtenthaler and Buschmann 2001).
Calculation of inhibition ratio
Cell concentrations, OD650, chl a, and carotenoid concentrations of the samples were determined as described above. The inhibition ratio (IR, %) of pyrogallol on M. aeruginosa under each treatment was calculated by comparing each of these parameters in treated cultures with that of the corresponding control, according to Eq. 1,
where Pt is the value measured for each parameter treated with pyrogallol and Pc is the value measured for the controls.
Assessment of pyrogallol autoxidation at different alkalinities
Microcystis aeruginosa cells in exponential phase were inoculated into 150 mL alkalinity-modified BG 11 medium and exposed to pyrogallol. Auto-oxidation dynamics of pyrogallol under the three alkalinity levels (0.09, 0.38, and 1.51 meq L−1) were tested. Pyrogallol and total phenolic compound (TPC) concentrations, dissolved oxygen (DO) concentrations, OD600 and OD440 were all measured. The TPC concentration in M. aeruginosa cultures represents the sum of the concentrations of pyrogallol and other oxidized products that contain phenolic hydroxyl groups. Changes in DO were measured to test for increases in oxygen consumption rates following exposure to pyrogallol, while measuring OD600 and OD440 respectively to represent changes of intermediate auto-oxidant and quinone end products following pyrogallol degradation (Ramasarma et al. 2014). Pyrogallol and TPC concentrations were sampled and measured immediately following pyrogallol addition (day 0) and on day 3. DO, and OD440 and OD600 were measured at 0, 10, 20, and 30 min following pyrogallol addition. The total alkalinity and pH of the culture solutions on day 0 and day 3 were also measured.
Quantification analysis of pyrogallol
Five milliliters of subsamples was collected and pyrogallol concentrations in the cultures were determined by solid phase extraction (SPE) and gas chromatography (GC) as described previously (Lu et al. 2016) but modified as follows: A subsample was centrifuged at 7100×g for 10 min. The supernatant was harvested and concentrated using an SPE cartridge (OASIS HLB, Waters, USA) and eluted with acetone then derivatized using bis(trimethylsilyl)trifluoroacetamide (BSTFA, Supelco, USA). The derivatized pyrogallol was then analyzed with an Agilent 6890 N GC fitted with an HP-5ms (30 m × 0.25 mm × 0.25 μm) capillary column. The carrier gas was He at a constant flow rate of 1 mL min−1. The GC was temperature programmed with an initial oven temperature of 80 °C for 1 min, then raised linearly to 280 °C at 15 °C min−1 with a final isotherm of 2 min. The injection port temperature was 280 °C. The sample was injected in splitless mode. The pyrogallol concentration measured immediately after addition to pure Milli-Q water at a concentration of 5.0 mg L−1 was 3.9 ± 0.08 mg L−1, indicating a recovery rate of using this method of about 80%.
Quantification analysis of total phenolic compounds
The TPC concentration in cultures was determined by the Folin-Ciocalteu colorimetric method, described previously (Wu et al. 2009). Briefly, 2 mL subsamples was mixed with 1 mL Folin-Ciocalteu reagent (Solarbio, USA) and incubated at room temperature for 5 min, followed by addition of 1 mL 10% Na2CO3. Samples were then incubated at 25 °C for 2 h, and the optical density measured at 765 nm. The results were calculated using the equation described by Wu et al. (2009) and expressed as the content of pyrogallol. The measured TPC concentration was 5.5 ± 0.08 mg L−1 immediately after pyrogallol addition to Milli-Q water when the actual concentration was 5.0 mg L−1, indicating the recovery of TPC using this method was about 110%.
Determination of DO, OD600, and OD440
The changes of DO concentrations in the cultures were determined with a portable dissolved oxygen analyzer (HQ40D, HACH, USA). The OD600 and OD440 were measured using an ultraviolet/visible light spectrophotometer.
