Abstract
The antioxidant responses of four green microalgae, i.e., Chlorella vulgaris, Chlorella sp., Selenastrum capricornutum and Scenedesmus quadricauda, under control, low (0.1 mg L−1) and high (1.0 mg L−1) nonylphenol (NP) concentration were studied. The antioxidant responses of microalgae to NP depended on both NP concentrations and exposure time. The effects of NP on antioxidant responses were most obvious on the first day of exposure and the effects decreased with prolonged exposure time. At low NP concentration, there were no significant changes in activities of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), or in glutathione (GSH) content, in all four species, while high concentration of NP led to different changes in these parameters. In NP-tolerant species, i.e., C. vulgaris and Chlorella sp., activities of SOD, CAT and POD increased remarkably when exposed to high NP concentration, while the increase was less evident or insignificant in Se. capricornutum and Sc. quadricauda, the two NP-sensitive species. On the other hand, the malondialdehyde (MDA) content declined gradually with increase in NP concentrations, particularly in C. vulgaris and Chlorella sp. Similarly, NP exposure caused an inhibition of glutathione peroxidase (GPX) activity in all four species. However, the changes of glutathione reductase (GR) and glutathione S-transferase (GST) activity did not seem to correlate with the NP tolerance of microalgae. These results suggested that various antioxidant mechanisms were involved in microalgae when exposed to NP, and the NP-tolerant species displayed more evident and rapid changes in some antioxidant responses than the NP-sensitive ones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, contamination by nonylphenol (NP), a microbial biodegradation product of nonylphenol ethoxylates (NPEOs), has raised public concern due to its persistence, toxicity and endocrine disrupting property (Vazquez-Duhalt et al. 2005; Soares et al. 2008). NP is frequently detected in all environmentals that directly or indirectly receive NPEOs and has been demonstrated to be one of the most commonly occurring contaminants. NP has been detected at higher concentrations than most of the other contaminants, particularly in wastewater effluents and sludge, due to the wide and long-term application of NPEOs (Kolpin et al. 2002; Soares et al. 2008). For instance, in untreated wastewater and seepage samples from Cape Cod in Massachusetts, USA, NP concentrations were above 1000 μg L−1, with the highest concentration up to 1350 μg L−1 (Rudel et al. 1998). The concentration of NP in aquatic environments is closely related with the discharge of effluents from wastewater treatment plants (Hale et al. 2000; Corsi et al. 2003). In river and lake waters in the Anoia and Cardener Tributary, Spain, NP concentrations ranged from below detection level to a very high concentration of 644 μg L−1 (Sole et al. 2000).
The toxicity of NP to organisms has been widely investigated, and the results have revealed that NP is quite toxic to aquatic organisms; however, the precise mechanism of NP toxicity remains unclear (Vazquez-Duhalt et al. 2005; Soares et al. 2008). It has been suggested that oxidative stress may be partially responsible for the toxicity following NP exposure, as NP has been shown to produce oxidative stress through either enhancing the generation of reactive oxygen species (ROS), and/or inhibiting the antioxidant defence mechanisms in cells (Okai et al. 2004; Gong and Han 2006; Wang and Xie 2007).
In plants, including algae, ROS are always formed by the inevitable leakage of electrons onto O2 from the electron transport activities of chloroplast, mitochondria, and plasma membrane during photosynthesis and respiration. As a consequence, various ROS, including singlet oxygen (1O2), superoxide radicals (O2 −·), hydroxyl radicals (OH−· ) and hydrogen peroxide (H2O2), are produced (Asada 1996). Under normal growth conditions, the production of ROS in cells is low and kept balanced by ROS scavenging systems (Powles 1984). However, both abiotic and biotic stresses are known to cause excess accumulation of ROS (Mittler 2002).
ROS react with biomolecules at varying degrees and may directly damage proteins, amino acids, nucleic acids, porphyrins, phenolic substances, etc. (Mittler 2002). In addition, OH−· causes peroxidative degradation of polyunsaturated fatty acid chains of membrane lipids, which is evident by the increased formation of malondialdehyde (MDA), a product of lipid peroxidation (Chitra et al. 2002). Among different ROS, OH−· is particularly harmful, and may lead to tissue injury and cell death. A tight control of ROS levels in plant cells is crucial for their normal growth and development (Mittler 2002). To defend the ROS-caused deleterious effects resulting from cellular oxidative stress, plants have evolved both non-enzymatic and enzymatic antioxidant defences, which are distributed in different cellular compartments (Mittler 2002). Among them, the metabolism of glutathione S-transferase (GSH) plays a crucial role in the antioxidant defence, the biotransformation of xenobiotics and the sensitivity of cells to various pollutants (Lei et al. 2003).
The antioxidant responses of microalgal cells to oxidative stress have been well documented, but the information on these responses seems to be meagre and widely scattered. For example, superoxide dismutase (SOD) activity has attracted more attention than the other antioxidant parameters, and its activity is generally stimulated by a variety of stresses (Nagalakshmi and Prasad 1998; Mallick and Rai 1999; Wang and Xie 2007). On the contrary, there are studies showing that the SOD activity was inhibited by environmental stresses (Vartak and Bhargave 1999), while others have reported that SOD activity remained unchanged under stress conditions and that there was no correlation between the resistance of microalgae to stresses and SOD activity (Choo et al. 2004; Lei et al. 2006). All these results indicate that the antioxidant response of microalgal cells to environmental stress is not only species-specific but is also dependent on the types and magnitude of the stresses.
Few studies have reported the antioxidant response of plants, including algae, exposed to NP, although NP has been shown to produce oxidative stress and enhances ROS generation in animal cells (Chitra and Mathur 2004; Gong and Han 2006). Wang and Xie (2007) showed that high concentrations of NP (1 and 2 mg L−1) caused increases in the activities of SOD and GST, as well as reduced glutathione (GSH) levels, in the cyanobacterium Microcystis aeruginosa, suggesting that both enzymatic and non-enzymatic systems were involved in the antioxidant response and detoxification ability of microalgae to NP.
