Abstract
Ruminants could be the most suitable domestic animals to be supplemented with seaweeds as the rumen ecosystem might provide the animal the ability to use these feed resources by breaking down the complex polysaccharides. The objective of the present in vivo study was to determine the digestibility and the effects on the immune system of one green (Ulva rigida) and one red (Gracilaria vermiculophylla) seaweed cultivated in an integrated multitrophic aquaculture system (IMTA) and included in the diet of sheep at a supplementing level up to 25%. Both seaweeds showed lower dry matter digestibilitity than alfalfa hay, the organic matter digestibility of U. rigida being higher than that of G. vermiculophylla. The studied seaweeds had similar fiber and energy digestibility. Seaweed supplementation did not influence hematological parameters, reactive oxygen species production by neutrophils, nor lymphocytic response to T and B cells mitogens. The low fiber digestibility of selected seaweeds would be the major constraint to their use in high amounts in ruminant diets. Dietary seaweed supplementation has no deleterious effect on the immune function of cells mediating innate and acquired immunity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Livestock production is increasing fast for feeding a burgeoning human population. According to the FAO, it is expected that global meat and dairy production will more than double by 2050 (Steinfeld et al. 2006). This huge increase for animal products will require increasing amounts of feed supplies; thus, the identification of novel feeds is essential for the development of the livestock sector.
Seaweeds have been used to feed livestock since immemorial times in coastal regions, in times of feed scarcity (Balasse et al. 2005), and animals will naturally consume some quantity of seaweeds if they are available, such as on coastal farms. Generally, seaweeds are markedly rich in organic minerals, complex carbohydrates, proteins and low molecular weight nitrogenous compounds, lipids, vitamins, volatile compounds, and pigments (Makkar et al. 2016). Due to the chemical diversity and complexity of polysaccharides that may account to 25–75% of algal dry weight (Jiménez-Escrig and Sánchez-Muniz 2000), herbivorous animals and especially ruminants may be well suited to be fed on seaweeds as the rumen ecosystem might provide the animal the ability to use seaweeds by breaking down the complex polysaccharides. Brown algae have been the most intensively studied and exploited in animal feeding due to their large size, ease of harvesting, and mineral profile (especially iodine) (Rey-Crespo et al. 2014), but they have lower nutritional value than red and green algae due to their lower protein content (Makkar et al. 2016). Limited data are available on the in vivo digestibility of seaweeds for ruminants (Makkar et al. 2016), and their nutritive value varies with the species, geographic area, season of the year, environmental conditions (Ito and Hori 1989), and the nutrient content of the medium where they are cultivated (Azevedo et al. 2015).
More recently, seaweeds have been also evaluated as a prebiotic promoter (Ramnani et al. 2012) due to their content on bioactive substances with broad biological activities (Kumar et al. 2008; Santos et al. 2015). This is particularly important, as the exponential growth of the world population has contributed to the industrialization of food animal production that promoted major increases in livestock productivity largely due to the genetic progress and the development of diets tailored to specific stages of production. However, animal selection for increased production with little or no emphasis on health traits has led to a declining breeding success, increasing incidence of health problems, and declining longevity (Oltenacu and Algers 2005).