Determination of alkalinity and pH values
Alkalinity was determined using the acid-base titration method (Lei 2006). Hydrochloric acid standard solution was used to titrate water samples with two acid-base indicators. The first indicator, phenolphthalein, changed from red to colorless when titrated to a pH of 8.3, and the hydroxide (OH−) and carbonate (C\( {\mathrm{O}}_3^{2-} \)) ions were neutralized. The second indicator, methyl orange-aniline blue, changed from orange-yellow to light purple when titrated to a pH range of 4.4–4.5, and all bicarbonate (HC\( {\mathrm{O}}_3^{-} \)) ions were neutralized. The total alkalinity and the relative concentrations of each component of the total alkalinity including hydroxide (OH−), bicarbonate (HC\( {\mathrm{O}}_3^{-} \)), and carbonate (C\( {\mathrm{O}}_3^{2-}\Big) \) were calculated according to the amount of hydrochloric acid consumed by the two-step titration. The pH of the cultures was measured with a portable pH analyzer (HQ40D, HACH, USA).
Statistical analyses
All data were analyzed with Microsoft Excel and R (R Development Core Team 2011). Differences among the three alkalinities in the control and pyrogallol treatment and differences among the control and pyrogallol treatments on the same day were analyzed respectively by a one-way ANOVA, and multiple comparisons were performed using the Tukey HSD test at p < 0.05 (Bates et al. 2012). Pearson correlation coefficient was chosen for the correlation analysis of alkalinity and OD650, cell concentrations and concentrations of photosynthesis pigments for the second experiment.
Results
Effects of pyrogallol on the alkalinity-adapted M. aeruginosa cultures
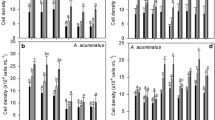
After pre-adapting M. aeruginosa in alkalinity-modified BG 11 medium over three cell cycles, different responses of M. aeruginosa towards varying alkalinities were observed over the 3-day culture period (Fig. 1). The OD650 and cell concentrations at alkalinities of 0.38 and 1.51 meq L−1 were significantly higher than those at 0.09 meq L−1 (p < 0.05). However, the chl a and carotenoid concentrations were significantly higher at lower alkalinities (0.09 and 0.38 meq L−1, p < 0.05). The optimal alkalinity level for this M. aeruginosa strain was 0.38 meq L−1 in the present study.
OD650 (a), cell concentrations (b), chl a (c), and carotenoid concentrations (d) of alkalinity-adapted M. aeruginosa cultures in the control and pyrogallol treatments at each alkalinity level in expt. I. Significant differences are indicated by different lowercase letters in the control and different uppercase letters in the pyrogallol treatments (one-way ANOVA, p < 0.05)
The OD650, cell concentration, chl a, and carotenoid concentrations in the pyrogallol treatments were all lower (p < 0.05) compared with the controls on day 3 (Fig. 1), suggesting a negative effect of pyrogallol. Additionally, OD650, cell concentrations, chl a, and carotenoid concentrations in all pyrogallol-treated cultures at 1.51 meq L−1 were lower after 3 days than on day 0.
The mean inhibition ratio for pyrogallol-treated M. aeruginosa cells calculated from OD650, cell concentration, chl a, and carotenoid concentrations of alkalinity-adapted M. aeruginosa cells on day 3 increased as alkalinity increased from 0.09 to 1.51 meq L−1 (Fig. 2). The mean inhibition ratios were highest in the highest alkalinity treatment (1.51 meq L−1), i.e., up to 74%, 80%, 73%, and 87% for OD650, cell concentration, chl a, and carotenoid concentration respectively.