Our previous study demonstrated that the toxicity of NP to microalgae was highly species-specific. No evident toxic effects in Chlorella vulgaris, Chlorella sp., Selenastrum capricornutum and Scenedesmus quadricauda were observed when the NP concentration was at 0.1 mg L−1; no significant or slightly toxic effects were found in C. vulgaris and Chlorella sp. when exposed to 1 mg L−1 NP, but the growth of Se. capricornutum and Sc. quadricauda was seriously inhibited under 1 mg L−1 NP (unpublished data). The present study aims to (1) measure the GSH content, antioxidant enzymes activities and lipid peroxidation in four different green microalgal species, including C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda; (2) determine whether the exposure of microalgae to NP would induce oxidative stress and lead to the modification of their antioxidant parameters and (3) compare the NP-induced changes of these parameters among four different microalgal species and identify the main antioxidant parameter(s) involved in NP tolerance.
Materials and methods
Four microalgal species were used in the present study. Chlorella vulgaris was purchased from Carolina Biological Supply Company, USA, while Scenedesmus quadricauda (UTEX76) and Selenastrum capricornutum (UTEX1648) were obtained from the culture collection of algae at the University of Texas at Austin (UTEX), USA. Chlorella sp. was isolated from polluted water in Hong Kong. Axenic stock cultures of these four microalgal species were cultivated in 250-mL Erlenmeyer flasks containing 100-mL Bristol medium (BM) (C. vulgaris, Chlorella sp., Sc. quadricauda) and soil extract medium (SE) (Se. capricornutum) prepared as described by James (1978) and Song and Liu (1999). All the materials and culturing media were autoclaved at 121 °C for 20 min and then cooled to room temperature.
Preparation of mass culture
The axenic mass culture of these four microalgal species was carried out in 2-L Erlenmeyer flasks; each flask contained 1 L BM for C. vulgaris, Sc. quadricauda and Chlorella sp. or SE medium for Se. capricornutum. The culture was illuminated with cool, fluorescent light at an irradiance of 60 μmol photons m−2 s−1 at the surface of the culture medium (measured with a LI-250 Light meter, LI-COR, Inc. US), with a 16/8 h light/dark cycle in an environmental chamber at 25 ± 1 °C. The culture was aerated with 0.2 μm filtered air at a rate of 35 mL min−1. The culture was kept in exponential phase of growth by repeatedly sub-culturing with freshly prepared medium at about a 4-day interval. Before each experiment, the cultures were verified for axenity by streaking on 1.5% agar plates containing 1 g L−1 yeast extract and 2 g L−1 tryptone (prepared with corresponding algal culturing media). Prior to the start of the experiment, the microalgal cells at the mid-exponential phase of growth were harvested by centrifugation at 5000×g for 10 min at 25 °C. The cell pellets were then washed twice with sterilized, deionized water as described by Gao et al. (2011).
Experimental setup
An appropriate amount of cell pellets of each microalgal species was re-suspended in 100-mL sterilized medium (in a 250-mL Erlenmeyer flask) to give an initial chlorophyll a (chl a) concentration of 1 mg L−1. Stock solution of NP (Sigma-Aldrich, USA) was prepared by dissolving NP in methanol to a concentration of 1 g L−1 and stored at −20 °C for at most 3 months. NP stock solution was spiked into the culture to give a final NP concentration of 0.1 (low NP) and 1.0 mg L−1 (high NP). In all NP treatments, an appropriate volume of methanol was added to each flask to give a final methanol concentration of 0.1% (v/v). Flasks without the addition of NP, but with 0.1% methanol (v/v), were used as the control. Each treatment, as well as the control, was performed in triplicate. The cultures were incubated at 25 °C in an environmental chamber on a rotary shaker with a speed of 160 rpm, at a light intensity of 40 μmol photons m−2 s−1 (at the surface of the culture), with a 16/8 h light/dark cycle. At time intervals of 1, 4 and 7 days, three flasks from the control or each treatment were retrieved. The growth of microalgal cells were determined by following the changes of chlorophyll a concentration of the cultures. For the determination of chlorophyll a concentration, 5 mL culture was harvested by centrifugation at 5000×g for 10 min. The supernatant was discarded and the microalgal pellet was re-suspended in 5 mL of 95% methanol, incubated at 60 °C in a water bath for 5 min and centrifuged again for 5 min. The absorbance of the supernatant at 665 and 652 nm was determined with an Agilent 8453 UV-visible spectrophotometer. The chlorophyll a concentration of the supernatant was calculated following the formula described by Porra et al. (1989):
where A 665 and A 652 refer to the absorbance of the extract at wavelengths of 665 and 652 nm, respectively.
Extraction of antioxidant enzymes, GSH and MDA
After the flasks were retrieved, the cells were harvested by centrifugation at 5000×g for 15 min at 4 °C. The cell pellets were then transferred to a 2-mL vial (Biospec Products, USA), washed with deionized distilled water and centrifuged again, then stored at −80 °C for further analysis. For the measurement of SOD, CAT, POD, GR, GST and GPX activities, the pellets were re-suspended in 1 mL of 0.1 M sodium phosphate buffer (pH 7.0) containing 0.1 mM Na2EDTA and 1% polyvinylpyrrolidone (PVP40). For the determination of GSH and MDA content, cell pellets were re-suspended in 1-mL Tris–HCl buffer (50 mM, pH 7.5). The cells were ruptured in a 2-mL vial by mini-beadbeater (Biospec Products, USA) with zirconia/silica beads (Biospec products with a diameter of 0.5 mm) five times, each for 20 s at a high speed of 4600 rpm. Prior to each beating, the vials containing the suspension were incubated in ice to avoid overheating the sample during the bead-beating process. The homogenate was then centrifuged at 12,000×g for 20 min at 4 °C. The supernatant was considered as a cell-free enzyme extract and stored at 4 °C before any assay. The protein concentration of the extract was determined according to the method described by Bradford (1976) with bovine serum albumin (BSA) as the protein standard.
