Abstract
We tested the interactive effects of increased temperature and nutrient (ammonium, NH4+) levels on physiological properties such as photosynthetic rates, NH4+ uptake rates, relative growth rates, chlorophyll fluorescence, and tissue nutrient contents in Ulva linza Linnaeus. The experiments were conducted at four temperatures (LT, low temperature (15 °C); MT, medium temperature (20 °C); CT, control temperature (25 °C); and HT, high temperature (30 °C)) and three NH4+ concentrations (LN, low nutrient (4 μM); MN, medium nutrient (60 μM); and HN, high nutrient (120 μM)). The interaction between temperature and NH4+ levels influenced the photosynthetic rates, NH4+ uptake rates, relative growth rates, photosynthetic efficiency, tissue nitrogen contents, and C:N ratios in algal tissues. Temperature strongly affected the photosynthetic rates, NH4+ uptake rates, and photosynthetic efficiency. Nutrient enrichment increased the photosynthetic rates, nutrient uptake rates, relative growth rates, photosynthetic efficiency, tissue nitrogen contents, and tissue C:N ratios. Our study results could help understand the physiological responses of U. linza under future ocean environmental conditions such as ocean warming and eutrophication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CO2 concentration has increased from 280 ppm in the pre-industrial era to the current level of 400 ppm because of anthropogenic activities following the Industrial Revolution (IPCC 2014). Many studies predict CO2 concentrations will double their current levels by the year 2100 (Roleda et al. 2012; IPCC 2014). According to the IPCC report (2014), global average temperature will increase by 0.6 to 2.0 °C, based on projections of the climate change model. If no effort is made to curb CO2 emissions, then global average temperature will increase by 2.6 to 4.8 °C (IPCC 2014). In addition, seawater temperatures could increase from 1.9 to 5.8 °C by the end of the twenty-first century under elevated CO2 conditions (IPCC 2014).
Seawater temperature is an important factor for macroalgae survival, growth, reproduction, morphology, and metabolism (Lüning and Neushul 1978; Davison 1991; Wernberg et al. 2010; Rothäusler et al. 2011; Martínez et al. 2012). Temperature change could influence the activity of enzymes including photosynthetic C and N assimilation, ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) activity, and nitrate reductase formation (Raven and Geider 1988; Davison 1991; Yoshida et al. 1999; Berges et al. 2002). Elevated seawater temperature can have a positive effect on some macroalgae (Fan et al. 2014; Zou and Gao 2014). However, ocean warming has negatively affected changing of biomass, productivity, growth, structure of community, and physiological performance of macroalgae (Raven and Geider 1988; Barry et al. 1995; Yesson et al. 2015; Ji et al. 2016; Kay et al. 2016). Macroalgae also experience high or low disruptive stresses in the form of cellular and subcellular damage (Davison and Pearson 1996; Eggert 2012). In addition, biogeographical distribution of macroalgae has moved from the tropical and temperate regions towards the poles because of ocean warming (Wernberg et al. 2011; Díez et al. 2012).
Eutrophication is also an acute environmental problem in the coastal areas that experience ocean warming (Lohman and Priscu 1992; Fei 2004). Elevated concentrations of nitrogen (N) and phosphorus (P) could increase macroalgal growth and biomass (Luo et al. 2012; Hurd et al. 2014; Li et al. 2016). Under natural conditions, nitrogen and phosphorus are factors limiting algal productivity (Dring and Dring 1991). Nitrogen is an important constituent of many compounds such as Rubisco, the main enzyme in photosynthesis (Dawes and Koch 1990); phosphorus has various chloroplast functions, including use in ATP generation, photosynthetic protein production, and enzyme phosphorylation (Zer and Ohad 2003). However, Carpenter (2008) reported that nitrogen and phosphorus could cause ocean eutrophication. Excessive nutrient input causes severe blooming of macroalgae such as Ulva spp., Chaetomorpha spp., Cladophora spp., and Sargassum spp., the latter two being responsible for what are known as green and golden seaweed tide, respectively (Taylor et al. 2001; Cohen and Fong 2006; Ye et al. 2011; Smetacek and Zingone 2013; Li et al. 2016). Bloom-forming species have a negative ecological impact because the decomposition of biomass in the water column decreases oxygen levels in the benthic environment (Lomstein et al. 2006; Wang et al. 2009). In addition, elevated nutrient concentrations have been shown to decrease biodiversity, affect marine habitats, and change ecosystem functioning (Yang et al. 2005; Liu et al. 2009; Mineur et al. 2015).
