Abstract
Hypertension affects about a quarter of the global population and is a major risk factor in cardiovascular diseases. Current pharmacological treatments are considered useful but lead to secondary effects that end in low compliance. In this work, a transplastomic Chlamydomonas reinhardtii strain producing three antihypertensive peptides (AHP) as a fusion protein has been developed. The chimeric protein was coded by a synthetic gene and transferred to the plastome in a site-specific integration approach. Expression was mediated by the rbcL promoter. Molecular analysis confirmed the presence of the transgene inserted in the chloroplast genome. An ELISA quantification assay indicated that the amount of recombinant protein is 34.4 ng per mg of freeze-dried biomass. The RPLKPW and AINPSK peptides were identified by HPLC after in vitro digestion form biomass of the transplastomic C. reinhardtii strain. The antihypertensive effect of the recombinant protein was demonstrated after intragastric administration of the genetically modified strain to spontaneously hypertensive rats (SHR) at a dose of 10 mg of recombinantAHP3 protein per kg of body weight with the maximal decrease in blood pressure 6 h post-administration. These results suggested that this transplastomic strain can be used to obtain a large quantity of antihypertensive peptides which could be useful for the production of functional foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is a major risk factor for the development of serious diseases, which include myocardial infarction, coronary heart disease, and nephropathy (WHO 2016). In recent years, a number of antihypertensive peptides (AHP) have been isolated, purified, and identified from different natural food sources (Abubakar et al. 1998; FitzGerald and Meisel 2000; Shah 2000).
In spite of the different biological pathways regulating blood pressure in living organisms, hypotensive peptides mainly act on the renin-angiotensin system (RAS), which starts by the conversion of angiotensinogen (secreted from the liver) to the prehypertensive hormone angiotensin I (an inactive decapeptide, NRVYIHPFHL) by the action of renin that is a proteolytic enzyme secreted by the kidney. Angiotensin I is further converted to angiotensin II (an active octapeptide, NRVYIHPF) by the action of the angiotensin converting enzyme (ACE), which is a metallopeptidase with two zinc-active catalytic sites (Soffer 1976). Angiotensin II (Ang II) raises blood pressure by acting directly on blood vessels, sympathetic nerves, and adrenal glands. These effects arise from the interaction of Ang II with two main receptors: angiotensin type 1 (AT1) and type 2 (AT2) receptors (Zhuo et al. 2013). By directly binding to AT1 receptors, Ang II causes vasoconstriction in vascular smooth muscle cells, promoting sodium and water reabsorption in the kidney, and eventually elevating blood pressure (Kovács et al. 2002). However, excessive stimulation of the AT1 receptor will induce a counterbalance mechanism that is delivered by the AT2 receptor, which mediates vasodilation upon activation and releases nitric oxide (NO) (Stankevicius et al. 2003; Zhuo et al. 2013).
Moreover, the ACE can also inactivate the vasodilator bradykinin (RPPGFSPFR), which possesses hypotensive activity (Okamoto et al. 1995; Diplock et al. 1999). Bradykinin acts through two receptors, type 1 and 2. Both receptors induce the production of NO and prostacyclin in endothelial cells, which are potent vasodilators (Reaves et al. 2003). Consequently, the ACE has a critical dual role in blood pressure control. Overall, the activation of this enzyme would result in an increase of systematic vascular resistance and an elevated blood pressure. Thus, inhibition of the ACE is the therapeutic target for antihypertensive drug development or preferably non-pharmacological (food-derived peptides) methods of prevention and management of hypertension (Hackenthal et al. 1990). Bioactive peptides derived from food sources are generally short-chain peptides with 2–12 amino acids that are regarded as safer and cost-effective alternatives for the prevention and treatment of hypertension (Champagne 2006).
ACE-inhibitory peptides bind to the active sites of the ACE (Natesh et al. 2003). The blocking effect of ACE inhibitors in the angiotensin II synthesis causes vasodilatation and a decrease in blood pressure via inhibition of the kininase 2 enzyme; also, the breakdown of bradykinin is inhibited (Johnston and Risvanis 1997). These kinds of peptides generally consist of specific amino acids. It has been reported that the presence of some amino acids such as tryptophan (W), proline (P), lysine (K), isoleucine (I), leucine (L), valine (V), tyrosine (Y), phenylalanine (F), and arginine (R) has strong influence on ACE binding (López-Fandiño et al. 2006; Murray and FitzGerald 2007; Guang and Phillips 2009; Iwaniak et al. 2014; ). For example, peptides like IY, LW, IKW, LKPNM, and LKP have shown potent ACE-inhibitory activity after oral administration in SHR (spontaneously hypertensive rats); these peptides have been obtained by purification of enzymatic digests of bonito muscle (Katsuo-bushi). Their IC50 (the concentration of an ACE inhibitor needed to inhibit 50% of the ACE activity) were 2.1, 6.8, 0.21, 2.4, and 0.32 μM, respectively (Fujita et al. 2000). Similarly, the AINPSK peptide, derived from a tryptic digest of bovine αS2-casein, shows ACE-inhibitory activity. Their IC50 was 60 μM (Tauzin et al. 2002).