Toxicity of pyrogallol to M. aeruginosa without alkalinity pre-adaptation
The OD650, cell concentrations, chl a, and carotenoid concentrations in the three pyrogallol treatments were significantly lower than those for the control from day 4 to day 12 (p < 0.05). Pyrogallol toxicity increased with increasing alkalinity (Fig. 3). As for the pyrogallol treatment at the highest alkalinity level (1.51 meq L −1), OD650, cell concentrations, chl a, and carotenoid concentrations declined gradually from day 2, being lower than other treatments and control. By the end of the experiment, values were only 0.06, 8.8 × 103 cells mL−1, 3.33 μg L−1, and 1.12 μg L−1 respectively. There was a significantly negative correlation between alkalinity and OD650 (r = − 0.50, p < 0.001), cell concentrations (r = − 0.39, p < 0.001), chl a (r = − 0.43, p < 0.001), and carotenoid concentrations (r = − 0.43, p < 0.001).
Rapid auto-oxidation process of pyrogallol under varying alkalinities
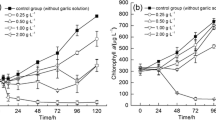
Extracellular pyrogallol concentrations decreased more rapidly after addition into cultures with higher alkalinity. Pyrogallol was added at an initial concentration of 5.0 mg L−1, but the extracellular concentration immediately fell to 1.99 mg L−1 in the low alkalinity (0.09 meq L−1) treatment, to 0.05 mg L−1 in the medium alkalinity (0.38 meq L−1) treatment, and to 0.03 mg L−1 in the high alkalinity (1.51 meq L−1) treatment as shown in Table 2. Concentrations of TPC (the sum of pyrogallol and its autoxidation products containing phenolic hydroxyl groups) also decreased with the increase of alkalinity, but the rate of decrease was much lower than for pyrogallol itself. The measured TPC concentration in the cultures was significantly higher than pyrogallol concentrations at each alkalinity immediately after pyrogallol addition, ranging from 2.96 mg L−1 at the alkalinity of 0.09 meq L−1 to 1.54 mg L−1 at the alkalinity of 1.51 meq L−1 (p < 0.05). Pyrogallol and TPC concentrations on day 3 were also measured and were below 0.03 and 0.80 mg L−1 respectively.
The changes in DO concentrations and the production of intermediate auto-oxidants and quinone end products (measured by changes in OD600 and OD440) during the first 30 min demonstrated rapid auto-oxidation of pyrogallol following addition to the culture medium (Fig. 4). The autoxidation rate was faster with higher alkalinity. The most rapid DO reduction and the most rapid increase of OD600 and OD440 occurred within 10 min following pyrogallol addition. Increased OD600 measures the production of intermediate degradation products and it declined after 10 min. Conversely, OD440 which measures the production of the quinone end products increased during the first 30 min.
The changes of alkalinity and pH values
The measured alkalinity values in the M. aeruginosa cultures were consistent with the calculated levels in the treatments, and they slightly increased from day 0 to day 3 for each treatment (p > 0.05, Table 3). The percentage of bicarbonate (HC\( {\mathrm{O}}_3^{-} \)) in total alkalinity decreased with increasing alkalinity on day 0, but was 100% for each treatment on day 3.
The initial pH of cultures ranged from 6.83 to 8.51 increasing with increasing alkalinity levels (Table 3). For the two lower alkalinity treatments, pH levels increased by 1.13 and 0.26 units respectively from day 0 to day 3, but decreased by 0.59 units for the highest alkalinity treatment.
Discussion
This study showed a positive correlation between alkalinity and pyrogallol toxicity with the lowest toxicity in the 0.09 meq L−1 treatment and the highest toxicity in the 1.51 meq L−1 treatment, in both adapted and un-adapted M. aeruginosa cultures. This study is the first to show that increasing alkalinity correlated with increased pyrogallol toxicity to M. aeruginosa. This trend was found to be more obvious over time during our 12-day experiment. Specifically, the ambient alkalinity conditions of systems with M. aeruginosa present must be taken into account as an important factor in the evaluation of pyrogallol toxicity. The cyanobacterial control efficacy of pyrogallol might be underestimated in the lab experiment using BG 11 culture medium with an alkalinity of 0.38 meq L−1 and enhanced in those waterbodies with higher alkalinity levels. The study also provided a potential way to improve the efficacy of pyrogallol as an algicide to control M. aeruginosa, that is, to elevate ambient alkalinity levels to some extent.