Measurement of antioxidant parameters
All enzyme activities and GSH content were measured at 37 °C with assay kits purchased from Nanjing Jian Cheng Bioengineering Institute (China). The superoxide dismutase (SOD, EC 1.15.1.1) activity was determined as the inhibition of cytochrome c reduction followed the principles as described by Mishra et al. (1993), with some modifications. The reaction mixture (total volume 3.0 mL) consisted of 50 mM potassium phosphate buffer (pH 7.8) containing 0.1 mM EDTA, 50 μM xanthine, 20 μM cytochrome c and a suitable amount of enzyme extract. The reaction was initiated by the addition of 50 μL of 25 U mL−1 xanthine oxidase to the reaction buffer, and the absorbance at 550 nm was recorded. One unit of SOD was defined as the amount of enzyme required for 50% inhibition of cytochrome c reduction under the specified conditions. The catalase (CAT, EC 1.11.1.6) activity was determined according to Goth (1991), with some modifications. In brief, 0.2 mL of the enzyme extract was added to 1.1 mL of the substrate solution made up of 100 μL of 50 mM H2O2 and 1.0 mL of 60 mM sodium-potassium phosphate buffer (pH 7.0) and incubated for 2 min. The enzymatic reaction was initiated by the addition of the enzyme extract and stopped with addition of 1.0 mL of 50 mM ammonium molybate. The absorbance of the reaction mixture at 405 nm was measured and one unit of CAT activity was expressed as 1 μmol H2O2 L−1 mg−1 protein s−1. The peroxidase (POD, EC 1.11.1.7) activity was determined following the method described by Montavon and Bortlik (2004) and one unit of POD activity was expressed as 1 μg H2O2 mg−1 protein min−1. The reduced GSH content was determined using 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) as the substrate as described by Anderson (1985), with some modifications. An aliquot of extract was deproteinized by adding an equal volume of 4% (w/v) sulfosalicylic acid, and after centrifugation, 0.5 mL of the supernatant was mixed with 2 mL of reaction buffer made up of 1.75 mL of 0.1 M phosphate buffer (pH 7.5, containing 0.067 mM EDTA) and 0.25 mL of 6 mM DTNB (in 1% citric acid); the absorbance at 412 nm was measured after 5 min and the content of GSH was calculated based on a linear standard curve. The glutathione S-transferase (GST, EC 2.5.1.18) assay was performed according to a method modified by Grant et al. (1989), using 1-chloro, 2,4-dinitrobenzene (CDNB) as the substrate and GSH as the cofactor. One unit of GST activity was defined as the amount of the enzyme that catalyses the conversion of 1 μM CDNB per min. Glutathione reductase (GR, EC 1.6.4.2) activity was assayed by the oxidation of NADPH, and a decrease in the absorbance at 340 nm (extinction coefficient 6.2 mM cm−1) was determined, as described by Rao (1992). GR activity was expressed as nmol of NADPH oxidized mg−1 protein min−1. Glutathione peroxidase (GPX, EC 1.11.1.9) assay was performed according to the method modified from Splittgerber and Tappel (1979). One unit of GPX activity was defined as the consumption of 1 μmol GSH mg−1 protein min−1. The MDA concentration was measured according to the method described by Heath and Packer (1968), and the absorbances at 450, 532 and 600 nm were determined. The concentration of MDA was calculated according to the formula described by Zhao et al. (1991):
where A 450 , A 532 and A 600 refer to the absorbance of the reaction mixture at wavelengths of 450, 532 and 600 nm, respectively.
Statistical analysis
The mean and standard deviation of three replicates from each treatment, as well as the control, at each sampling time of each species, were calculated. A parametric three-way analysis of variance (ANOVA) was used to test any differences of each antioxidant parameter among different microalgal species, sampling times and NP concentrations. If the interaction factors of algal species and the other two factors were significant, the effect of NP concentrations on antioxidant parameters of each microalgal species was determined by a two-way ANOVA, with times and NP concentrations as the two main factors. If the interaction factor was significant, a one-way ANOVA was used to test the NP effect. If the statistical test was significant at p ≤ 0.05, a Tukey test was employed to find out where the difference occurred. All tests were carried out using SPSS 16.0 for Windows (SPSS Inc., USA).
Results
Effect of NP on growth
Figure 1 shows the effects of different NP concentrations on the growth of C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda. The effects of NP on growth varied significantly among species, exposure times and NP concentrations (Tables 1 and 2). At low NP concentration (0.1 mg L−1), no significant inhibitory effect on the growth of C. vulgaris, Chlorella sp. and Se. capricornutum was observed during all the experiment periods, while the growth of Sc. quadricauda, under low NP treatment, slightly decreased as compared to its control after 4 days. However, when the NP concentration was increased to 1.0 mg L−1, different patterns were observed among the four species. For C. vulgaris and Chlorella sp., the chlorophyll concentration of the culture at 1 mg L−1 NP treatment after a 4-day exposure decreased to 89 and 52% of their corresponding controls, respectively, while no significant effect was found between the 1.0 mg L−1 NP treatment and the control on both day 1 and day 7. For Se. capricornutum and Sc. quadricauda, the decreases in growth were found at all exposure times at the high NP concentration (1.0 mg L−1). Even after 7 days, the growth of Se. capricornutum and Sc. quadricauda under high NP treatment could not recover to the control level (Fig. 2 and Table 2). These results demonstrated that C. vulgaris was the most tolerant to NP, followed by Chlorella sp., while Sc. quadricauda and Se. capricornutum were more sensitive to NP than the two Chlorella species.