The objectives of the present study were to determine the in vivo digestibility of one green (Ulva rigida) and one red (Gracilaria vermiculophylla) seaweed cultivated in an integrated multitrophic aquaculture system (IMTA) and included in the diet of sheep up to a level of 25% (as fed), as well as to examine wether these algae may have deleterious effects on immunity status of the animal or if they could instead be considered immunity enhancers. Gracilaria is one of the most cultivated genera of seaweeds around the world (Yarish and Pereira 2008), G. vermiculophylla being a nonindigenous Asian red alga, and a dominant Gracilaria species in the Ria de Aveiro, Portugal, where it reproduces throughout the year and attains high rates growth success under a wide range of environmental conditions (Abreu et al. 2011b). The green algae of the genus Ulva are a group of edible algae widely distributed in a variety of habitats (Peña-Rodríguez et al. 2011). Culture of seaweeds increasingly contributes to supply the worldwide seaweed demand, as natural stocks of seaweeds are insufficient. The integration of seaweed culture with existing aquaculture operations (IMTA) has been successfully achieved in land-based-contained systems. These systems have potential to decrease costs as the production of seaweeds can be achieved by utilizing ammonia, phosphate, and CO2 from aquatic animal waste water, converting them into potentially valuable biomass. Effluents can recirculate back to the fish ponds or be discharged into the environment without negative impact (Neori et al. 2004). Many seaweed species may be suitable for bioremediation of aquaculture effluents (Lawton et al. 2013). Gracilaria species are efficient biofilters due to their capacity to uptake N (Abreu et al. 2011a). Ulva species are also ideal candidates for bioremediation of aquaculture effluents due to their high growth rates, broad environmental tolerance, low susceptibility to epiphytism (de Paula Silva et al. 2008; Mata et al. 2010), and high ability to absorb inorganic phosphorous and nitrogen (Mata et al. 2010).
Seaweeds
Seaweeds used in the present experiment (U. rigida and G. vermiculophylla) were cultivated in an IMTA system by ALGAPlus (Ílhavo, Portugal) as described by Domingues et al. (2015). After harvesting, seaweeds were rinsed with freshwater to remove epiphytes, detritus, and sand, subsequently dried for 8–10 h in a drying tunnel at 25 °C, and transported to the laboratory, where they were ground at 10 mm, and kept at the room temperature until usage.
In vivo digestibility
To estimate in vivo digestibility of the two seaweed species, three male and three female cross Merino sheep weighing 46 ± 2.0 and 42 ± 4.6 kg body weight (BW), respectively, at the start of the study were used. Animals were placed in individual metabolism crates with head gates and a steel grid floor beneath which there were sloped grids that allow the separation of urine from feces, respectively collected in scree-topped plastic boxes and plastic boxes. Animals were randomly allocated within sex to one of the three experimental diets (as fed) in a replicated 3 × 3 Latin square design with 15 days per period (10 days for diet adaptation and 5 days for total feces collection): 100% ground (10 mm) alfalfa hay (AH), 75% AH and 25% U. rigida (ULV), and 75% AH and 25% G. vermicullophyla (GRA). All diets were supplemented (30 g kg−1 diet) with a mineral-vitamin premix (all values per kg of DM: vitamin A 226,660 IU, vitamin D3 33,330 IU, vitamin E 1330 mg, Zn 1660 mg, Mn 1000 mg, Fe 333 g, Co 3.5 mg) formulated according to the mineral composition of the seaweeds (Cabrita et al. 2016). A maximum objective of daily diet DM intake allowed was set at 20 g per kg BW. After weighing and sampling the leftovers of alfalfa hay from the previous day, if present, seaweed was fed at 0900 hours and hay at 0930 hours in order to guarantee the total consumption of the seaweed. In the afternoon, seaweed was given at 1700 hours and hay at 1730 hours when the seaweed was already eaten. Animals had free access to clean water. In the last 5 days of each experimental period, total feces voided by each animal were daily collected, dried at 65 °C in a forced air oven until constant weight, bulked, subsampled, and ground to pass a 1-mm sieve for subsequent laboratory analysis. Representative samples of feeds and refusals, when present, were daily collected and dried at 65 °C for later analysis. Animals were weighed in the first and last days of each period at the same hour.