However, not all of these peptides have the same action mechanism, for example the RPLKPW peptide is a nitric oxide-dependent vasorelaxing peptide. Its antihypertensive effect was demonstrated after oral administration in SHR at dose of 0.1 mg kg−1 with the maximal decrease in blood pressure 4 h post-administration. This peptide was first isolated from a chymotryptic digest of ovalbumin; afterwards, it was chemically synthetized and purified by reversed-phase chromatography (Yamada et al. 2002).
All of these AHP derived from food have been object of considerable attention since they exert significant effects in the decrease blood pressure and are considered safe for humans, thus having a potential use as nutraceuticals (Clare and Swaisgood 2000; Fujita et al. 2000). In most of the cases, the bioactive peptides are in a latent state as part of the sequence of a precursor protein and need to be released to become active. The releasing mechanism is typically mediated by enzymatic digestion, mainly by trypsin and pepsin (Puchalska et al. 2012). Concerning the production, most of the AHP are obtained by means of enzymatic hydrolysis. However, due to the low content of AHP in natural food proteins, few reports are available about the commercial production of AHP (Yamamoto et al. 2003). Industrial production of proteins by recombinant DNA technology has gained a prominent success; it could be the most promising method for mass production of AHP. The advantages of genetic engineering techniques to prepare AHP include higher protein yield and lower cost when compared to traditional enzymatic digestion from natural food protein (Yabuta et al. 1995).
Chlamydomonas reinhardtii is considered a convenient platform for the production of valuable proteins. In particular, the use of chloroplast-based expression systems has gained great interest due to the high recombinant protein levels achieved thus far (Kumar and Daniell 2004; Fletcher et al. 2007; Mayfield et al. 2007; Wang et al. 2008). The ability of propagating this alga in full containment to rapidly generate stable transgenic lines and the GRAS (generally recognized as safe) category assigned by the FDA makes the chloroplast of C. reinhardtii an attractive biofactory (Franklin and Mayfield 2004). In the present study, a C. reinhardtii strain-producing AHP is reported. The antihypertensive activity was assessed in SHR after intragastric administration of freeze-dried biomass.
Materials and methods
Plasmid construction
Three peptides with antihypertensive activity previously reported (RPLKPW, LKPNM, and AINPSK) were chosen to design a fusion protein. Between each peptide, one to four amino acid linkers were included, corresponding to gastrointestinal cleavage sites for trypsin, pepsin, and chymotrypsin (Fig. 1a). A histidine tag was included at the N-terminus to facilitate the detection of the recombinant protein. The gene, named AHP3, was synthesized by GenScript USA Inc., (NJ, USA) following codon optimization for expression in the chloroplast of C. reinhardtii, including NcoI flanking restriction sites to facilitate cloning. The resulting gene was cloned into the p463 expression vector at the unique NcoI restriction site (Chlamydomonas connection, http://www.chlamy.org/).
a Nucleotide and traduced amino acid sequence of the AHP3 chimera cloned in the p463 vector. The AHP3 synthetic gene was optimized for chloroplast expression in C. reinhardtii. NcoI restriction sites were included at the ends. rbs: Ribosome binding site. b Schematic representation of the p463-AHP3 vector. The AHP3 gene is under the control of the rbcL promoter. The regions encoding the spectinomycin resistance gene (aadA), the 3′ rbcL untranslated region (UTR), the ribosome binding sites (rbs), and the homologous recombination region (tscA) are indicated. The regions used to construct internal oligonucleotides into the AHP3 and aadA genes are represented by left and right arrows. c Presence of the AHP3 gene and d aadA gene in C. reinhardtii transformants. Figures represent the outcome of PCR reactions carried out for detection of AHP3 and aadA genes in genomic DNA samples isolated from C. reinhardtii putatively transgenic. Lane 1: molecular weight marker. Lanes 2–5: putative transformed lines (Cr.1 to Cr.4). Lane 6: untransformed line (Wild Type: WT). Lane 7: positive control (p463-AHP3)
The p463 vector (Fig. 1b) contains the rbcL promoter, the 5′ and 3′ rbcL untranslated region (UTR), the spectinomycin resistance gene (aadA), and the homologous recombination region tscA (Dreesen et al. 2010). The rbcL (ribulose bisphosphate carboxylase large subunit) was selected because of its association with the most expressed region in C. reinhardtii (Klein et al. 1994); the rbcL promoters and their respective UTRs have been used successfully before (Hallmann 2007; Manuell et al. 2007; Surzycki et al. 2009).
After cloning, one positive clone (selected by restriction analysis and sequencing), was used to perform a biolistic procedure (p463-AHP3). All these procedures were performed using standard molecular cloning techniques (Sambrook et al. 1989).