Ulva spp. are green tide forming species with traits of opportunistic species, which is found near the coastal areas. Opportunistic macroalgae have higher growth and nutrient uptake under optimal environmental conditions than do other macroalgae (Taylor et al. 2001; Nelson et al. 2008). Many studies have been conducted using Ulva spp. under various environmental conditions such as different temperatures, CO2 levels, nutrient concentrations, light intensities, and/or salinities (Figueroa et al. 2014a, b; Stengel et al. 2014; Cui et al. 2015; Kang et al. 2016; Gao et al. 2016a, b; Kang and Chung 2017). Previous studies indicated that the physiological responses of Ulva spp. change differently depending on the species. For example, U. australis was shown to display different physiological responses under elevated CO2/pH and nutrient concentrations (Kang and Chung 2017; Reidenbach et al. 2017). Cui et al. (2015) indicated that U. prolifera, U. compressa, U. flexuosa, and U. linza were influenced differently under various temperatures and light intensities. Therefore, research needs to identify specific information on the physiology of Ulva species. Our study focused on U. linza Linnaeus (Ulvales, Chlorophyta), which several researches have studied under different environmental conditions (Kim et al. 2011; Luo et al. 2012; Kang et al. 2016), but not under combinations of temperature and ammonium (NH4+) concentrations.
In this study, we used NH4+ as the nitrogen form. Ulva spp. grow faster under NH4+ than under nitrate conditions (NO3−) (Ale et al. 2011; Li et al. 2019). In addition, NH4+ is the preferred nitrogen form for macroalgae because, in comparison with NO3−, less energy is required to assimilate its nitrogen (McGlathery et al. 1996; Pedersen and Borum 1996; Runcie et al. 2003). The utilization of NO3− by macroalgae involves the reduction of NH4+ by nitrate reductase activity (NRA) (Syrett 1981; Teichberg et al. 2007).
The objective of this study was to examine the physiological activities of U. linza under elevated temperature and nutrient concentrations. In addition, we tried to determine the interactions between ocean warming and eutrophication in physiological responses of this alga. Therefore, we measured the oxygen evolution rates during photosynthesis, rates of nutrient uptake, rates of relative growth, chlorophyll fluorescence, and nutrient contents in tissues of this alga.
Materials and methods
The samples of U. linza were collected from Cheongsapo, South Korea (35°09′N, 129°11′E) in September 2017. At this sampling site temperature was 25.80 ± 0.50 °C, salinity was 33.50 ± 0.2 ‰, and pH was 8.10 ± 0.12. Temperature and salinity were measured with a YSI Pro 2030 meter (YSI, USA) and pH values were measured with a YSI Pro 10 meter (YSI, USA). Samples were transported to the laboratory and washed several times with 0.20 μm filtered seawater to remove all epiphytes. After washing, the samples were kept in a culture room in filtered seawater at 20 °C with 80 μmol photons m−2 s−1 under a 12:12 light:dark cycle. The samples of U. linza were acclimated 3 days before the experiments. For each treatment, samples (1 g) were placed in 500 mL of filtered seawater. The multi-factorial design experiment was set up with four temperature conditions (LT; low temperature (15 °C), MT; medium temperature (20 °C), CT; control temperature (25 °C), and HT; high temperature (30 °C)) and three NH4+ concentrations (LN; low nutrient (4 μM), MN; medium nutrient (60 μM) and HN; high nutrient (120 μM)). Of the four temperature treatments, 15 and 20 °C were used for the optimal growth condition (Taylor et al. 2001). The temperature 25 °C was used to reflect the summer seawater temperature in the coastal areas of Cheongspo, Korea (KHOA 2017). The upper 30 °C condition represented the summer seawater temperature at Cheongsapo predicted to be recorded with increasing global temperature (4 °C), based on the IPCC report (2014). NH4+ nutrient levels followed Kang and Chung (2017). Other experimental conditions such as temperature, light intensity and light period followed the above acclimation conditions. Each experimental condition had four replicates. The temperature conditions were maintained in the incubator throughout the experiments. To support the NH4+ concentrations, we added NH4Cl to the filtered seawater. The medium was changed every two days to prevent nutrient depletion.
The photosynthetic rates (μmol O2 g−1 FW h−1) and NH4+ uptake rates (μmol NH4+ g−1 FW h−1) were measured 12 h after the beginning of the experiment. The photosynthetic rates (μmol O2 g−1 FW h−1) were measured with a Clark-type microelectrode oxygen sensor (Unisense, Denmark). The oxygen sensor was calibrated by mixing a solution of sodium ascorbate (C6H7NaO6) and sodium hydroxide (NaOH), and it detected photosynthetic rates in less than 1 s.
The NH4+ uptake rates (μmol NH4+ g−1 FW h−1) were determined based on the average amount that disappeared from the culture medium over the incubation period of 12 h. The measurement method followed Parsons et al. (1984). The following equation was used to calculate the NH4+ uptake rate:
where Si is the initial concentration of NH4+, Sf is the final concentration of NH4+ after T hours of incubation, vol is the volume of the culture medium, and W is the fresh weight of each sample.