Analysis of immunological parameters
In the last day of each experimental period, blood samples were collected from the jugular vein for hemogram and proteinogram analysis and leukocyte isolation. Polymorphonuclear (PMN) and peripheral blood mononuclear cells (PBMC) were isolated by a single-step density gradient separation procedure. Briefly, whole blood diluted (1:2) in PBS was layered on a double gradient of Histopaque 1077 over histopaque 1119 (Sigma) and centrifuged at 400×g for 30 min at room temperature. Peripheral blood mononuclear cells were recovered from the interface between Histopaque-1077 and the plasma, and PMN were collected from the interface between Histopaque-1119 and Histopaque-1077. Platelets were removed by low-speed centrifugation during cell washing. The success of PBMC and PMN isolation was evaluated upon standard Hemacolor staining. Briefly, cytospins of the isolated cells were methanol fixed, stained with Hemacolor Solution 2 and 3 (Merck, Germany) washed by running tap water, mounted in Entellan (Merck), and observed through a microscope.
Production of reactive oxygen species
Purified PMN (1 × 106) cells were stimulated with 10 μM phorbol-myristate acetate (PMA) for 5 min at 37 °C and 5% CO2. Cells without stimulus were used as controls of basal reactive oxygen species (ROS) production. Reactive oxygen species production was measured by using the Superoxide Detection kit (Enzo Life Sciences) and analyzed in an EPICS XL flow cytometer (Beckman-Coulter). Flow cytometry data were analyzed with FlowJo software (version 10.1). Reactive oxygen species production was evaluated by determining the mean fluorescence intensity (MFI) of the orange fluorescence emitted due to superoxide production within cells.
Proliferation assays
Isolated PBMC were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) using the CellTrace CFSE Cell Proliferation Kit, for flow cytometry (ThermoFischer Scientific). CFSE-labeled cells (5 × 104) were cultured in RPMI complete medium (RPMI 1640 supplemented with 50 U mL−1 penicillin, 50 μg mL−1 streptomycin, 1 % HEPES buffer, 10 % FCS, and 5 μM 2-mercaptoethanol, all from Sigma) in 96-well round-bottomed culture plates without stimulus or stimulated with 2.5 μg mL−1 Lipopolysaccharide (LPS; Sigma) or 2.5 μg mL−1 Concanavalin A (ConA; Sigma) for 72 h at 37 °C and 5 % CO2 in a humidified atmosphere. Cultured nonlabeled PBMC were used to define cell’s autofluorescence. Cell proliferation was measured by flow cytometry through successive halving of the fluorescence intensity of CFSE. Two parameters were used to assess cell proliferation: the percentage of cells that divided at least once and the proliferation index, defined by the equation \( \frac{\sum_0^iNi}{\sum_0^i\frac{Ni}{2^i}} \), where i is the generation number (0 is the undivided population) and Ni corresponds to the number of cells in generation i.
Interferon-γ measurement
The concentration of IFN-γ in 72 h-cell culture supernatants from nonstimulated and LPS- or ConA-stimulated PBMC was determined by using the Bovine IFN-γ ELISA development kit (Mabtech), according to the manufacturer’s instructions. The monoclonal antibodies of the kit cross-react with IFN-γ from sheep.
Analytical methods
Ground (1 mm) samples of feeds, refusals, and feces were analyzed for DM by drying samples at 105 °C for 24 h in a forced air oven (AOAC 1990). Representative samples of each feed (bulked for period) and of feces (bulked by period and animal) were subjected to analysis of ash (ID 942.05) (AOAC 1990), ether extract (EE; ID 920.39) (AOAC 1990), and neutral detergent fiber (NDF; with α-amylase and without sodium sulfite) (Robertson and Van Soest 1981; Van Soest et al. 1991). Samples of feeds were also analyzed for Kjeldahl N (ID 954.01) (AOAC 1990), acid detergent fiber (ADF), and acid detergent lignin (ADL) (Robertson and Van Soest 1981; Van Soest et al. 1991). Crude protein (CP) was determined as Kjeldahl N × 6.25 for hay and Kjeldahl N × 5.0 for seaweeds (Angell et al. 2016). Neutral detergent fiber and ADL were expressed exclusive of residual ash. Gross energy (GE) of feeds and feces was determined in an adiabatic bomb calorimeter (Werke C2000, IKA, Germany). All chemical analyses were run in duplicate.