Chlamydomonas reinhardtii transformation
Wild-type C. reinhardtii strain CC-137 (mt+) obtained from the Chlamydomonas Center (http://www.chlamy.org/) was used in transformation experiments. Cells were grown in Tris-acetate-phosphate (TAP) medium. Liquid cultures were kept in an incubation shaker under fluorescent white light (80 μmol photons m−2 s−1) (16 h light/8 h dark cycles) at 25 °C; cell growth was continued until the late logarithmic phase. Chloroplast transformations were performed by particle bombardment using DNA-coated gold particles prepared as described elsewhere (Daniell et al. 2004). The bombardment parameters used were those described by Campos-Quevedo et al. (2013). Chloroplast transplastomic lines were identified by growing cultures in TAP media containing 100 mg L−1 spectinomycin as selective agent.
PCR analysis
Genomic DNA from four putative transformed colonies developed in selective media was extracted according to Goldschmidt-Clermont (1991); DNA from an untransformed strain (WT) was used as negative control. PCR analysis was performed with specific primers for the AHP3 and aadA genes to amplify 180 and 790 bp amplicons, respectively. After 7 min of initial denaturation at 95 °C, the samples were subjected to 35 PCR cycles under the following conditions: 95 °C for 40 s, 55 °C for 45 s, and 72 °C for 50s, with a final extension at 72 °C for 10 min. The PCR products were electrophoresed in 2 or 1% agarose gels. To prove site specific integration of the expression cassette and homoplasmy, another PCR was performed with primers annealing on the tscA (primer R1) and ch1N regions (primer R2), which are located flanking the expected insertion site.
ELISA and Western blot analyses
Soluble protein extracts from untransformed and four transformed C. reinhardtii lines were obtained using a buffer containing 50 mM Tris-HCl, pH 8, 40 mM NaCl, 0.1% Tween 20, and 1× Complete protease inhibitor cocktail (Roche, IN, USA), following the procedure previously described by Campos-Quevedo et al. (2013). Protein concentration was measured by the Bradford assay (1976). The recombinant protein was quantified by ELISA analysis using lyophilized biomass of C. reinhardtii. Microtiter plates were coated overnight at 4 °C with total soluble protein (TSP) (100 mg well−1) from either wild type or transplastomic C. reinhardtii strains, along with a standard curve prepared with known amounts of the pure RPLKPW peptide (produced by chemical synthesis, GenScript Inc., USA). The wells were washed with PBST, blocked with milk at 6%, and incubated with an antiRPLKPW polyclonal antibody produced in rabbit, overnight at 4 °C (dilution 1:1000). After a washing step, the plates were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at 37 °C (dilution 1:3000). The colorimetric reaction was developed with an ABTS substrate solution (Sigma, USA). The optical density was registered at a wavelength of 415 nm in a microplate reader (Glomax-Multi, Promega). The amount of recombinant protein was expressed as nanogram of recombinant protein per milligram of lyophilized biomass.
For Western blot analysis, 20 μg of total soluble protein (TSP) was denatured by the addition of SDS-PAGE loading buffer; the samples were boiled for 5 min and resolved in a 12% polyacrylamide gel. The proteins were then transferred onto a PVDF membrane by ultra-fast transfer (Trans-Blot Turbo, BIO-RAD, USA). After blocking with a 5% BSA solution, the blot was incubated with primary antibodies directed against the His tag (1:2000 dilution; GenScript USA Inc., USA). A horseradish peroxidase (HRP)-coupled antimouse secondary antibody was used (1:10,000 dilution; Sigma, USA). The signal was detected by chemiluminescence using the Pierce ECL Western blotting Substrate following the manufacturer’s instructions (Thermo Scientific, USA).
In vitro digestion and identification by HPLC
The in vitro simulation of gastrointestinal digestion is a very useful tool to assess the stability and bioavailability of peptides with biological activity; for this experiment, lyophilized biomass was used. Fifty milligrams of biomass from WT and transplastomic C. reinhardtii strains were dispersed in 25 mL of saline solution, setting the pH value to 2. Pepsin was added at a 1:50 ratio (enzyme:substrate, w/w). The reaction was incubated for 90 min at 37 °C under stirring. Afterwards, the pH of the samples was adjusted to 7.5. Trypsin and chymotrypsin were added at 1:200 (w/w) ratios and the reaction was incubated for 180 min at 37 °C under stirring. Digestion was stopped by boiling the reaction medium for 10 min and samples were withdrawn for peptides identification by HPLC. The procedure was as follows: 100 μL of each sample from the in vitro digestion along with the pure peptides RPLKPW, LKPNM, and AINPSK (used as standards) were analyzed with an HPLC system (Agilent Technologies, USA) equipped with a silica C-18 column and a diode array detector. The column thermostat was set to 35 °C. The mobile phase consisted of 0.1% trifluoroacetic acid in water changing to 0.1% trifluoroacetic acid in acetonitrile at a flow rate of 0.3 mL min−1. At the end of the gradient, the column was washed with 0.1% trifluoroacetic acid in acetonitrile and equilibrated to the initial condition for 10 min. Detection was carried out at 215 nm.