The growth, chlorophyll fluorescence and carbon (C) and nitrogen (N) contents in algal tissue were measured after 14 days of the experiment. Ulva linza growth was determined at the end of the experiment. The relative growth rates (% day−1) were calculated as follows:
where W1 is the initial fresh weight, W2 is the final fresh weight after 14 days, and T is the cultivation period (14 days).
Chlorophyll fluorescence was measured with a pulse amplitude modulation fluorometer (Diving-PAM, Walz, Germany) at the end of the experiment. The maximum quantum yield of photosystem II was measured as follows:
where Fv/Fm is the photosynthetic efficiency, as measured using saturating pulse under dark-adaptation, Fm is the maximum fluorescence after dark-adaptation, and Fo is the minimum fluorescence after dark-adaptation. Samples were placed in the leaf-clip holders and kept in the dark for 15 min before measuring chlorophyll fluorescence.
Carbon and nitrogen contents in algal tissue were analyzed at the end of the experiment, using samples of U. linza. The samples were dried at 60 °C for 48 h and then ground to a powder. Carbon and nitrogen contents (%) in the tissue were analyzed using an elemental analyzer (Vario-Micro Cube, Elementar Analysensysteme GmbH, Germany). In addition, we calculated C:N ratio on a molar basis.
A two-way analysis of variance (ANOVA) was conducted on all experimental data. Before the statistical analysis was performed, all data were tested for normality and homogeneity. Tukey’s tests were used to compare the treatments. A p value of 0.05 represented significant difference among treatments. All statistical analyses were performed with the SPSS program version 23.0 (IBM, USA).
Results
Photosynthetic rates (μmol O2 g−1 FW h−1) were affected by temperature and NH4+ conditions. In addition, the samples of U. linza were influenced by the interactive effects of temperature and NH4+ concentrations (Table 1). After the 12-h experimental period, the photosynthetic rates (μmol O2 g−1 FW h−1) ranged from 38.10 ± 3.76 to 89.38 ± 8.04 μmol O2 g−1 FW h−1 (Fig. 1). The minimum value was found at LTLN and the maximum value was observed at MTHN. When the temperature remained constant, the photosynthetic rates increased significantly with increasing NH4+ concentrations (p < 0.05). In the case of the HN, the MT treatment had significantly higher photosynthetic rates than did the treatments at CT and HT (p < 0.05).
The rates of NH4+ uptake (μmol NH4+ g−1 FW h−1) were influenced by temperature, NH4+ levels, and the combined effects of temperature and NH4+ levels (Table 1). The minimum NH4+ uptake rate was 0.16 ± 0.02 μmol NH4+ g−1 FW h−1 at HTLN; the maximum rate was 5.93 ± 0.05 μmol NH4+ g−1 FW h−1 at MTHN (Fig. 2). At LN, the NH4+ uptake rates were not significantly different under any temperature conditions (p > 0.05). However, the rate was significantly different between treatments at LT and over LT under LN condition (p < 0.05).
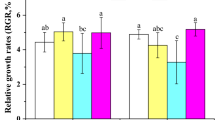
The relative growth rates were measured after 14 days of incubation. The relative growth rates (% day−1) were affected by NH4+ and the combined effects of temperature and NH4+ combinations (Table 1). The relative growth rates ranged from 5.36 ± 0.38 to 8.07 ± 0.12% day−1 (Fig. 3). The minimum relative growth rate was observed at LTLN; the maximum value was observed at MTHN. When the temperature was the same, relative growth rates under MN and HN were significantly higher than that under LN (p < 0.05), but the values were not significantly different between the MN and HN (p > 0.05).
Photosynthetic efficiency (Fv/Fm), as measured by chlorophyll fluorescence after 2 weeks of the experiment, was influenced by temperature, NH4+, and the interactive effects of temperature and NH4+ (Table 1). Fv/Fm ranged from 0.61 ± 0.01 to 0.76 ± 0.02 (Fig. 4). Fv/Fm was lowest at CTLN and highest at MTHN. In addition, Fv/Fm values increased significantly in elevated NH4+ concentrations at all temperatures (p < 0.05), but were not significantly different between MN and HN (p > 0.05). At the HN condition, the Fv/Fm values were significantly different between CT and HT (p < 0.05).
The carbon content in algal tissues (%) was not affected by any culture treatments (Table 1). The tissue carbon contents ranged from 24.40 ± 0.39 to 24.94 ± 0.08% (Fig. 5). Carbon content was lowest at LTMN and highest at MTHN, but there was no significant difference among the culture conditions (p > 0.05).