This study demonstrated that pyrogallol was more effectively auto-oxidized under higher alkalinity levels. Previous studies have shown that auto-oxidized breakdown products of pyrogallol can also enhance the toxicity of pyrogallol to M. aeruginosa (Nakai et al. 2000; Lu et al. 2016). The oxidation products of pyrogallol in sequence were pyrogallol-ortho-quinone, purpurogallin, purpurogallin-quinone, and complex polymers. The concentration dynamics of these quinone breakdown products can be measured spectrophotometrically at OD600 and OD440 respectively (Ramasarma et al. 2014). Pyrogallol and TPC concentrations in the culture solutions decreased, while intermediate products and quinone end products increased with alkalinity. It can be deduced that the concentration of the proton acceptor, i.e., bicarbonate, increased with increased alkalinity, causing a decrease in the concentration of phenolic hydroxyl groups and an increase of the concentration of quinonoid carbonyl groups. It has been reported that the quinones are around 1000-fold more toxic to algae than phenolic acids (Pillinger et al. 1994). Additionally, the rapid decrease in DO concentrations indicated the production of more oxygen radicals, i.e., H2O2, during the process of pyrogallol autoxidation, which are capable of affecting oxidative damage to organisms (Liu et al. 2007; Ramasarma et al. 2014). Therefore, the enhanced toxicity of pyrogallol to M. aeruginosa might be due to production of quinones coupled with the presence of oxygen radicals produced under higher alkalinities.
As expected, the pH value of the cultures on day 0 was higher at higher alkalinity levels and ranged from 6.83 to 8.51. Higher toxicity occurred at higher alkalinity and higher initial pH levels. A previous study has shown that pyrogallol toxicity to a toxic M. aeruginosa strain increased when the initial pH was increased from 8.0 to 9.0 (Liu et al. 2007). However, it has also been reported that polyphenol toxicity was enhanced via auto-oxidation when the pH value was higher than 7.0, but decreased due to oxidative polymerization when the pH value was higher than 8.3 (Bährs et al. 2014). The highest pyrogallol toxicity indicated oxidative polymerization did not happen at the highest alkalinity level, although the initial pH value exceeded 8.3 in the present study. It might be due to enhanced pH buffering by higher alkalinity. Although the pH increased with time due to the growth and photosynthesis of M. aeruginosa for the two lower alkalinity levels (0.09 and 0.38 meq L−1) which reflects previous studies (Krüger et al. 2012; Tann et al. 2016), it decreased with time at the high alkalinity level (1.51 meq L−1). The pH value in the three pyrogallol treatments ranged from 7.76 to 7.96 on day 3. Higher pH buffering capacity at higher alkalinity levels reduced the pH value of the cultures into the range that favored auto-oxidation instead of oxidative polymerization.
Microcystis aeruginosa strains with either sbtA or bicA genes that are responsible for different bicarbonate uptake systems have been shown to behave differently at varying dissolved inorganic carbon (DIC) conditions (Sandrini et al. 2014). Rapid adaptive changes in the genotype composition in response to changes in inorganic carbon availability ensure that Microcystis is capable of living in a wide range of alkalinities and therefore pH and can result in dominance of the phytoplankton community with increasing carbon concentrations (Sandrini et al. 2016). The alkalinity in Microcystis-dominated Lake Taihu, for example, ranged from 13.8 to 52.8 μeq L−1 during 1991 and 1999 (Chen et al. 2003), which was much lower than the highest alkalinity level in the present study. A dense cyanobacterial bloom dominated by M. aeruginosa was present in the main lake basin of Lake Krugersdrift, South Africa, during 2005 and 2006, where the alkalinity in the two bloom sites was more than 50-fold higher than Lake Taihu at 2.58 and 2.70 meq L−1 (Oberholster et al. 2009), and in an alkaline Hungarian lake, where alkalinity was as high as 13.58 meq L−1 (Bell et al. 2018). The higher frequency of cyanobacteria dominance was positively related to higher nutrient concentrations and higher alkalinity during an analysis of phytoplankton data and habitat conditions from 1500 lakes in 20 European countries (Maileht et al. 2013). Our findings indicated that it may be possible to control harmful M. aeruginosa using plant polyphenols, e.g., pyrogallol, under higher alkalinity conditions.