Antihypertensive activity assay
Fourteen week-old spontaneously hypertensive rats (five male per group) with about 200–250 g of body weight (BW) were obtained from FES Iztacala (Mexico). The SHR were fed with standard food and accessed water freely. The rats were considered hypertensive when their systolic blood pressure (SBP) was higher than 150 mm Hg. Lyophilized biomass from untransformed (WT) and transplastomic lines was intragastrically administered using a metal cannula at a dose of 10 mg of recombinant AHP3 protein per kilogram of body weight.
The corresponding lyophilized biomass from WT and transplastomic C. reinhardtii strains was suspended in 4 mL of distilled water. Distilled water administration (4 mL) served as the negative control. The SBP was measured by the tail-cuff method with a programed noninvasive blood pressure controller (LE 5067 automatic blood pressure computer, Letica Scientific Instrument, PanLab, Spain). Blood pressure was measured before administration and at 2, 4, 6, and 8 h post-administration.
Changes in SBP were calculated as the mean values of three measurements obtained before and after administration. The results were expressed as mean values ± standard error of the mean (SEM) for a minimum of five rats. The differences between the groups were assessed by the Dunnett’s test and considered significant for p < 0.05 and p < 0.01.
Results
Design of the synthetic gene and plasmid construction
The synthetic gene (AHP3) was designed for optimal expression in the chloroplast of C. reinhardtii by considering codon usage in this microalga. The chimeric polypeptide contains two repetitions of the peptides RPLKPW, LKPNM, and AINPSK, previously reported as antihypertensives. We also included the sequence 5′-AGGAGG-3′ as ribosome-binding site (rbs) at the 5′ and 3′ ends since we expected a bicistronic arrangement, where the rbcL promoter drives the expression of both the AHP3 gene and the aadA marker gene; therefore, we also included an rbs at the 3′ end to be useful for the aadA gene. Considering that chloroplasts possess a prokaryotic-like translation machinery, many chloroplast mRNAs have a Shine-Dalgarno (SD)-like sequence that serves as an rbs, one of the commonest SD motifs being the sequence AGGAGG (Kozak 1999; Omotajo et al. 2015). This sequence has been used in previous works (Campos-Quevedo et al. 2013; Beltrán-López et al. 2016; Ochoa-Méndez et al. 2016).
The synthetic gene was cloned into the unique NcoI site from the p463 vector, which contains the tscA gene corresponding to a chloroplast region to mediate homologous recombination between the insertional plasmid and the chloroplast genome of C. reinhardtii (Fig. 1b). The presence of the synthetic gene AHP3 in the p463 expression vectors was successfully confirmed by restriction analysis and verified by sequencing. Insert orientation, size, and integrity of the reading frame were properly detected in these analyses.
Selection of C. reinhardtii chloroplast transformants
Chlamydomonas reinhardtii chloroplast transformation was conducted by particle bombardment as described above using the construction p463-AHP3. The transformants were rescued on spectinomycin-containing medium. After subjecting six bombarded plates to five selective rounds, several resistant clones were rescued, whereas, the wild-type strain became yellowish and died. Four lines were selected for molecular characterization.
PCR of C. reinhardtii transformants
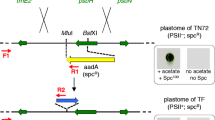
In order to determine the presence of the transgene in C. reinhardtii transformants, a PCR analysis was carried out in the four spectinomycin resistant clones. The expected 180 and 790 bp amplicons were obtained with specific primers for AHP and aadA genes, respectively, in both putative transformed C. reinhardtii clones and the positive control, while absent in the wild type. Figure 1c and d shows the four positive clones. Additionally, a PCR was performed using R1 and R2 primers, which align on the insertion site flanking regions (tscA and ch1N genes) to determine if the integration of foreign gene had occurred by homologous recombination in the chloroplast genome at the elected site (Fig. 2a, b). As shown in Fig. 2c, an expected fragment of 2272 bp was observed in the four transformed strains, indicating a homoplasmic event, while an 850-bp amplicon was obtained in the wild type strain.