The tissue nitrogen contents (%) were influenced by NH4+ levels and the combined effects of temperature and NH4+ level (Table 1). The tissue nitrogen contents ranged from 1.52 ± 0.11 to 2.20 ± 0.04% (Fig. 6). Nitrogen content was lowest at MTLN and highest at MTHN. When the temperature remained constant, the tissue nitrogen content increased under elevated NH4+ levels (p < 0.05). When NH4+ levels were the same, tissue nitrogen contents were not significantly different among temperature conditions (p > 0.05).
The C:N ratios in tissue were affected by NH4+ and the interactive effects of temperature and NH4+ level (Table 1). The tissue C:N ratios ranged from 11.32 ± 0.24 to 16.15 ± 0.92 (Fig. 7). The ratio was lowest at MTHN and highest at MTLN. At the MT condition, the ratios were significantly different under elevated NH4+ conditions (p < 0.05). At other temperatures, there were not significantly different between MN and HN (p > 0.05). In the case of HN, the values were significantly different between LT and MT conditions (p < 0.05).
Discussion
Previous studies indicated that the photosynthetic rates of Ulva spp. increased under elevated temperature or nutrient concentrations (Zou and Gao 2014; Li et al. 2016; Kang and Chung 2017). In our study, the photosynthetic rate was highest at MTHN. Photosynthesis in Ulva sp. was highest at 20 to 25 °C (Murase et al. 1994). Also, the elevated nutrient concentrations increased photosynthetic rates in Ulva spp. (Kang et al. 2016; Kang and Chung 2017). Li et al. (2016) showed that the photosynthetic rates of U. prolifera were increased at elevated nitrogen and phosphorus levels. In case of the increased nutrient concentrations in the seawater, macroalgae could easily take up nutrients because of higher nutrient availability (Yu and Yang 2008). The higher availability of nutrients could increase photosynthesis in U. linza because this species is an opportunistic and bloom-forming species found near coastal areas. In addition, Zou and Gao (2014) indicated that photosynthetic rates increase under elevated temperatures at high nutrient concentrations.
The rate of NH4+ uptakes were influenced by temperature, NH4+ treatment, and the combined effects of temperature and NH4+ treatments. Temperature is an important factor in the nutrient uptake of algae (Gao et al. 2018). Fan et al. (2014) indicated that macroalgae could have different nutrient requirements under various temperature conditions. Our result shows that the NH4+ uptake rate was highest at MTHN. Fan et al. (2014) also showed that the nutrient uptake rate of U. prolifera was highest at 20 °C. In addition, the rate increased at higher temperatures (25 and 30 °C) when compared to control the temperature condition (15 °C) at 60 and 120 μM NH4+ (Fan et al. 2014). The NH4+ uptake rates of Ulva spp. increased under elevated NH4+ concentrations. Many researches have indicated that increased nutrient concentrations could elevate the nutrient uptake rate of Ulva spp. (Luo et al. 2012; Kang and Chung 2017; Reidenbach et al. 2017). Seawater has high nutrient concentrations; so, macroalgae could more easily and efficiently perform nutrient uptake in seawater than in other environments (Yu and Yang 2008; Runcie et al. 2003). In addition, the morphological and opportunistic characteristics of U. linza could increase nutrient uptake rates under elevated nutrient concentrations (Littler 1980; Wallentinus 1984). The high nutrient uptake rate could affect the metabolism of macroalgae because they generate Rubisco and ATP using nitrogen and phosphorus, respectively (Dawes and Koch 1990; Zer and Ohad 2003).
The relative growth rates of our samples were not affected by elevated temperature conditions. However, several studies have indicated that temperature affects macroalgal growth (Mantri et al. 2011; Gao et al. 2016a, b, 2017; Chen et al. 2018). Temperature is an important factor affecting the physiology of algal metabolism and growth (Zou and Gao 2013, 2014). Mantri et al. (2011) found that seaweed growth could accelerate immediately under elevated temperatures because of increased metabolism. Taylor et al. (2001) found that Ulva species could grow over a broad temperature range (10–30 °C). The growth rates of Ulva spp. were highest at 15–20 °C and decreased over 20 °C (Taylor et al. 2001). Cui et al. (2015) also observed that U. linza grew the fastest at 20 °C. However, our results suggest that relative growth rates did not significantly decrease over 20 °C under constant NH4+ concentrations. Therefore, there may be several environmental conditions influencing U. linza growth. In contrast, the relative growth rate of Ulva sp. has been reported to increase under various nutrient concentrations (Zou and Gao 2014; Li et al. 2016; Kang and Chung 2017; Ober and Thornber 2017; Reidenbach et al. 2017). Kang et al. (2016) mentioned that the growth of U. linza increased more under elevated nutrient concentrations at a low salinity (10 ‰) condition than under a high salinity one (30 ‰). In addition, the relative growth rates of U. linza were not only affected by temperature but also by the interaction between temperature and NH4+. Therefore, in our culture conditions, the NH4+ level was the main factor affecting growth in U. linza. Lotze and Worm (2002) have reported that elevated temperature and nutrient levels have a combined effect on the growth of green algae.