Calculations and statistical analysis
The apparent digestibility (g kg−1) of dietary constituints (DM; organic matter, OM; NDF; and GE) was calculated according to the following equation (intake and output of nutrients in kilograms): apparent nutrient digestibility = (1 − (fecal nutrient / total nutrient intake)) × 1000. The apparent digestibility (g kg−1) of the seaweeds was calculated by difference and by animal, considering the apparent digestibility of the alfalfa hay measured in each period.
Data were tested for normality using the Kolmogorov-Smirnov test. Reactive oxygen species production in nonstimulated cells and IFN-γ production under LPS stimulation were subjected to inverse transformation and PMA-stimulated cells to square transformation to achieve a normal distribution of the data.
The experimental design was a replicated 3 × 3 Latin square. Data were analyzed using the general linear model of SPSS (IBM SPSS statistics V22.0, USA). The model was:
where Y ijkl = response variable, μ = mean, S i = the fixed effect of square, c j(i) = the fixed effect of animal nested within square, p k = the fixed effect of period, D l = the fixed effect of diet, and e ijkl = the experimental error. Significance is declared at P ≤ 0.05. Trends are discussed at 0.05 < P < 0.10.
Results
Chemical composition
The chemical composition of the experimental feeds is listed in Table 1. Alfalfa hay presented 186 g kg−1 CP, 372 g kg−1 NDF, and 73.0 g kg−1 ADL (DM basis). Gracilaria vermiculophylla and U. rigida presented, respectively, 359 and 470 g kg−1 ash, 202 and 123 g kg−1 CP, 183 and 221 g kg−1 NDF, and 12.8 and 9.58 MJ kg−1 of GE (DM basis).
Feed intake and in vivo digestibility
Daily DM intake of total diet, alfalfa hay, and of each studied seaweed is given in Table 2. Animals ingested 24 and 25% more of the diet without seaweed than the diets supplemented with G. vermiculophylla and U. rigida, respectively (P < 0.001). With the control diet, animals achieved the maximum allowed level of intake of 20 g DM kg−1 of BW, but when fed diets supplemented with G. vermiculophylla and U. rigida, they were not beyond 17.6 to 17.2 g DM kg−1 BW, respectively. There were no differences between the intake of alfalfa hay or seaweed among diets GRA and ULV.
Table 3 presents the apparent digestibility coefficients of DM, OM, NDF, and GE of total diets and of the studied seaweeds. The DM digestibility of diets with 25% of G. vermiculophylla and U. rigida was respectively 4 and 6% lower than the control diet with no seaweed supplementation (P = 0.009). The digestibility of OM and GE of the AH and ULV diets were similar and higher than that of GRA diet (more 5% for OM digestibility, P = 0.005, and 2.5 and 1% for GE digestibility, respectively, for AH and ULV diets, P = 0.006). Fiber digestibility of GRA and ULV diets were respectively 6 and 9% lower than that of AH diet (P = 0.019).
The DM digestibility of G. vermiculophylla was 13% higher than that of U. rigida, the values for these seaweeds being, respectively, 15 and 25% lower than the DM digestibility of the alfalfa hay. Conversely, the OM digestibility of U. rigida was similar to that of the alfalfa hay, and 36% higher than G. vermiculophylla. Neutral detergent fiber and GE digestibility were not different between seaweeds and averaged, respectively, 267.8 and 522.9 g kg−1 DM for U. rigida and 290.4 and 558.3 g kg−1 DM for G. vermiculophylla.
Analysis of immunological parameters
The values of all measured hematological parameters were within the normal range for sheep. As it can be observed in Table 4, experimental treatments had no significant effects (P > 0.05) on the cell blood count parameters or on the concentration of serum proteins.