Schematic representation of the C. reinhardtii chloroplast transformation. The tscA region of the chloroplast genome is used for homologous recombination between the recombinant vector and the C. reinhardtii chloroplast genome. Arrows indicate the primers used in PCR. a Untransformed chloroplast containing the tscA and ch1N genes. b Transformed chloroplast containing the AHP3 gene. c PCR analysis of C. reinhardtii transformants to detect homoplastic lines using the R1 and R2 primers. Lane 1: wild type strain (WT). Lanes 2–5: transformed C. reinhardtii strains. Lane 6: molecular weight marker
ELISA and Western blot analyses
Quantitative ELISA against the RPLKPW peptide was carried out using an antiRPLKPW polyclonal antibody. Absorbance of the soluble protein extracts from the wild type (WT) used as control was almost equal to that registered as background of the plate wells. The reported values are averages of three independent experiments showing standard deviations. AHP3 contents ranged from 30.8 to 34.4 ng of recombinant protein per mg of lyophilized biomass in the transformed lines (Fig. 3a). According to the statistical analysis (p < 0.05 Dunnett’s test), there was no significant difference between the lines transformed with the p463-AHP3. Since the highest amount of recombinant protein was achieved by the transplastomic line Cr.1 (34.4 ng per mg of lyophilized biomass), this line was used for subsequent analysis.
a ELISA of the recombinant AHP3 protein expressed in C. reinhardtii (Cr.1 to Cr.4). Reactivity of AHP3 was determined with the anti-RPLKPW polyclonal antibody. These values are averages of three independent experiments. An asterisk (*) indicates no statistically significant difference (p < 0.05) between lines. b Western blot analysis using total soluble protein (TSP). The recombinant AHP3 protein was detected with the antiHis -6× antibody. The size of the molecular weight markers is shown on the right. Lane 1: transplastomic Cr.1 strain with the p463-AHP3 construction. Lane 2: wild type strain
The Western blot analysis confirms the presence of the recombinant protein in the transplastomic line Cr.1 using the antiHis-tag antibody. As shown in Fig. 3b, a 12-kDa band similar to the expected theoretical molecular weight was observed, while in the untransformed strain (WT), no band was detected.
Peptides identification by reversed-phase chromatography
The identification of the antihypertensive peptides was achieved using the retention times for the pure peptides. Based on the chromatogram and the specific retention times for the pure peptides, the identification of the peptides RPLKPW and AINPSK after in vitro digestion of biomass from the transplastomic Cr.1 strain was established; nonetheless, the LKPNM peptide was not found. We carried out a bioinformatic search of these peptides on the predicted proteome from the nuclear (NW_001843471.1), chloroplasts (NC_005353.1), and mitochondrion (NC_001638.1) genome sequences. According to the bioinformatic search, only the LKPNM peptide was found in the expected nuclear proteome, specifically into the fructose-bisphosphate aldolase gene (A8JCY4). However, this peptide was not identified by reversed-phase chromatography (Table 1). It can be hypothesized that the amino acid sequence of this peptide is sensitive to the action of proteases or to the acidity of the medium, which can cause its degradation.
Antihypertensive activity of the recombinant protein
The antihypertensive activity of the recombinant protein produced in C. reinhardtii was evaluated in spontaneously hypertensive rats (SHR). Lyophilized biomass from the untransformed (WT) and the transplastomic line was given by intragastric administration to SHR using a metal cannula at a dose of 10 mg of recombinant AHP3 protein per kg of body weight. Distilled water was administered to a group of SHR as control. As expected, the water did not show a decrease in the systolic blood pressure (SBP) at all the evaluated times. Although the readings fluctuated, all of them returned to base levels at 8 h.
As observed in Fig. 4, there is a slight decrease in blood pressure when the untransformed strain was administered. According to the literature, there is a large number of antihypertensive peptides reported, including the peptides VWIS, RIY, IKW, LKP, and IPP (Nakamura et al. 1995; Fujita et al. 2000; Tauzin et al. 2002; Marczak et al. 2003). Analyzing the predicted proteome of C. reinhardtii, we found some of these peptides, for example, the VWIS peptide is part of the cytochrome b protein (access number P23662). In the ATP synthase (Q42687 and P26526) from the chloroplast, we found the peptides LKP and RIY. Several peptides such as IPP and RIY were also identified in cilia- and flagella-associated proteins (A8IB22, A8ITV9, A8JAF2, A8ICS9, and A8IU92). These same peptides are in the dynein regulatory complex (Q39610, Q39565, Q9XHH2, P0DL09, A8JAM0, Q39578, Q9SMH3, Q39610, and Q9MBF8), which is theoretically a prosperous source of antihypertensive peptides. In the phosphoenolpyruvate carboxylase, we found the LKP and IPP peptides (P81831 and P81831). The RIY peptide was also found in the arylsulfatase (P14217) and photosystem II protein D1 (P09752). Into the fructose-bisphosphate aldolase 1 (Q42690) and light-independent protochlorophyllide reductase (P29683), we identified the LKP peptide. Finally, in the proteins ferredoxin–NADP reductase from the chloroplast (P53991) and in a flap endonuclease (A8J2Z9), the IPP peptide was found. We can assume that the slight decrease in blood pressure in the untransformed strain is due to the presence of these endogenous peptides; however, their effect is not enough to significantly decrease blood pressure, probably due to the amount of administered biomass or because these peptides undergo degradation by gastrointestinal proteases of the SHR.