Conclusions
In summary, this study showed that alkalinity affected pyrogallol toxicity against a toxic strain of M. aeruginosa. Increasing the alkalinities from 0.09 to 1.51 meq L−1 enhanced the toxicity of pyrogallol to M. aeruginosa in both alkalinity pre-adapted and un-adapted cultures, for a range of growth parameters and pigment concentrations. The study demonstrated more efficient auto-oxidation of pyrogallol under higher alkalinity levels, causing a rapid decrease of the concentration of phenolic hydroxyl groups and an increase of the concentration of quinonoid carbonyl groups, which were more toxic to M. aeruginosa cells. The study has enhanced our understanding of the interactive effects of an environmental parameter, i.e., alkalinity, and plant allelochemicals on the resilience of M. aeruginosa blooms. The results of this study provide important background information for the development of a more effective and environmentally friendly technique to control harmful cyanobacterial blooms using plant-derived allelochemicals.
References
Bates D, Maechler M, Bolker B (2012) Lme4: linear mixed-effects models using S4 classes. R package version: 0.999999-0. R Foundation for Statistical Computing, Vienna
Bährs H, Laue P, Chakrabarti S, Steinberg CEW (2014) Plant polyphenols: do they control freshwater planktonic nuisance phototrophs? In: Watson R (ed) Polyphenols in plants: isolation, purification and extract preparation. Academic Press, Massachusetts, USA, pp 87–98
Bährs H, Putschew A, Steinberg CEW (2013) Toxicity of hydroquinone to different freshwater phototrophs is influenced by time of exposure and pH. Environ Sci Pollut Res 20:146–154
Bell TAS, Sen-Kilic E, Felföldi T, Vasas G, Fields MW, Peyton BM (2018) Microbial community changes during a toxic cyanobacterial bloom in an alkaline Hungarian lake. Antonie van Leeuwenhoek 111:2425–2440
Carvalho L, Miller CA, Scott EM, Codd GA, Davies PS, Tyler AN (2011) Cyanobacterial blooms: statistical models describing risk factors for national-scale lake assessment and lake management. Sci Total Environ 409:5353–5358
Carvalho H, Mcdonald C, de Hoyos C, Mischke U, Phillips G, Borics G, Poikane S, Skjelbred B, Solheim AL, Wichelen JV, Cardoso AC (2013) Sustaining recreational quality of European lakes: minimizing the health risks from algal blooms through phosphorus control. J Appl Ecol 50:315–323
Chen Y, Qin B, Teubner K, Dokulil MT (2003) Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J Plankton Res 25:445–453
Dunnivant FM (2005) Environmental laboratory exercises for instrumental analysis and environmental chemistry, John Wiley & Sons, Inc. NJ, USA
Gao YN, Dong J, Fu QQ, Wang YP, Chen C, Li JH, Li R, Zhou CJ (2017a) Allelopathic effects of submerged macrophytes on phytoplankton. Allelopath J 40:1–22
Gao YN, Ge FJ, Zhang LP, He Y, Lu ZY, Zhang YY, Liu BY, Zhou QH, Wu ZB (2017b) Enhanced toxicity to the cyanobacterium Microcystis aeruginosa by low-dosage repeated exposure to the allelochemical N-phenyl-1-naphthylamine. Chemosphere 174:732–738
Geddie AW, Hall SG (2019) The effect of salinity and alkalinity on growth and the accumulation of copper and zinc in the Chlorophyta Ulva fasciata. Ecotoxicol Environ Saf 172:203–209
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20
Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, Visser PM (2018) Cyanobacterial blooms. Nat Rev Microbiol 16:471–483