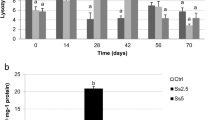
A putative influence of seaweed supplementation in immunological parameters characteristic of both innate and adaptive immunity was assessed using cells isolated from the peripheral blood. As shown in Fig. 1, PMA-stimulated PMN produced markedly higher amounts of superoxide anion when compared to the nonstimulated ones. Nevertheless, no differences could be observed in this parameter among animals fed with any of the diets (Table 4). Lymphocyte proliferative response to classic T and B lymphocyte mitogens ConA and LPS was assessed as a surrogate marker for adaptive immunity competence. A marked proliferative response was induced in PBMC upon stimulation with ConA. Nonetheless, it was not different among animals fed with AH, GRA, or ULV diets either when proliferation was measured as the percentage of cells that divided at least once or by the proliferation index. Stimulation of PBMC with LPS resulted in a minor proliferative effect that did not differ considerably from controls and that was similar among diet groups (Table 5 and Fig. 2). The lack of significant differences in the lymphocyte proliferative response to ConA could in part be attributed to the high variability observed, which was already reported in sheep (Wattegedera et al. 2004). The amounts of the pro-inflammatory cytokine IFN-γ measured in culture supernatants of nonstimulated control cells were very low, as could be expected. Conversely, ConA-stimulated cells produced significantly higher amounts of IFN-γ than the nonstimulated cells (P < 0.05). However, no differences could be observed in IFN-γ production between PBMC isolated from control diet-fed animals and seaweed-supplemented diet-fed sheep, regardless of the seaweed used (Table 5). No effect was observed upon LPS stimulation, as compared to controls, and no significant differences were observed among diet groups. Altogether, these results indicated that seaweed supplementation did not influence hematological parameters, ROS production by neutrophils, and the lymphocytic response to mitogens. This provides preliminary evidence indicating that these seaweed diet supplementations have no deleterious or enhancing effects on basic immune functions of cells mediating innate and acquired immunity.
Flow cytometry analysis of PBMC proliferation using CFSE-labeling. a Representative example showing gated CFSE-positive PBMC cells nonstimulated or stimulated with LPS or ConA, obtained from an AH diet-fed animal. b Histograms showing the percentage of cells that divided at least once. c Histograms showing the number of generations traced by CFSE fluorescence dilution. Numbers above the peaks represent the number of times the respective cell population divided. Red lines correspond to the model sum of the blue lines which correspond top calculated generation peaks
Discussion
Nutritive value
The chemical composition of the alfalfa hay used in the present study agrees with the values reported by FEDNA (2010) for a high quality alfalfa hay. Seaweeds drawn from the water are a rich source of minerals, their content being higher than those reported for edible land plants (Rupérez 2002). Ash, which broadly represents mineral content (Cabrita et al. 2016), was the greatest constituent in both seaweeds, being higher than the values observed in an earlier study (Cabrita et al. 2016) with Ulva sp. and G. vermiculophylla originated from the same IMTA system (25.0% DM for Ulva sp., and 27.8% DM for G. vermiculophylla).
Due to the high and more constant levels of nutrients in the medium, IMTA systems can improve the productivity and nutritional quality of the seaweeds, when compared to the naturally harvested ones (Abreu et al. 2009). Ulva rigida presented lower CP content than G. vermiculophylla. However, higher protein content (up to 34% in U. lactuca, and 49% in Gracilaria) has been reported in seaweed produced in IMTA systems (Schuenhoff et al. 2003; Shields and Lupatsch 2012; Silva et al. 2015). Differences among studies could be explained by the culture conditions applied. For instance, biomass production and nutrient removal from seaweeds are negatively related to the cultivation densities in the system, temperature, and light being the main environmental factors affecting the growth and nutrient removal (Abreu et al. 2011a).
The low lipid content of both seaweeds is in agreement with several studies (Khairy and El-Shafay 2013; Mouritsen et al. 2013). The high ash and the low lipid contents observed in the studied seaweeds were reflected in their low GE content, supporting the work by Hind et al. (2014) who referred that energy potential of U. lactuca is limited by its high mineral content. Indeed, earlier work observed higher energy content for U. lactuca (15.2 MJ GE kg−1 DM, Felix and Brindo 2014; estimated digestible energy of 10.2 MJ kg−1 DM, Ventura and Castañón 1998).