Antihypertensive activity of the recombinant protein after intragastric administration of 10 mg of recombinant AHP3 protein per kilogram of body weight to spontaneously hypertensive rats. Solid circle, transplastomic strain of C. reinhardtti; blank circle, untransformed strain (WT); solid inverted triangle, water (negative control). *p < 0.05, **p < 0.01
The greater decrease in SBP occurs with the administration of the transplastomic Cr.1 line. Four hours after intragastric administration, the SBP began to decay, reaching the largest decrease after 6 h; this difference was statistically significant when compared to the WT group (p < 0.05, p < 0.01 Dunnett’s test). Similar to the other groups, the SBP return to the baseline 8 h post-administration of biomass.
Discussion
Hypertension affects about a quarter of the human population (Meisel 2004) and is a major, yet controllable, risk factor in cardiovascular disease and related complications. Potent synthetic ACE inhibitors are currently commercialized for blood pressure regulation. Nevertheless, these synthetic compounds yield side effects such as coughing, taste disturbances, and skin rushes. Food-derived ACE inhibitor peptides represent an alternative to these drugs since they are safer. However, the unavailability of large-scale technologies for their production limits the commercialization of food-derived bioactive peptides (Agyei and Danquah 2011).
Previous studies have pursued the expression of AHP in different platforms. In 2009, Rao et al. expressed an antihypertensive peptide multimer in Escherichia coli BL21. The recombinant protein, expressed by IPTG induction, reached a maximum concentration of 39 mg L−1 culture. The release of highly active fragments from the multimer was confirmed by simulated gastrointestinal digestion. In addition, it was demonstrated that the digested protein reduced blood pressure of SHR after oral administration at a dose of 10 mg kg−1. However, the protein was expressed mostly as inclusion bodies, which requires solubilization, purification by cation-exchange chromatography under denaturing conditions, followed by refolding along with size-exclusion chromatography, and gradual dialysis.
In another study, Liu et al. (2007) reported the expression of a milk-derived antihypertensive peptide also in Escherichia coli BL21. The KVLPVP peptide was linked to produce a chimeric protein carrying six tandem repeats spaced with the specific cleavage site for clostripain. The authors reported that the production of this protein reached 170 mg L−1 culture. The recombinant protein was separated by ultrafiltration and reversed-phase HPLC. The systolic blood pressure of SHR was dramatically decreased when the peptide was orally administered at a 0.3 mg kg−1 dose. Although these reports represent an effort to obtain AHP, a purification protocol is needed, leading to higher costs.
In another approach, Yang et al. (2006) developed transgenic rice plants that accumulated an antihypertensive peptide as a fusion protein with the rice storage protein glutelin. The engineered peptide was expressed under the control of endosperm-specific glutelin promoter and specifically accumulated in seeds. They verified that the oral administration of transgenic rice seeds significantly reduced the systolic blood pressure in SHR using a dose of 470 μg kg−1, which is much higher than the effective dose reported for reduction of blood pressure of SHR, which is of 100 μg kg−1 (Yamada et al. 2002). The possible explanation for this result is that the seeds may not be easily digested and assimilated in an uncooked form in the digestive organs.
In this study, the design and expression of an antihypertensive polypeptide containing the sequences RPLKPW, LKPNM, and AINPSK in C. reinhardtii is reported. This expression platform is highly convenient since this microalga has many features that are desirable in a commercial recombinant protein expression system, including high growth rate in low-cost media, and short time for generating transgenic lines (Mayfield et al. 2007; Rosales-Mendoza et al. 2012). Moreover, it exhibits superior photosynthetic efficiency, approximately three times more efficient than higher plants (Shimizu 1996). Chlamydomonas reinhardtii also offers the advantage that it falls into the GRAS category, which means that the biomass containing the recombinant protein can be directly used as food additive in functional foods as well as in drugs for treating and preventing hypertension without the need of purification or thermal processes. Given these features, a substantial reduction of the production costs can be envisioned.
The transformation strategy was based on the use of the rbcL promoter to drive the transgene expression. ELISA and Western blot analyses confirmed the presence of the recombinant protein. After a simulated intestinal digestion, only two of the three antihypertensive peptides were identified by reversed-phase HPLC. Further studies are required to determine if the missing peptide was degraded by proteolytic enzymes or acid hydrolysis.
The antihypertensive effect of the recombinant protein was demonstrated after intragastric administration of the transplastomic strain to SHR at a dose of 10 mg of recombinant AHP3 protein per kg of body weight with the maximal decrease in blood pressure 6 h post-administration, reaching almost normal levels of blood pressure. These findings suggest a significant potential for using C. reinhardtii as platform for the production and delivery of bioactive peptides for hypertension treatment.