Jones GJ, Orr PT (1994) Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res 28:871–876
Kahara SN, Vermaat JE (2003) The effect of alkalinity on photosynthesis–light curves and inorganic carbon extraction capacity of freshwater macrophytes. Aquat Bot 75:217–227
Körner S, Nicklisch A (2002) Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes. J Phycol 871:862–871
Krüger T, Hölzel N, Luckas B (2012) Influence of cultivation parameters on growth and microcystin production of Microcystis aeruginosa (Cyanophyceae) isolated from Lake Chao (China). Microb Ecol 63:199–209
Lei YZ (2006) Environmental chemistry experiment of aquaculture water. China Agriculture Press, Beijing
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:F4.3.1–F4.3.8
Liu BY, Zhou PJ, Tian JR, Jiang SY (2007) Effect of pyrogallol on the growth and pigment content of cyanobacteria-blooming toxic and nontoxic microcystis aeruginosa. Bull Environ Contam Toxicol 78:499–502
Lu Y, Wang J, Yu Y, Su W, Kong FX (2013) Inhibition of Camellia sinensis (L.) O. Kuntze on Microcystis aeruginosa and isolation of the inhibition factors. Biotechnol Lett 35:1029–1034
Lu Z, Sha J, Tian Y, Zhang XZ, Liu BY, Wu ZB (2017) Polyphenolic allelochemical pyrogallic acid induces caspase-3(like)-dependent programmed cell death in the cyanobacterium Microcystis aeruginosa. Algal Res 21:148–155
Lu Z, Zhang Y, Gao Y, Liu BY, Sun XM, He F, Zhou QH, Wu ZB (2016) Effects of pyrogallic acid on Microcystis aeruginosa: oxidative stress related toxicity. Ecotoxicol Environ Saf 132:413–419
Lu ZY, Liu B Y, He Y, Chen Z L, Zhou Q H, Wu Z B (2014) Effects of daily exposure of cyanobacterium and chlorophyte to low-doses of pyrogallol. Allelopath J 34:195–205
Maileht K, Nõges T, Nõges P, Ott I, Mischke U, Carvalho L, Dudley B (2013) Water colour, phosphorus and alkalinity are the major determinants of the dominant phytoplankton species in European lakes. Hydrobiologia 704:115–126
Marcé R, Obrador B, Morguí J-A, Riera JL, López P, Armengol J (2015) Carbonate weathering as a driver of CO2 supersaturation in lakes. Nat Geosci 8:107–111
Miller T, Mackay W (1980) The effects of hardness, alkalinity and pH of test water on the toxicity of copper to rainbow trout (Salmo gairdneri). Water Res 14:129–133
Nakai S, Asaoka S, Okuda T, Nishijima W (2014) Growth inhibition of Microcystis aeruginosa by allelopathic compounds originally isolated from Myriophyllum spicatum: temperature and light effects and evidence of possible major mechanisms. J Chem Eng Japan 47:488–493
Nakai S, Inoue Y, Hosomi M, Murakami A (2000) Myriophyllum spicatum -released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res 34:3026–3032
Nguyen BT, Rittmann BE (2016) Effects of inorganic carbon and pH on growth kinetics of Synechocystis sp. PCC 6803. Algal Res 19:363–369
Oberholster PJ, Botha AM, Ashton PJ (2009) The influence of a toxic cyanobacterial bloom and water hydrology on algal populations and macroinvertebrate abundance in the upper littoral zone of Lake Krugersdrift, South Africa. Ecotoxicology 18:34–46
Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: causes, consequences, and controls. Microb Ecol 65:995–1010
Pillinger JM, Cooper JA, Ridge I (1994) Role of phenolic compounds in the antialgal activity of barley straw. J Chem Ecol 20:1557–1569
R Development Core Team R (2011) R: a language and environment for statistical computing. R Found Stat Comput 1:409