Effect of NP on the growth of C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda after 1, 4 and 7 days of exposure (mean and standard deviation of three replicates are shown. The mean with different letters at each exposure time for each algal species indicated that they were significantly different at p ≤ 0.05 according to one-way ANOVA test. NS not significant)
Effect of NP on the SOD activity of C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda after 1, 4 and 7 days of exposure (mean and standard deviation of three replicates are shown. The mean with different letters at each exposure time for each algal species indicated that they were significantly different at p ≤ 0.05 according to one-way ANOVA test. NS not significant)
Effect of NP on SOD activity
Under the control condition, the SOD activity varied among species (p < 0.001), as well as times (p < 0.001). On the first day of the experiment, the highest SOD activity was observed in Sc. quadricauda, followed by Se. capricornutum and C. vulgaris, and Chlorella sp. displayed the lowest SOD activity (Fig. 2). NP treatments changed the SOD activity of algal cells, and these changes were highly dependent on microalgal species, NP concentrations and exposure times (Table 1). For C. vulgaris and Chlorella sp. under the high NP concentration (1.0 mg L−1), the SOD activity increased significantly after a 1-day exposure, with increases of 64 and 214%, as compared to their control, respectively (Fig. 2). After prolonged exposure time (4 and 7 days), the effects of NP at 1 mg L−1 on the SOD activity of these two Chlorella species disappeared. For Sc. quadricauda, although no apparent change was observed among control, low and high NP treatments after 1- and 4-day exposure, a significant increase was found after a 7-day exposure at 1 mg L−1 NP concentration. In Se. capricornutum, the SOD activity increased gradually with the increase of NP concentrations after a 1-day exposure, but after 4 days, the SOD activities of both low and high NP treatments were significantly inhibited by NP in comparison with that of control (Fig. 2).
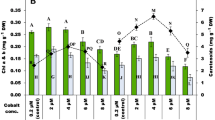
Effect of NP on CAT activity
Similar to SOD, the CAT activity of the four species under the control condition varied significantly from species to species (p < 0.001) and times (p < 0.001) (Table 1). On the first day of the experiment, the highest CAT activity was observed in the control of C. vulgaris, followed by Sc. quadricauda, while Chlorella sp. and Se. capricornutum displayed similar low CAT activity (Fig. 3). The NP concentrations and exposure time exerted different influences on the CAT activity of all the four species (Tables 1 and 2 and Fig. 3). The most significant changes in CAT activity following NP exposure were found in C. vulgaris and Chlorella sp. at high NP treatment (1.0 mg L−1) on the first day of the experiment, with 2.16- and 1.89-fold increases as compared to that of their corresponding control, respectively. No significant change was found at the low NP treatment (0.1 mg L−1) as compared to control in all the four species during the experiment. For C. vulgaris, the increase in CAT activity following NP exposure at both low and high NP concentrations disappeared after 4 and 7 days. Similarly, the CAT activity of Chlorella sp. between two NP treatments and control was also comparable after 7 days (Fig. 3).
Effect of NP on the CAT and POD activity of C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda after 1, 4 and 7 days of exposure (mean and standard deviation of three replicates are shown. The mean with different letters at each exposure time for each algal species indicated that they were significantly different at p ≤ 0.05 according to one-way ANOVA test. NS not significant)
On the other hand, although the CAT activity of Se. capricornutum treated with low NP was comparable to the control, the CAT activity was significantly stimulated at the high NP concentration during the experiment. On the contrary, a significant decrease in the CAT activity of Sc. quadricauda at 1.0 mg L−1 NP treatment after 1- and 4-day exposures was found, but no significant difference was observed among control, low and high NP treatment on day 7 (Fig. 3).
Effect of NP on POD activity
The POD activity of the four species under the control condition varied greatly from species to species (p < 0.001), as well as with times (p < 0.001) (Table 1). On day 1, the highest and similar POD activity was observed in the control of Sc. quadricauda, and C. vulgaris, followed by Se. capricornutum. Chlorella sp. was shown to have the lowest POD activity under the control condition (Fig. 3). Upon exposed to different NP concentrations, the four species displayed different responses in POD activity. The POD activity of C. vulgaris increased gradually with the increase in NP concentrations after 1 day of exposure, but such an increase disappeared after both 4- and 7-day exposures. Compared to C. vulgaris, the increase in POD activity of Chlorella sp. and Se. capricornutum was only observed at the high NP concentration (1.0 mg L−1) after a 1-day exposure. Similar to C. vulgaris, the increase in POD activity of Chlorella sp. was not observed after prolonged exposure time (days 4 and 7). For Se. capricornutum, the increase in POD activity under 1 mg L−1 NP concentration after a 4-day exposure was more obvious than that after a 1-day exposure. On the contrary, the POD activity of Sc. quadricauda was significantly inhibited by both low and high NP concentrations after 1 and 4 days of exposure, and such inhibitory effect of NP on POD activities disappeared after 7 days (Fig. 3). These results suggested that the effect of NP on POD activity varied significantly with NP concentration, species and exposure time (p < 0.001).
Effect of NP on GSH content
The levels of reduced GSH content varied significantly among the four species grown under the control condition (p < 0.001) and times (p < 0.001). Under the control condition, Chlorella sp. displayed the highest GSH content, followed by C. vulgaris, while the other two species, Se. capricornutum and Sc. quadricauda, displayed much lower GSH level in comparison to the two Chlorella species (Fig. 4). Exposed to NP led to significant changes in GSH content, depending on algal species and exposure times (Fig. 4 and Tables 1 and 2). For C. vulgaris, the GSH content increased by about 70% as compared to that of control when exposed to high NP concentration (1.0 mg L−1) after a 1-day exposure, but no significant difference was observed between the control and two NP treatments thereafter. For Chlorella sp., the GSH content gradually increased with the increase of NP concentrations after 1- and 4-day exposures, while the GSH content at these two NP treatments was comparable to that of control on day 7. Compared to the response of two Chlorella species, the effect of NP treatments on GSH content of Se. capricornutum was not significant after the first day of exposure but decreased significantly after 4- and 7-day exposures under both low and high NP concentrations. NP treatments had no notable effect on the GSH content of Sc. quadricauda throughout the experiment (Fig. 4).
Effect of NP on the GSH content and GR activity of C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda after 1, 4 and 7 days of exposure (mean and standard deviation of three replicates are shown. The mean with different letters at each exposure time for each algal species indicated that they were significantly different at p ≤ 0.05 according to one-way ANOVA test. NS not significant)
Effect of NP on GR activity
Under the control condition, GR levels varied significantly among different species (p < 0.001), but remained relatively constant with growth time. On the first day of the experiment, C. vulgaris and Chlorella sp. grown under the control condition showed similar and relatively high GR activities as compared to Se. capricornutum and Sc. quadricauda (Fig. 4). When exposed to NP for 1 day, the GR activities of C. vulgaris and Sc. quadricauda increased significantly under the high NP concentration (1 mg L−1), with an increase by 120 and 96% as compared to that of their corresponding control, respectively. After 4 and 7 days of exposure, increases in the GR activity of C. vulgaris were still observed, but GR activity of Sc. quadricauda was inhibited by both low and high NP concentrations as compared to their control on day 4. After 7 days of exposure, no significant effect on the GR activity was found in Sc. quadricauda. On the other hand, the GR activities of Chlorella sp. and Se. capricornutum decreased significantly under both low and high NP concentrations after a 1-day exposure, but the NP effect on GR activities of these two species became insignificant thereafter (after 4 and 7 days of exposure) (Fig. 4). These results displayed that the NP effect on GR activity was dependent on microalgal species, NP exposure times and NP concentrations (p < 0.001).