References
Abubakar A, Saito T, Kitazawa H, Kawai Y, Itoh T (1998) Structural analysis of new antihypertensive peptides derived from cheese whey protein by proteinase K digestion. J Dairy Sci 81:3131–3138
Agyei D, Danquah K (2011) Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol Adv 29:272–277
Beltrán-López JI, Romero-Maldonado A, Monreal-Escalante E, Bañuelos-Hernández B, Paz-Maldonado LM, Rosales-Mendoza S (2016) Chlamydomonas reinhardtii chloroplasts express an orally immunogenic protein targeting the p210 epitope implicated in atherosclerosis immunotherapies. Plant Cell Rep 35:1133–1141
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Campos-Quevedo N, Rosales-Mendoza S, Paz-Maldonado LMT, Martinez-Salgado L, Guevara-Arauza JC, Soria-Guerra RE (2013) Production of milk-derived bioactive peptides as precursor chimeric proteins in chloroplasts of Chlamydomonas reinhardtii. Plant Cell Tissue Organ Cult 113:217–225
Champagne CM (2006) Dietary interventions on blood pressure: the dietary approaches to stop hypertension (DASH) trials. Nutr Rev 64:S53–S56
Clare DA, Swaisgood HE (2000) Bioactive milk peptides: a prospectus. J Dairy Sci 83:1187–1195
Daniell H, Ruiz O, Dhingra A (2004) Chloroplast genetic engineering to improve agronomic traits. In: Pena L (ed) Transgenic plants: methods and protocols, vol 286. Humana Press Inc., Totowa, pp 111–137
Diplock AT, Charleux JL, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, Stahl W, Vina-Ribes J (1999) Functional food science and defense against reactive oxidative species. Br J Nutr 80:S77–112
Dreesen IA, Charpin-El Hamri G, Fussenegger M (2010) Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol 145:273–280
FitzGerald RJ, Meisel H (2000) Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br J Nutr 84:S33–S37
Fletcher SP, Muto M, Mayfield SP (2007) Optimization of recombinant protein expression in the chloroplasts of green algae. Adv Exp Med Biol 616:90–98
Franklin SE, Mayfield SP (2004) Prospects for molecular farming in the green alga Chlamydomonas. Curr Opin Plant Biol 7:159–165
Fujita H, Yokoyama K, Yoshikawa M (2000) Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J Food Sci 65:564–566
Goldschmidt-Clermont M (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker for site-directed transformation of Chlamydomonas. Nucl Acids Res 19:4083–4089
Guang C, Phillips RD (2009) Plant food-derived angiotensin I converting enzyme inhibitory peptides. J Agric Food Chem 57:5113–5120
Hackenthal E, Paul M, Ganten D, Taugner R (1990) Morphology, physiology and molecular biology of renin secretion. Physiol Rev 70:1067–1116
Hallmann A (2007) Algal transgenics and biotechnology. Transgenic Plant J 1:81–98
Iwaniak A, Minkiewicz P, Darewicz M (2014) Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr Rev Food Sci Food Saf 13:114–134
Johnston CI, Risvanis J (1997) Preclinical pharmacology of angiotensin II receptor antagonists. Am J Hypertens 10:306–310
Klein U, Salvador M, Bogorad L (1994) Activity of the Chlamydomonas chloroplast rbcL gene promoter is enhanced by a remote sequence element. Proc Nat Acad Sci USA 91:10819–10823
Kovács G, Peti-Peterdi J, Rosivall L, Darwin Bell P (2002) Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT1 receptors. Am J Physiol Renal Physiol 282:F301–F306
Kozak M (1999) Initiation of translation in prokaryotes and eukaryotes. Gene 234:187–208
Kumar S, Daniell H (2004) Engineering the chloroplast genome for hyperexpression of human therapeutic proteins and vaccine antigens. Meth Mol Biol 267:365–383
Liu D, Sun H, Zhang L, Li S, Qin Z (2007) High-level expression of milk-derived antihypertensive peptide in Escherichia coli and its bioactivity. J Agric Food Chem 55:5109–5112
López-Fandiño R, Otte J, van Camp J (2006) Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int Dairy J 16:1277–1293
Manuell AL, Beligni MV, Elder JH, Siefker DT, Tran M, Weber A, McDonald TL, Mayfield SP (2007) Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotech J 5:402–412
Marczak ED, Usui H, Fujita H, Yang YJ, Yokoo M, Lipkowski AW, Yoshikawa M (2003) New antihypertensive peptides isolated from rapeseed. Peptides 24:791–798
Mayfield SP, Manuell AL, Chen S, Wu J, Tran M, Siefker D, Muto M, Marin-Navarro J (2007) Chlamydomonas reinhardtii chloroplasts as protein factories. Curr Op Biotech 18:126–133
Meisel H (2004) Multifunctional peptides encrypted in milk proteins. Biofactors 21:55–61