Schreiber and Bilger (1993) state that chlorophyll fluorescence studies are a powerful tool for analyzing photosynthesis. Fv/Fm is a particularly valuable measure of photosynthetic efficiency (Hanelt et al. 1995). Many studies have been conducted on photosynthetic efficiency under different temperatures or nutrient levels (Padilla-Gamino and Carpenter 2007; Zou and Gao 2014; Kang and Kim 2016). The photosynthetic efficiency of our samples was affected by temperature. Kang and Kim (2016) reported that warming temperatures do not affect the photosynthetic efficiency of U. australis. Padilla-Gamino and Carpenter (2007) show that the photosynthetic efficiency of Asparagopsis taxiformis decreased under elevated temperature. However, the photosynthetic efficiency in their study possibly changed seasonally because of acclimatization to natural seawater temperatures in different seasons. Therefore, we need to determine the pattern of photosynthetic efficiency of U. linza in different seasons. The photosynthetic efficiency was also affected by NH4+ levels. Photosynthetic efficiency increases with increasing nitrogen concentrations in macroalgae (Dawes and Koch 1990). In the case of U. australis, photosynthetic efficiency increased at higher NH4+ concentrations (Kang and Chung 2017; Reidenbach et al. 2017). However, Kang et al. (2016) found that the photosynthetic efficiency of U. linza was not affected by elevated nitrate concentrations. Therefore, the photosynthetic efficiency of U. linza changes with various nitrogen sources and concentrations.
The tissue carbon and nitrogen contents and C:N ratios of U. linza were not affected by different temperatures. A similar result was also was found in U. australis (Kang and Kim 2016). Our results, however, show that NH4+ concentration affects tissue nitrogen content and C:N ratio. The C:N ratio in tissues is a good index of the physiological status of macroalgae and can be used as an indicator of macroalgae status (Vergara et al. 1993; Kang et al. 2011). When nutrients were abundant, the C:N ratio of U. australis decreased (Kang and Chung 2017; Reidenbach et al. 2017; Ober and Thornber 2017). Ober and Thornber (2017) stated that C:N ratios decrease under higher nutrient treatments, and this, in turn, increases the tissue quality of samples. Under sufficient nutrient concentrations, macroalgae could possibly assimilate more nutrients compared with the control nutrient level (Reidenbach et al. 2017). Gómez-Pinchetti et al. (1998) indicated that macroalgae might store the nitrogen in their tissue under abundant nutrient conditions. We found that tissue nitrogen content and C:N ratio were significantly affected by the interaction between temperature and NH4+ concentrations. Although temperature and NH4+ concentration had a significant interactive effect on the C:N ratio of U. linza, NH4+ concentration is the main driver of C:N ratio because the temperatures were not affected any culture treatments.
In conclusion, the U. linza was positively affected by increased temperatures and nutrient concentrations. The photosynthetic rates, NH4+ uptake rates, and photosynthetic efficiency were affected by temperature, NH4+ concentration, and the interaction between temperature and NH4+ concentration. The relative growth rates, tissue nitrogen contents, and C:N ratios were affected by NH4+ concentration and the combined effects of temperature and NH4+ concentration. The tissue carbon contents, however, were not affected by any culture condition. According to our study, the physiological responses of U. linza could increase under future ocean conditions. This phenomenon could be a serious problem near the coastal areas by inducing the formation of green tides. On the contrary, this species could have a bioremediation capacity because of its fast growth and high nutrient uptake rate. Therefore, both characteristics of U. linza have to be considered when judging whether it represents a harmful macroalga or a potential solution to coastal environmental problems.