Ramasarma T, Rao AVS, Devi MM, Omkumar RV, Bhagyashree KS, Bhat SV (2014) New insights of superoxide dismutase inhibition of pyrogallol autoxidation. Mol Cell Biochem 400:277–285
Rippka R, Deruelles J, Waterbury JB (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Sandrini G, Ji X, Verspagen JMH, Tann RP, Slot PC, Luimstra VM, Schuurmans JM, Matthijs HCP, Huisman J (2016) Rapid adaptation of harmful cyanobacteria to rising CO2. Proc Natl Acad Sci 113:9315–9320
Sandrini G, Matthijs HCP, Verspagen JMH, Muyzer G, Huisman J (2014) Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. ISME J 8:589–600
Shao J, Wu Z, Yu G, Peng X, Li RH (2009) Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (Cyanobacteria): from views of gene expression and antioxidant system. Chemosphere 75:924–928
Somridhivej B, Boyd CE (2017) Likely effects of the increasing alkalinity of inland waters on aquaculture. J World Aquac Soc 48:496–502
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. John Wiley & Sons. Inc., New York
Tann RP, Sandrini G, van Beusekom SAM, Matthijs HCP, Huisman J (2016) Diel variation in gene expression of the CO2-concentrating mechanism during a harmful cyanobacterial bloom. Front Microbiol 7:551
Techer D, Fontaine P, Personne A, Viot S, Thomas M (2016) Allelopathic potential and ecotoxicity evaluation of gallic and nonanoic acids to prevent cyanobacterial growth in lentic systems: a preliminary mesocosm study. Sci Total Environ 547:157–165
Vestergaard O, Sand-Jensen K (2000) Alkalinity and trophic state regulate aquatic plant distribution in Danish lakes. Aquat Bot 67:85–107
Wang J, Liu Q, Feng J, Lv JP, Xie SL (2016) Effect of high-doses pyrogallol on oxidative damage, transcriptional responses and microcystins synthesis in Microcystis aeruginosa TY001 (Cyanobacteria). Ecotoxicol Environ Saf 134:273–279
Wu ZB, Gao YN, Wang J, Liu BY, Zhou QH, Zhang YY (2009) Allelopathic effects of phenolic compounds present in submerged macrophytes on Microcystis aeruginosa. Allelopathy J 23:403–410
Xiao X, Huang H, Ge Z, Rounge TB, Shi JY, Xu XH, Li RB, Chen YX (2014) A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): characterization and comparison of their anti-cyanobacterial activities. Environ Microbiol 16:1238–1251
Ye L, Qian J, Jin S, Zuo SP, Mei H, Ma SM (2014) Algicidal effects of four Chinese herb extracts on bloom-forming Microcystis aeruginosa and Chlorella pyrenoidosa. Environ Technol 35:1150–1156
Zhu J, Liu B, Wang J, Gao YN, Wu ZB (2010) Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat Toxicol 98:196–203
Acknowledgments
The authors thank Professor John Beardall for the valuable discussions about the chemistry of alkalinity in water.
Funding
This work was supported by the National Natural Science Foundation of China (31700405, 31500380), Major Science and Technology Program in Henan Province (182102110196), and Breeding Project of Henan Normal University (HNU20180073). Professor Michele Burford was supported by Australian Research Council grant LP120100284.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Y., Fu, Q., Lu, J. et al. Enhanced pyrogallol toxicity to cyanobacterium Microcystis aeruginosa with increasing alkalinity. J Appl Phycol 32, 1827–1835 (2020). https://doi.org/10.1007/s10811-020-02096-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02096-2