Effect of NP on GPX activity
Under the control condition, the levels of GPX activity varied significantly among the four algal species (p < 0.001) and times (p < 0.001). On the first day of the experiment, C. vulgaris demonstrated the highest GPX activity, followed by Chlorella sp. and Se. capricornutum, while Sc. quadricauda showed the lowest GPX activity (Fig. 5). When exposed to NP, the GPX activities of all four microalgal species decreased significantly after 1 day of exposure but to different magnitude. The GPX activities of C. vulgaris and Se. capricornutum declined gradually with the increase of NP concentrations, while the decrease in GPX activities of Chlorella sp. and Sc. quadricauda under both low and high NP concentrations was comparable, indicating that high NP concentrations did not show more adverse effects than the low one in Chlorella sp. and Sc. quadricauda. With prolonged exposure times, the GPX activities of Chlorella sp., Se. capricornutum and Sc. quadricauda were comparable to that of their corresponding control. However, an increase in GPX activity of C. vulgaris was observed on day 4 (Fig. 5).
Effect of NP on the GST and GPX activity of C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda after 1, 4 and 7 days of exposure (mean and standard deviation of three replicates are shown. The mean with different letters at each exposure time for each algal species indicated that they were significantly different at p ≤ 0.05 according to one-way ANOVA test. NS not significant)
Effect of NP on GST activity
The GST activity of the cells of the four species grown under the control condition varied significantly among the four species (p < 0.001), but its activity remained relatively constant over time (Fig. 5). When grown under control conditions, C. vulgaris had the highest GST level, followed by Chlorella sp., while Se. capricornutum and Sc. quadricauda had the similar but much lower GST levels as compared to that of the two Chlorella species. After being exposed to NP, no significant changes in GST activities were observed in C. vulgaris throughout the experiment, but significant increases in GST activities of Chlorella sp. and Se. capricornutum were observed when exposed to NP after 1 and 4 days of exposure. The increase in the GST activity of Chlorella sp. was dose-dependent, while the increases in Se. capricornutum and Sc. quadricauda were observed only under the high NP concentration after 1 and/or 4 days of exposure (Fig. 5). Table 1 shows the effects of microalgal species, exposure times and NP concentrations on GST. Their interactions were all significant (p < 0.001), indicating that the GST response in microalgae was very complicated.
Effect of NP on lipid peroxidation
Under the control condition, the MDA content, an indicator of lipid peroxidation level, varied significantly among different species (p < 0.001) and times (p < 0.001). Chlorella vulgaris had a much higher MDA content than the other three species, followed by Se. capricornutum, while Chlorella sp. and Sc. quadricauda contained the lowest and similar MDA content (Fig. 6). Following exposure to NP, the changes of MDA content were species-specific and time-dependent. In C. vulgaris, the MDA content decreased gradually with the increase of NP concentrations after 1 day but such decrease was only observed at the high NP concentration on day 4 and the NP effect disappeared at the end of experiment (day 7). On the other hand, after a 1-day exposure, no significant change was observed in Chlorella sp. and Se. capricornutum under both low and high NP concentration as compared to their control, while the MDA content of Sc. quadricauda decreased under 1.0 mg L−1 NP. After 4 and 7 days, the MDA content of Chlorella sp., Se. capricornutum and Sc. quadricauda decreased under both low and high NP concentrations as compared to control, although no significant difference was observed between low and high NP treatments in these two species (Fig. 6).
Effect of NP on the MDA content of C. vulgaris, Chlorella sp., Se. capricornutum and Sc. quadricauda after 1, 4 and 7 days of exposure (mean and standard deviation of three replicates are shown. The mean with different letters at each exposure time for each algal species indicated that they were significantly different at p ≤ 0.05 according to one-way ANOVA test. NS not significant)
Discussion
Antioxidant responses of microalgae to NP exposure
The modification of antioxidant parameters of the four microalgal species following the NP exposure in the present study suggested that microalgal cells suffered from oxidative stress when exposed to NP, although to different extent (Figs. 1, 2, 3, 4, 5 and 6 and Table 2). The changes in SOD activities of microalgal species indicated that SOD contributed to the elimination of the toxic effects of superoxide resulted from NP exposure. The increase in the activities of CAT and POD in C. vulgaris, Chlorella sp. and Se. capricornutum demonstrated that these enzymes were also involved in the removal of H2O2 produced from the dismutation of superoxide catalysed by SOD or direct production via photorespiration. This was in agreement with the observation from Wang and Xie (2007). CAT is present in peroxisomes and mitochondria, while POD is located in cytosol, cell wall, vacuole and extracellular spaces (Reddy et al. 2005). The function of CAT is to decompose H2O2 to water and oxygen, whereas POD consumes H2O2 to generate phenoxy compounds that are polymerized to produce cell wall components, which have a broad specific for phenolic substrate and a high affinity to H2O2 than CAT (Reddy et al. 2005; Mishra et al. 2006). In the present study, the POD and CAT activities, as well as the chlorophyll concentration, in Sc. quadricauda, decreased when exposed to NP and did not show any recovery, even at the end of the experiment (day 7) (Figs. 1 and 3 and Table 2), indicating that CAT and POD were very sensitive to NP-induced toxicity and failed to produce more antioxidant enzymes to remove H2O2 in this microalgal species. H2O2 can also be removed by GPX or ascorbate peroxidase (APX) in Asc-GSH cycle. However, the GPX activities of all the four species declined under both low and high NP concentrations, suggesting that this enzyme was sensitive to NP-induced toxicity and might not be involved in the ROS scavenging process. However, the downregulation of GPX might lead to the upregulation of other H2O2-scanvenging enzymes, such as CAT, POD and APX (Selote and Khanna-Chopra 2010), as indicated by the increase in CAT and POD activities in C. vulgaris and Chlorella sp. and Se. capricornutum, following NP exposure in the present study.