References
Ale MT, Mikkelsen JD, Meyer AS (2011) Differential growth response of Ulva lactuca to ammonium and nitrate assimilation. J Appl Phycol 23:345–351
Barry JP, Baxter CH, Sagarin RD, Gilman SE (1995) Climate-related, long-term faunal changes in a California rocky intertidal community. Science 267:672–675
Berges JA, Varela DE, Harrison PJ (2002) Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar Ecol Prog Ser 225:139–146
Carpenter SR (2008) Phosphorus control is critical to mitigating eutrophication. Proc Natl Acad Sci 105:11039–11040
Chen B, Zou D, Du H, Ji Z (2018) Carbon and nitrogen accumulation in the economic seaweed Gracilaria lemaneiformis affected by ocean acidification and increasing temperature. Aquaculture 482:176–182
Cohen RA, Fong P (2006) Using opportunistic green macroalgae as indicators of nitrogen supply and sources to estuaries. Ecol Appl 16:1405–1420
Cui J, Zhang J, Huo Y, Zhou L, Wu Q, Chen L et al (2015) Adaptability of free-floating green tide algae in the Yellow Sea to variable temperature and light intensity. Mar Pollut Bull 101:660–666
Davison IR (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27:2–8
Davison IR, Pearson GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32:197–211
Dawes CJ, Koch EW (1990) Physiological responses of the red algae Gracilaria verrucosa and G. tikvahiae before and after nutrient enrichment. Bull Mar Sci 46:335–344
Díez I, Muguerza N, Santolaria A, Ganzedo U, Gorostiaga JM (2012) Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuar Coast Shelf Sci 99:108–120
Dring MJ, Dring MH (1991) The biology of marine plants. Cambridge University Press, Cambridge
Eggert A (2012) Seaweed responses to temperature. In: Wiencke C, Bischof K (eds) Seaweed biology. Springer, Berlin, pp 47–66
Fan X, Xu D, Wang Y, Zhang X, Cao S, Mou S, Ye N (2014) The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: implications for the explosion in green tides. J Appl Phycol 26:537–544
Fei X (2004) Solving the coastal eutrophication problem by large scale seaweed cultivation. Hydrobiologia 12:145–151
Figueroa FL, Barufi JB, Malta EJ, Conde-Álvarez R, Nitschke U, Arenas F, Mata M, Connan S, Abreu MH, Marquardt R, Vaz-Pinto F, Konotchick T, Celis-Plá PSM, Hermoso M, Ordoñez G, Ruiz E, Flores P, de los Ríos J, Kirke D, Chow F, Nassar CAG, Robledo D, Pérez-Ruzafa Á, Bañares-España E, Altamirano M, Jiménez C, Korbee N, Bischof K, Stengel DB (2014a) Short-term effects of increasing CO2, nitrate and temperature on three Mediterranean macroalgae: biochemical composition. Aquat Biol 22:177–193
Figueroa FL, Conde-Álvarez R, Barufi JB, Celis-Plá PSM, Flores P, Malta EJ, Stengel DB, Meyerhoff O, Pérez-Ruzafa Á (2014b) Continuous monitoring of in vivo chlorophyll a fluorescence in Ulva rigida (Chlorophyta) submitted to different CO2, nutrient and temperature regimes. Aquat Biol 22:195–212
Gao X, Endo H, Nagaki M, Agatsuma Y (2016a) Growth and survival of juvenile sporophytes of the kelp Ecklonia cava. Fish Sci 82:623–629
Gao G, Zhong Z, Zhou X, Xu J (2016b) Changes in morphological plasticity of Ulva prolifera under different environmental conditions: a laboratory experiment. Harmful Algae 59:51–58
Gao G, Clare AS, Rose C, Caldwell GS (2017) Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar Pollut Bull 114:439–447
Gao G, Clare AS, Rose C, Caldwell GS (2018) Ulva rigida in the future ocean: potential for carbon capture, bioremediation, and biomethane production. GCB Bioenergy 10:39–51
Gómez-Pinchetti JL, del Campo FE, Díez PM, Reina GG (1998) Nitrogen availability influences the biochemical composition and photosynthesis of tank-cultivated Ulva rigida (Chlorophyta). J Appl Phycol 10:383–389
Hanelt D, Uhrmacher S, Nultsch W (1995) The effect of photoinhibition on photosynthetic oxygen production in the brown alga Dictyota dichotoma. Plant Biol 108:99–105
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology. Cambridge University Press, Cambridge
IPCC (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York
Ji Y, Xu Z, Zou D, Gao K (2016) Ecophysiological responses of marine macroalgae to climate change factors. J Appl Phycol 28:2953–2967
Kang JW, Chung IK (2017) The effects of eutrophication and acidification on the ecophysiology of Ulva pertusa Kjellman. J Appl Phycol 29:2675–2683
Kang EJ, Kim KY (2016) Effects of future climate conditions on photosynthesis and biochemical component of Ulva pertusa (Chlorophyta). Algae 31:9–59