Ulva rigida had a higher fiber content than G. vermiculophylla, but lower than alfalfa hay. Unlike terrestrial plants, seaweeds have complex cell wall polysaccharides that greatly differ among seaweed classes and species (Pereira et al. 2009). Green algae are rich in soluble ulvans of the family of sulfated polysaccharides (Domozych et al. 2012), while carrageenans (Michel et al. 2006), agars, and porphyran (Correc et al. 2011) are the major matrix polysaccharides of red algae.
When fed the control diet, animals achieved the maximum allowed level of intake of 20 g DM kg−1 BW, but when supplemented with seaweeds, the DM intake decreased by around 20%. The lower seaweed palatability have been atributed to the existence of secondary metabolites (Cronin and Hay 1996) or to the high mineral content (Cabrita et al. 2016). This could be overcome by decreasing the level of dietary inclusion of seaweeds that in the present study was on average 24.5 and 23.7% (DM basis) respectively for G. vermiculophylla and U. rigida. Indeed, the actual low supply of these seaweeds makes lower levels of supplementation more realistic.
In the present study, in vivo digestibility was measured after 10 days for animal adaptation to the diet. This adaptation or preliminary period is designed to ensure that feed residues of the previous diet are eliminated before starting the feces collection, that a stable rumen population is established, and that the animals are eating approximately the same daily amount of feed and at the same time (Rymer 2000). The recommended length of time for the adaptation period varies from 4 to 14 days (Rymer 2000), and it is expected that its lengthening would improve the accuracy and precision of measurements. However, due to animal welfare concerns, efforts should be made to shorten the experimental periods as much as possible. When animals are fed ad libitum, where more variation in daily intake and excretion exists, the adaptation period should be at least 12 days long, but it could be shortened if following the worldwide recommendations to feed restrictively when measuring digestibility to decrease the variability of digestion and excretion processes (Farenzena et al. 2016), as done in the present study.
The digestibility values determined for alfalfa hay are in close agreement with those reported earlier (Carvalho et al. 2005). Both seaweeds presented lower DM digestibility than alfalfa hay, with the OM digestibility of U. rigida being similar to that of the alfalfa hay and higher than that of G. vermiculophylla, which was similar to the values reported for meadow hay, rice, rye, and wheat straw (Fonseca et al. 1998). A direct comparison of the values herein obtained with the literature is difficult because of the limited data regarding in vivo digestibility, in sacco degradability, and energy values of seaweeds for ruminants. A similar OM digestibility was obtained by Ventura and Castañón (1998) for U. lactuca (621 g kg−1 DM), but the values of OM digestibility reported in the current study were lower than that referred for a Laminaria digitata and Laminaria hyperborea mixture measured in vitro (78.3%) by Hansen et al. (2003). In the study by Arieli et al. (1993) with young rams, the in vivo energy digestibility of U. lactuca (60%) was similar to the value obtained in the present study, but the digestible energy value (9.1 MJ kg−1 DM) was higher. The results obtained suggest that the main constraint to the use of these seaweeds at high dietary inclusion rates is their low fiber digestibility and their high mineral content. As far as we know, this is the first report of seaweed in vivo fiber digestibility. Typically, seaweeds have low amounts of cellulose (around 4%) and are rich in specific polysaccharides. Despite ulvans from green algae being potentially hydrolysable to bioactive oligosaccharides (Andrieux et al. 1998), ulvan lyases have only been isolated in marine environments (Barbeyron et al. 2011) and in Proteobacteria species found in soil (Collén et al. 2011). Similarly, the hydrolysis of galactans from red algae requires enzymes predominantly encoded in genomes of marine microbes, but less frequent or even absent in bacteria that hydrolyze polysaccharides from land plants (Hehemann et al. 2010). These could be hydrolyzed by the rumen microbial population producing methane and acetic acid (Williams et al. 2013). However, differences on seaweed digestibility between studies could be explained not only by the composition of the algae but also by the adaptation of the animal to this particular feed (Makkar et al. 2016). Indeed, Orpin et al. (1985) found that 13% of the culturable bacteria from seaweed-fed sheep grew on alginate, 71% on laminarin, 13% on fucoidan, and 99% on mannitol, while the percentages obtained from pasture-fed animals were significantly lower (2, 32, 0, and 0%, respectively). Differences in the ability to hydrolyze mannitol between seaweed-fed or grass-fed animals were also observed in other studies (Ahmed et al. 2013). Seaweed feeding seems to change rumen microflora substantially, not including phycomycete fungi or cellulolytic bacteria, but ciliate protozoa (e.g., Dasytricha ruminantium, Entodinium species) and lactate-utilizing bacteria (e.g., Streptococcus bovis, Selenomonas ruminantium, Butyrivibrio fibrisolvens) (Orpin et al. 1985); thus, a stepwise increase in the levels of seaweeds in the diet may enable rumen microbes to adapt and, thus, enhance energy availability from these complex carbohydrates (Makkar et al. 2016).