GSH is one of the most important components of the antioxidant defence systems in cells. GSH not only directly reacts with ROS under oxidative stress conditions but is also a substrate in the GPX and Asc-GSH cycle (Mittler 2002; Mishra et al. 2006). GSH is also involved in the detoxification process of xenobiotics in microalgae, catalysed by GST (Lei et al. 2003). In the present study, the GSH levels in C. vulgaris and Chlorella sp. increased significantly, but they remained unchanged in Sc. quadricauda, or even declined in Se. capricornutum, when exposed to NP, suggesting that the responses of GSH to NP-induced oxidative stress varied among different microalgal species. When exposed to an oxidative stress, GSH is utilized by cells and/or converted to GSSG by either the GPX of Asc-GSH cycle to combat the excessive ROS. It may also be used for the detoxification process, independently or in addition to combating excessive ROS. This, then, may lead to a depletion of GSH and to the decline of the GSH/GSSG ratio, which in turn may trigger the synthesis of more GR. The function of GR is to reduce GSSG back to reduced GSH, maintaining GSH/GSSG ratio, which is crucial for functioning of the antioxidant system, as well as for the primary detoxification pathways in plant cells (Mishra et al. 2006). In the present study, the GSH content increased significantly in the NP-tolerant species, namely, C. vulgaris and Chlorella sp., but significantly decreased, or remained unchanged, in the NP-sensitive species, including Se. capricornutum and Sc. quadricauda (Fig. 4 and Table 2). The GR activity increased significantly in C. vulgaris but declined in Chlorella sp. and Se. capricornutum. These results suggested that the antioxidant enzymes of microalgal cells responded in a coordinated way to combat NP-induced stress, to some extent, allowing the cells to tolerate NP toxicity. The increase in GSH content of C. vulgaris, an NP-tolerant species, was probably due to the increase in GR activity, accompanied with the decrease in GPX activity, while the decrease in GSH content of Se. capricornutum, an NP-sensitive species, might be caused by a decline in GR activity and an increased GSH consumption, as indicated by the increase in GST activity. The unchanged GSH content in Sc. quadricauda might result from the balance between the production of GSH by GR and NP consumption by GPX and GST; therefore, it did not produce more GSH to combat excessive ROS. The present study suggested that more GSH could be produced in the NP-tolerant species through increased GR activity (Figs. 4 and 5).
The relationships among different antioxidant parameters, under NP stress, varied among different microalgal species (Figs. 2, 3, 4, 5 and 6 and Tables 1 and 2). After 24 h of exposure, the SOD, CAT and POD activities in all species increased except in Sc. quadricauda, which had relatively higher constitutive SOD activity as compared to Se. capricornutum and Chlorella sp. The GST activities increased in Se. capricornutum, Sc. quadricauda and Chlorella sp. except in C. vulgaris, which had a high constitutive GST activity than the two NP-sensitive species, namely, Se. capricornutum and Sc. quadricauda. The GSH content in the two NP-tolerant species, i.e., C. vulgaris and Chlorella sp., increased following NP exposure, while the GSH content in the two NP-sensitive species remained unchanged. The increase in GSH content in C. vulgaris and Chlorella sp. was accompanied with the increase in GR activities. The GPX activities in all these species decreased. After a prolonged exposure time, these antioxidant parameters recovered gradually but the degree of recovery differed among microalgal species (Table 2).
One of the important consequences of excessive ROS is to induce chain-like peroxidation of polyunsaturated fatty acid chains of membrane lipids, resulting in the increased formation of MDA, a product of lipid peroxidation (Chitra et al. 2002). However, in the present study, the MDA content of the four microalgal cells, particularly C. vulgaris and Chlorella sp., declined gradually with increases in NP concentrations (Fig. 6), indicating that ROS, including O2 −· and H2O2 generated, following the NP exposure could be efficiently scavenged by the antioxidant defence system and prevented the peroxidation of lipid. This also explained why the NP-induced decline in growth (chlorophyll a concentration) in these two NP-tolerant species was recovered at the end of the experiment. However, in NP-sensitive species, although the MDA content also declined following the NP exposure, their growth did not recover at the end of the experiment (day 7) (Fig. 1 and Table 2), suggesting that the accumulation of ROS and lipid peroxidation might not have been the main reasons involved in the NP-induced injury to Sc. quadricauda and Se. capricornutum.
The present study also demonstrated that the changes in the antioxidant parameters in microalgal cells displayed a time-dependent recovery, especially in NP-tolerant species, with most of the antioxidant parameters recovering completely at the end of the experiment (Table 2 and Figs. 2, 3, 4, 5 and 6). These results suggested that the NP detoxification mechanisms, in addition to the ROS defence systems, may occur in C. vulgaris and Chlorella sp., leading to the gradual elimination of NP in the cells with time. The increase in GST activity in cells of Chlorella sp., Se. capricornutum and Sc. quadricauda suggested that the main detoxification process of NP in the cells of these microalgal species was likely through GSH conjugation by GST, a major phase II detoxification enzyme (Tang et al. 1998; Lei et al. 2003). Although the GST activity of C. vulgaris remained unchanged following NP exposure, the recovery of growth and antioxidant parameters after prolonged NP exposure indicated the decrease of NP toxicity with time, which might be due to its high constitutive GST activities. C. vulgaris showed the highest GST activities among the four microalgal species under the control condition (Fig. 5). It is also possible that other enzymes might be involved in the NP detoxification process. For instance, Nakajima et al. (2007) found that phenol glucosyl transferase (GTs), in addition to GST, was involved in the metabolism of xenobiotics in the phase II detoxification process in microalgae. Recently, POD was also suggested to be involved in the xenobiotics detoxification process (Stiborova et al. 2000). Therefore, the enzymes involved in the degradation/transformation of NP in C. vulgaris should be further investigated.