Kang YH, Park SR, Chung IK (2011) Biofiltration efficiency and biochemical composition of three seaweed species cultivated in a fish-seaweed integrated culture. Algae 26:97–108
Kang EJ, Kim JH, Kim K, Kim KY (2016) Adaptations of a green tide forming Ulva linza (Ulvophyceae, Chlorophyta) to selected salinity and nutrients conditions mimicking representative environments in the Yellow Sea. Phycologia 55:210–218
Kay LM, Schmidt AL, Wilson KL, Lotze HK (2016) Interactive effects of increasing temperature and nutrient loading on the habitat-forming rockweed Ascophyllum nodosum. Aquat Bot 133:70–78
KHOA (2017) Korea hydrographic and oceanographic agency. Busan, Korea http://www.khoa.go.kr/
Kim JH, Kang EJ, Park MG, Lee BG, Kim KY (2011) Effects of temperature and irradiance on photosynthesis and growth of a green-tide-forming species (Ulva linza) in the Yellow Sea. J Appl Phycol 23:421–432
Li S, Yu K, Huo Y, Zhang J, Wu H, Cai C, Liu Y, He P (2016) Effects of nitrogen and phosphorus enrichment on growth and photosynthetic assimilation of carbon in a green tide-forming species (Ulva prolifera) in the Yellow Sea. Hydrobiologia 776:161–171
Li H, Zhang Y, Chen J, Zheng X, Liu F, Jiao N (2019) Nitrogen uptake and assimilation preferences of the main green tide alga Ulva prolifera in the Yellow Sea, China. J Appl Phycol 31:625–635
Littler MM (1980) Morphological form and photosynthetic performances of marine macroalgae: tests of a functional/form hypothesis. Bot Mar 23:161–166
Liu D, Keesing JK, Xing Q, Shi P (2009) World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar Pollut Bull 58:888–895
Lohman K, Priscu JC (1992) Physiological indicator of nutrient deficiency in Cladophora (Chlorophyta) in the Clark Fork of the Columbia River, Montana. J Phycol 28:443–448
Lomstein BA, Guldberg LB, Neubauer ATA, Hansen J, Donnelly A, Herbert RA, Viaroli P, Giordani G, Azzoni R, de Wit R, Finster K (2006) Benthic decomposition of Ulva lactuca: a controlled laboratory experiment. Aquat Bot 85:271–281
Lotze HK, Worm B (2002) Complex interactions of climatic and ecological controls on macroalgal recruitment. Limnol Oceanogr 47:1734–1741
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and Central California. Mar Biol 45:297–309
Luo MB, Liu F, Xu ZL (2012) Growth and nutrient uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquat Bot 100:18–24
Mantri VA, Singh RP, Bijo AJ, Kumari P, Reddy CRK, Jha B (2011) Differential response of varying salinity and temperature on zoospore induction, regeneration and daily growth rate in Ulva fasciata (Chlorophyta, Ulvales). J Appl Phycol 23:243–250
Martínez B, Arenas F, Rubal M, Burgués S, Esteban R, García-Plazaola I, Figueroa FL, Pereira R, Saldaña L, Sousa-Pinto I, Trilla A, Viejo RM (2012) Physical factors driving intertidal macroalgae distribution: physiological stress of a dominant fucoid at its southern limit. Oecologia 170:341–353
McGlathery KJ, Pedersen MF, Borum J (1996) Changes in intracellular nitrogen pools and feedback controls on nitrogen uptake in Chaetomorpha linum (Chlorophyta). J Appl Phycol 32:393–401
Mineur F, Arenas F, Assis J, Davies AJ, Engelen AH, Fernandes F, Malta E-J, Thibaut T, Nguyen TV, Vaz-Pinto F, Vranken S, Serrão EA, De Clerck O (2015) European seaweeds under pressure: consequences for communities and ecosystem functioning. J Sea Res 98:91–108
Murase N, Maegawa M, Matsui T, Ohgai M, Katayama N, Saitoh M, Yokohama Y (1994) Growth and photosynthesis temperature characteristics of the sterile Ulva pertusa. Nippon Suisan Gakkaishi 65:625–630
Nelson TA, Haberlin K, Nelson AV, Ribarich H, Hotchkiss R, Van Alstyne KL, Buckingham L, Simunds DJ, Fredrickson K (2008) Ecological and physiological controls of species composition in green macroalgal blooms. Ecology 89:1287–1298
Ober GT, Thornber CS (2017) Divergent responses in growth and nutritional quality of coastal macroalgae to the combination of increased pCO2 and nutrients. Mar Environ Res 131:69–79
Padilla-Gamino JL, Carpenter RC (2007) Seasonal acclimatization of Asparagopsis taxiformis (Rhodophyta) from different biogeographic regions. Limnol Oceanogr 52:833–842
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, NewYork, USA, 173pp
Pedersen MF, Borum J (1996) Nutrient control of algal growth in estuarine waters. Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Mar Ecol Prog Ser 142:261–272
Raven JA, Geider RJ (1988) Temperature and algal growth. New Phytol 110:441–461