Murray BA, FitzGerald RJ (2007) Angiotensin converting enzyme inhibitory peptides derived from food proteins: biochemistry, bioactivity and production. Curr Pharm Des 13:773–791
Nakamura Y, Yamamoto N, Sakai N, Okubo O, Yamazaki S, Takano T (1995) Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci 78:777–783
Natesh R, Schwager SLU, Sturrock ED, Acharya KR (2003) Crystal structure of the human enzyme—lisinopril complex. Nature 421:1427–1429
Ochoa-Méndez CE, Lara-Hernández I, González LM, Aguirre-Bañuelos P, Ibarra-Barajas M, Castro-Moreno P, González-Ortega O, Soria-Guerra RE (2016) Bioactivity of an antihypertensive peptide expressed in Chlamydomonas reinhardtii. J Biotechnol 240:76–84
Okamoto H, Hanagata H, Kawamura Y, Yanagida F (1995) Anti-hypertensive substances in fermented soybean. Plant Foods Hum Nutr 47:39–47
Omotajo D, Tate T, Cho H, Choudhary M (2015) Distribution and diversity of ribosome binding sites in prokaryotic genomes. BMC Genomics 16(1):604. https://doi.org/10.1186/s12864-015-1808-6
Puchalska P, Marina ML, García MC (2012) Development of a reversed-phase high-performance liquid chromatography analytical methodology for the determination of antihypertensive peptides in maize crops. J Chromatogr A 1234:64–71
Rao S, Su Y, Li J, Xu Z, Yang Y (2009) Design and expression of recombinant antihypertensive peptide multimer gene in Escherichia coli BL21. J Microbiol Biotechnol 19:1620–1627
Reaves PY, Beck CR, Wang HW, Raizada MK, Katovich MJ (2003) Endothelial-independent prevention of high blood pressure in L-NAME-treated rats by angiotensin II type I receptor antisense gene therapy. Exp Physiol 88:467–473
Rosales-Mendoza S, Paz-Maldonado LM, Soria-Guerra RE (2012) Chlamydomonas reinhardtii as a viable platform for the production of recombinant proteins: current status and perspectives. Plant Cell Rep 31:479–494
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shah NP (2000) Effects of milk-derived bioactives: an overview. Br J Nutr 84:S3–S10
Shimizu Y (1996) Microalgal metabolites: a new perspective. Annu Rev Microbiol 50:442–444
Soffer RL (1976) Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu Rev Biochem 45:73–94
Stankevicius E, Kevelaitis E, Vainorius E, Simonsen U (2003) Role of nitric oxide and other endothelium-derived factors. Medicina 39:333–341
Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix JD, Ajam T, Surzycki S (2009) Factors effecting expression of vaccines in microalgae. Biologicals 37:133–138
Tauzin J, Miclo L, Gaillard JL (2002) Angiotensin-I converting enzyme inhibitory peptides from tryptic hydrolysate of bovine alpha (S2)-casein. FEBS Lett 531:369–374
Wang X, Brandsma M, Tremblay R, Maxwell D, Jevnikar AM, Huner N, Shengwu M (2008) A novel expression platform for the production of diabetes-associated autoantigen human glutamic acid decarboxylase (hGAD65). BMC Biotechnol 8(1):87. https://doi.org/10.1186/1472-6750-8-87
WHO (2016) A global brief on hypertension [WWW Document], n.d. URL http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/. Accessed 14 Apr 2016
Yabuta M, Suzuki Y, Ohsuye K (1995) Hyperproduction of a recombinant fusion protein of Staphylococcus aureus V8 protease in Escherichia coli and its processing by OmpT protease to release an active V8 protease derivative. Appl Microbiol Biotechnol 42:703–708
Yamada Y, Matoba N, Usui H, Onishi K, Yoshikawa M (2002) Design of a highly potent anti-hypertensive peptide based on ovokinin(2–7). Biosci Biotechnol Biochem 66:1213–1217
Yamamoto N, Ejiri M, Mizuno S (2003) Biogenic peptides and their potential use. Curr Pharm Des 9:1345–1355
Yang L, Tada Y, Masayuki P, Yamamoto HZ, Masaaki Y, Takaiwa F (2006) A transgenic rice seed accumulating an anti-hypertensive peptide reduces the blood pressure of spontaneously hypertensive rats. FEBS Lett 580:3315–3320
Zhuo JL, Ferrao FM, Zheng Y, Li XC (2013) New frontiers in the intrarenal renin angiotensin system: a critical review of classical and new paradigms. Front Endocr 4:166
Funding
This work was supported by the projects: Science Immersion 2015 and 2016 and FAI 2016 UASLP to RESG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Carrizalez-López, C., González-Ortega, O., Ochoa-Méndez, C.E. et al. Expression of multiple antihypertensive peptides as a fusion protein in the chloroplast of Chlamydomonas reinhardtii . J Appl Phycol 30, 1701–1709 (2018). https://doi.org/10.1007/s10811-017-1339-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1339-4