Relationships between antioxidant defence system and tolerance of microalgae to NP
In photosynthetic organisms, environmental factors, such as excess irradiation, aberrant temperatures and various pollutants, would create oxidative stresses through the increased production of ROS (Asada 1996). Under the oxidative stress condition, higher plants and algae respond to the oxidative stress by increasing their antioxidant defence systems, notably the antioxidant enzymes, such as SOD, APX or CAT, and antioxidant substances, including low-molecular-weight compounds like GSH (Bartosz 1997). It is believed that the tolerance of plants to environmental stress is closely related to their ability to scavenge ROS (Rao 1992; Choo et al. 2004). Generally, the stress-tolerant species have more effective defence systems against ROS than the stress-sensitive species (Buchanan et al. 2000), and many studies demonstrated that, in most cases, a series of antioxidant enzymes in the oxidative defence system works in combination to maintain the steady-state level of all types of ROS (Choo et al. 2004). This explains why the relationships between cell growth and individual antioxidant parameters were complicated and might not follow simple correlations in the present study. Nevertheless, the present study displayed that the NP-tolerant species generally responded to, and recovered more rapidly from NP-induced oxidative stress than the NP-sensitive species, as indicated by the changes in both growth and antioxidant parameters (Figs. 1, 2, 3, 4, 5 and 6 and Table 2).
As the major ROS scavenging mechanisms in plants include SOD, APX or CAT, the balance of SOD and CAT or APX is crucial for determining the steady-state levels of O2 −· and H2O2 (Mittler 2002). The present study showed that the ability of a microalga to remove O2 · was not directly related to its tolerance to NP-induced oxidative stress, as the two NP-sensitive species, Se. capricornutum and Sc. quadricauda, not only showed higher constitutive SOD activities than the two NP-tolerant species (C. vulgaris and Chlorella sp.) but also displayed similar responses under the NP-induced oxidative stress (Fig. 2), indicating that both NP-sensitive and tolerant species could remove superoxide efficiently. The present study demonstrated that CAT might play an important role in the tolerance of microalgal cells to the NP-induced oxidative stress, as its activity was highly correlated to the tolerance of microalgae to NP-induced stress. The NP-tolerant species, C. vulgaris and Chlorella sp., not only displayed a more rapid increase in CAT activity when exposed to NP but also showed a much higher increase in CAT activity than the two NP-sensitive species (Se. capricornutum and Sc. quadricauda) (Fig. 3 and Table 2).
The positive relation coefficient between GSH content and chl a concentrations (data not shown) suggested that GSH, together with the functioning of the GSH-related enzymes, played an important role in the microalgae’s tolerance to NP-induced oxidative stress. Under both control and two NP treatments, the two NP-tolerant species (C. vulgaris and Chlorella sp.) had a significantly higher GSH content than the two NP-sensitive species (Se. capricornutum and Sc. quadricauda), which were highly correlated to their higher constitutive and/or enhanced GR activities in NP-tolerant species when exposed to NP (Fig. 4). These results implied that the ability of microalgae to remove H2O2 through both enzymatic and non-enzymatic antioxidant defence systems might play a vital role in the NP-induced oxidative stress tolerance of microalgal cells (Figs. 3 and 4). It was also found that GST activity was related to the tolerance of microalgae, as the NP-tolerant species (C. vulgaris and Chlorella sp.) had much higher constitutive GST activities (Fig. 5). However, among the GSH-related enzymes, GPX and POD, the two H2O2-scavenging enzymes, seemed to have only a minor role in the tolerance of microalgal cells to NP-induced oxidative stress, probably due to their low ROS-removal ability as compared to CAT and APX (Figs. 3 and 5). In addition to ROS scavenging by antioxidant defence systems, plant cells responded to the oxidative stress by avoiding the production of ROS through physiological acclimation strategies or by excreting H2O2 into the surrounding environment (Choo et al. 2004; Gao and Tam 2011). The ROS accumulation in the microalgal cells following NP exposure should be further investigated for a more in-depth understanding of the relationship between the tolerance level of microalgae to NP and their ability to defend NP-induced oxidative stress.
Conclusions
The present study demonstrated that the antioxidant parameters not only differed significantly among C. vulgaris, Se. capricornutum, Sc. quadricauda and Chlorella sp. under the control condition (without NP addition), the responses of microalgae to NP also depended on NP concentrations and exposure times. The changes of the antioxidant parameters following NP exposure indicated that NP induced an oxidative stress to microalgal cells, and the effects of NP on the antioxidant responses were most obvious after the first day of exposure but gradually decreased with prolonged exposure times. Various antioxidant mechanisms were involved in the microalgae to combat NP toxicity. The two NP-tolerant species, C. vulgaris and Chlorella sp. displayed more evident and rapid changes in antioxidant responses than the two NP-sensitive ones, Se. capricornutum and Sc. quadricauda, especially under the high NP concentration.