Immunological parameters
Very little is known about putative effects of diet supplementation with seaweed or seaweed extracts on hematological or immunological parameters. The results presented here are in line with a previous report in which Ascophylum nodosum extract inclusion in the diet of goats did not affect white blood counts (Kannan et al. 2007). Evaluation of different immunological parameters also indicated that seaweed supplementation done in this study had no adverse or enhancing effects on immunity. Reactive oxygen species production is a major immune effector mechanism used by neutrophils (Amulic et al. 2012). Previous reports have shown a reduction in ROS production after ex vivo stimulation of monocytes or neutrophils with algae or their extracts (Jeon et al. 2012; Jeon et al. 2014). However, when lambs subjected to heat stress were fed with a diet supplemented with extracts of the brown alga A. nodosum, there was an enhancement on the ability of monocytes to produce ROS (Saker et al. 2004). Our results did not indicate that inclusion of seaweed in the sheep diet could majorly affect ROS production by PMN. Nevertheless, minor effects in this innate immune mechanism could have been missed due to sample size limitations. Indeed, according to the results obtained in the present study, a power analysis suggested a minimum sample size per treatment group of eight to 12 to ensure a minimum margin of error.
Here, the lymphocyte proliferative response to mitogens was used as a surrogate marker of acquired immunity to evaluate whether seaweed inclusion could affect the ex vivo function of lymphocyte cells. Ciliberti et al. (2015) reported that PBMC isolated from sheep fed with a diet supplemented with A. nodosum had impaired T cell proliferation responses when stimulated with the T cell mitogen phytohemagglutinin (PHA). The authors attributed the effect to the high content of eicosapentaenoic acid in that brown macroalga. Contrastingly, we show here that supplementation with a green and a red algae had no negative impact on the lymphocyte proliferation response to mitogens. The low levels of this polyunsaturated fatty acid in U. lactuca and G. vermiculophylla (van Ginneken et al. 2011; Imbs et al. 2012) could be accounting for these results.
Seaweeds are particularly rich in trace minerals like zinc, copper, chromium, and selenium or in vitamins E and β-carotenes (Mišurcová 2011). It was shown that zinc diet supplementation in lambs increased lymphocyte proliferation to ConA (Nagalakshmi et al. 2009), which was in accordance with the reduced lymphocyte response to T cell mitogens reported by Droke and Spears (1993) in a zinc deficiency study. Although G. vermicullophylla has a relatively high zinc content (Cabrita et al. 2016), diet supplementation with this seaweed did not result in altered lymphocyte proliferation. This might agree with another study showing that in vitro lymphocyte proliferation upon PHA stimulation in PBMC from steers fed control or zinc supplemented diets was not different (Spears and Kegley 2002). Selenium (Turner and Finch 1990; Cao et al. 1992) was shown to be important for adequate lymphoproliferative responses to T cell mitogens. The macroalgae used here are enriched in selenium (Cabrita et al. 2016), yet no improved lymphocyte proliferation was found. A putative immune-enhancer effect of seaweed supplementation on lymphocyte function would nevertheless be worth assessing in a condition of selenium-poor diet.