Reidenbach LB, Fernandez PA, Leal PP, Noisette F, McGraw CM, Revill AT, Hurd CL, Kübler JE (2017) Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS One 12:e0188389
Roleda MY, Morris JN, McGraw CM, Hurd CL (2012) Ocean acidification and seaweed reproduction: increased CO2 ameliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Glob Chang Biol 18:854–864
Rothäusler E, Gómez I, Karsten U, Tala F, Thiel M (2011) Physiological acclimation of floating Macrocystis pyrifera to temperature and irradiance ensures long-term persistence at the sea surface at mid-latitudes. J Exp Mar Biol Ecol 405:33–41
Runcie JW, Ritchie RJ, Larkum AW (2003) Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquat Bot 76:155–174
Schreiber U, Bilger W (1993) Progress in chlorophyll fluorescence research: major developments during the past years in retrospect. Progr Bot 54:151–173
Smetacek V, Zingone A (2013) Green and golden seaweed tides on the rise. Nature 504:84–88
Stengel DB, Conde-Álvarez R, Connan S, Nitschke U, Arenas F, Abreu H, Barufi JB, Chow F, Robledo D, Malta EJ, Mata M, Konotchick T, Nassar C, Pérez-Ruzafa Á, López D, Marquardt R, Vaz-Pinto F, Celis-Plá PSM, Hermoso M, Ruiz E, Ordoñez G, Flores P, Zanolla M, Bañares-España E, Altamirano M, Korbee N, Bischof K, Figueroa FL (2014) Short-term effects of CO2, nutrients and temperature on three marine macroalgae under solar radiation. Aquat Biol 22:159–176
Syrett PJ (1981) Nitrogen metabolism of microalgae. Can J Fish Aquat Sci 210:182–210
Taylor R, Fletcher RL, Raven JA (2001) Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate. Bot Mar 44:327–336
Teichberg M, Heffner LR, Fox S, Valiela I (2007) Nitrate reductase and glutamine synthetase activity, internal N pools, and growth of Ulva lactuca: responses to long and short-term N supply. Mar Biol 151:1249–1259
Vergara JJ, Niell FX, Torres M (1993) Culture of Gelidium sesquipedale (Clem.) born. Et Thur. In a chemostat system. Biomass production and metabolic responses affected by N flow. J Appl Phycol 5:405–415
Wallentinus I (1984) Comparisons of nutrient uptake rates for Baltic macroalgae with different thallus morphologies. Mar Biol 80:215–225
Wang Y, Zhou B, Tang X (2009) Effects of two species of macroalgae—Ulva pertusa and Gracilaria lemaneiformis—on growth of Heterosigma akashiwo (Raphidophyceae). J Appl Phycol 21:375–385
Wernberg T, Thomsen MS, Tuya F, Kendrick GA, Staehr PA, Toohey BD (2010) Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol Lett 13:685–694
Wernberg T, Russell BD, Moore PJ, Ling SD, Smale DA, Campbell A, Coleman MA, Steinberg PD, Kendrick GA, Connell SD (2011) Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J Exp Mar Biol Ecol 400:7–16
Yang H, Zhou Y, Mao Y, Li X, Liu Y, Zhang F (2005) Growth characters and photosynthetic capacity of Gracilaria lemaneiformis as a biofilter in a shellfish farming area in Sanggou Bay, China. J Appl Phycol 17:199–206
Ye NH, Zhang XW, Mao YZ, Liang CW, Xu D, Zou J, Zhuang Z, Wang QY (2011) ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecol Res 26:477
Yesson C, Bush LE, Davies AJ, Maggs CA, Brodie J (2015) Large brown seaweeds of the British Isles: evidence of changes in abundance over four decades. Estuar Coast Shelf Sci 155:167–175
Yoshida S, Hotsubo K, Kawamura Y, Murai M, Arakawa K, Takezawa D (1999) Alterations of intracellular pH in response to low temperature stresses. J Plant Res 112:225–236
Yu J, Yang YF (2008) Physiological and biochemical response of seaweed Gracilaria lemaneiformis to concentration changes of N and P. J Exp Mar Biol Ecol 367:142–148
Zer H, Ohad I (2003) Light, redox state, thylakoid-protein phosphorylation and signaling gene expression. Trends Biochem Sci 28:467–470
Zou D, Gao K (2013) Thermal acclimation of respiration and photosynthesis in the marine macroalga Gracilaria lemaneiformis (Gracilariales, Rhodophyta). J Phycol 49:61–68
Zou D, Gao K (2014) The photosynthetic and respiratory responses to temperature and nitrogen supply in the marine green macroalga Ulva conglobata (Chlorophyta). Phycologia 53:86–94
Funding
This work was financially supported by a grant from the National Institute of Fisheries Science (R2019009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.E., Kang, J.W. The interactive effects of elevated temperature and nutrient concentrations on the physiological responses of Ulva linza Linnaeus (Ulvales, Chlorophyta). J Appl Phycol 32, 2459–2467 (2020). https://doi.org/10.1007/s10811-019-02031-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-02031-0