References
Anderson MF (1985) Determination of glutathione and glutathione disulfide in biological samples. Meth Enzymol 113:548–555
Asada K (1996) Radical production and scavenging in the chloroplast. In: Baker NR (ed) Advances in photosynthesis, Photosynthesis and the Environment, vol 5. Kluwer, Dordrecht, pp 123–150
Bartosz G (1997) Oxidative stress in plants. Acta Physiol Plant 19:47–64
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville
Chitra KC, Mathur PP (2004) Vitamin E prevents nonylphenol induced oxidative stress in testis of rats. Indian J Exp Biol l42:220–223
Chitra KC, Latchoumycandane C, Mathur PP (2002) Effect of nonylphenol on the antioxidant system in epididymal sperm of rats. Arch Toxicol 76:545–551
Choo KS, Snoeijs P, Pedersen M (2004) Oxidative stress tolerance in the filamentous green algae Chladophora glomerata and Enteromorpha ahlneriana. J Exp Mar Biol Ecol 298:111–123
Corsi SR, Zitomer DH, Field JA, Cancilla DA (2003) Nonylphenol ethoxylates and other additives in aircraft de-icers, anti-icers, and waters receiving airport runoff. Environ Sci Technol 37:4031–4037
Gao QT, Tam NFY (2011) Growth, photosynthesis and antioxidant responses of two microalgal species, Chlorella vulgaris and Selenastrum capricornutum, to nonylphenol stress. Chemophere 82:346–354
Gao QT, Wong YS, Tam NFY (2011) Removal and biodegradation of nonylphenol by different Chlorella species. Mar Poll Bull 63:445–451
Gong Y, Han XD (2006) Nonylphenol-induced oxidative stress and cytotoxicity in testicular sertoli cells. Reprod Toxicol 22:623–630
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Grant DF, Bender DM, Hammock BD (1989) Quantitative kinetic assays for glutathione S-transferase and general esterase in individual mosquitoes using an EIA reader. Insect Biochem 19:741–751
Hale RC, Smith CL, de Fur PO, Harvey E, Bush EO, La Guardia MJ, Vadas GG (2000) Nonylphenols in sediments and effluents associated with diverse wastewater outfalls. Environ Toxicol Chem 19:946–952
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
James DE (1978) Culturing algae. Carolina Biological Supply Company, USA
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Lei AP, Wong YS, Tam NFY (2003) Pyrene-induced changes of glutathione-S-transferase activities in different microalgal species. Chemosphere 50:293–301
Lei AP, Hu ZL, Wong YS, Tam NFY (2006) Antioxidant responses of microalgal species to pyrene. J Appl Phycol 18:67–78
Mallick N, Rai LC (1999) Response of the antioxidant systems of the nitrogen fixing cyanobacterium Anabaena doliolum to copper. J Plant Physiol 155:146–149
Mishra NP, Mishra RK, Singhal GS (1993) Changes in the activities of antioxidant enzymes during exposure of intact wheat leaves to strong visible-light at different temperatures in the presence of protein-synthesis inhibitors. Plant Physiol 102:903–910
Mishra S, Srivastava S, Tripathi RD, Kumar R, Seth CS, Gupta DK (2006) Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65:1027–1039
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Montavon P, Bortlik K (2004) Evolution of robusta green coffee redox enzymatic activities with maturation. J Agric Food Chem 52:3590–3594
Nagalakshmi N, Prasad MNV (1998) Copper–induced oxidative stress in Scenedesmus bijugatus: protective role of free radical scavengers. Bull Environ Contam Toxicol 61:623–628
Nakajima N, Teramoto T, Kasai F, Sano T, Tamaoki M, Aono M, Kubo A, Kamada H, Azumi Y, Saji H (2007) Glycosylation of bisphenol a by freshwater microalgae. Chemosphere 69:934–941
Okai Y, Sato EF, Higashi-Okai K, Inoue M (2004) Enhancing effect of the endocrine disruptor para-nonylphenol on the generation of reactive oxygen species in human blood neutrophils. Environ. Health Perspect 112:553–556
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Powles SB (1984) Photoinhibition of photosynthesis induced by visible-light. Annu Rev Plant Physiol Plant Mol Biol 35:15–44
Rao MV (1992) Cellular detoxifying mechanisms determine the age-dependent injury in tropical trees exposed to SO2. J Plant Physiol 140:733–740
Reddy AM, Kumar SG, Jyonthsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60:97–104
Rudel RA, Melly SJ, Geno PW, Sun G, Brody JG (1998) Identification of alkylphenols and other estrogenic phenolic compounds in wastewater, seepage, and groundwater on Cape Cod, Massachusetts. Env Sci Technol 32:861–869
Selote DS, Khanna-Chopra R (2010) Antioxidant response of wheat roots to drought acclimation. Protoplasma 245:153–163
Soares A, Guieysse B, Jefferson B, Cartmell E, Lester JN (2008) Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int 34:1033–1049
Sole M, Lopez de Alda MJ, Castillo M, Porte C, Ladegaard-Pedersen K, Barcelo D (2000) Estrogenicity determination in sewage treatment plants and surface waters from Catalonian area (NE Spain). Env Sci Technol 34:5076–5083
Song L, Liu Y (1999) FACHB-collection: freshwater algae culture collection of the Institute of Hydrobiology, lists strains, 2nd edn. The Chinese Academy of Sciences, China, p 11
Splittgerber AG, Tappel AL (1979) Inhibition of glutathione peroxidase by cadmium and other metal ions. Arch Biochem Biophys 197:534–542
Stiborova M, Schmeiser HH, Frei E (2000) Oxidation of xenobiotics by plant microsomes, a reconstituted cytochrome P450 system and peroxidase: a comparative study. Phytochemistry 54:353–362
Tang J, Siegfried BD, Hoagland KD (1998) Glutathione-S-transferase and in vitro metabolism of atrazine in freshwater algae. Pestic Biochem Physiol l59:155–161
Vartak V, Bhargave S (1999) Photosynthetic performance and antioxidant metabolism in a paraquat–resistant mutant of Chlamydomonas reinhardtii L. Pestic Biochem Physiol 64:9–15
Vazquez-Duhalt R, Marquez-Rocha F, Ponce E, Licea AF, Viana MT (2005) Nonyphenol, an integrated vision of a pollutant. Appl Ecol Environ Res 4:1–25
Wang JX, Xie P (2007) Antioxidant enzyme activities of Microcystis aeruginosa in response to nonylphenols and degradation of nonylphenols by M. aeruginosa. Environ Geochem Health 29:375–383
Zhao S-J, Xu C-C, Zou Q, Meng Q-W (1991) Improvements of methods for measurement of malonialdehyde in plant tissues. Zhi Wu Sheng Li Xue Tong Xun 30:207–210
Acknowledgements
The work described in this paper was supported by the State Key Laboratory in Marine Pollution, City University of Hong Kong and an internal grant (Project No. 7004709). The authors greatly appreciate the reviewer for comprehensively reading and providing valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, Q.T., Wong, Y.S. & Tam, N.F.Y. Antioxidant responses of different microalgal species to nonylphenol-induced oxidative stress. J Appl Phycol 29, 1317–1329 (2017). https://doi.org/10.1007/s10811-017-1065-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1065-y