The pro-inflammatory cytokine IFN-γ is a major effector in cell-mediated immune responses to common intracellular pathogens affecting sheep, like Chlamydophila abortus and Toxoplasma gondii (Graham et al. 1995; Esteban-Redondo and Innes 1997). Despite the importance of IFN-γ, very few studies addressed the effect of the diet on the production of this inflammatory cytokine. In accordance with the lack of effect of seaweed supplementation observed in the present study, two previous studies showed no effect of A. nodosum supplementation on the IFN-γ production in either stimulated or nonstimulated sheep PBMC (Ciliberti et al. 2015) and on sheep Th1 responses (Caroprese et al. 2014).
The evidence reported here indicating that U. rigida and G. vermiculophylla diet supplementation have no deleterious effect on the immune function of cells mediating innate and acquired immunity further supports the potential of seaweed as feed ingredients for ruminant feeding. Exploring the studied and other immune parameters in longer feeding periods, in different physiological conditions or environmental challenges, such as infection, would be important to more accurately ascertain the safety or potential beneficial effects of the seaweed diet supplementation.
Conclusions
Digestibility of U. rigida and G. vermiculophylla was lower than that of alfalfa hay, the results suggesting that the main constraint to use these seaweeds as feed ingredients for ruminant animals being the low fiber digestibility. On the other hand, dietary supplementation with these seaweeds at 25% (as fed) did not affect the immune function of cells mediating innate and acquired immunity, which suggests that these two species are safe ingredients for ruminant nutrition.
References
Abreu MH, Pereira R, Buschmann AH, Sousa-Pinto I, Yarish C (2011a) Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium. J Exp Mar Biol Ecol 407:190–199
Abreu MH, Pereira R, Sousa-Pinto I, Yarish C (2011b) Ecophysiological studies of the non-indigenous species Gracilaria vermiculophylla (Rhodophyta) and its abundance patterns in Ria de Aveiro lagoon, Portugal. Eur J Phycol 46:453–464
Abreu MH, Varela DA, Henríquez L, Villarroel A, Yarish C, Sousa-Pinto I, Buschmann AH (2009) Traditional vs. integrated multi-trophic aquaculture of Gracilaria chilensis C. J. Bird, J. McLachlan & E. C. Oliveira: productivity and physiological performance. Aquaculture 293:211–220
Ahmed S, Minuti A, Bani P (2013) In vitro rumen fermentation characteristics of some naturally occurring and synthetic sugars. Ital J Anim Sci 12:359–365
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A (2012) Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30:459–489
Andrieux C, Hibert A, Houari AM, Bensaada M, Popot F, Szylit O (1998) Ulva lactuca is poorly fermented but alters bacterial metabolism in rats inoculated with human fecal flora from methane and non-methane producers. J Sci Food Agric 77:25–30
Angell AR, Mata L, Nys R, Paul NA (2016) The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J Appl Phycol 28:511–524
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Arlington
Arieli A, Sklan D, Kissil G (1993) A note on the nutritive value of Ulva lactuca for ruminants. Anim Sci 57:329.331
Azevedo G, Domingues B, Abreu H, Sousa-Pinto I, Feio G, Hilliou L (2015) Impact of cultivation of Mastocarpus stellatus in IMTA on the seaweeds chemistry and hybrid carrageenan properties. Carbohyd Polym 116:140–148
Balasse M, Tresset A, Dobney K, Ambrose SH (2005) The use of isotope ratios to test for seaweed eating in sheep. J Zool 266:283–291
Barbeyron T, Lerat Y, Sassi JF, Le Panse S, Helbert W, Collén PN (2011) Persicivirga ulvanivorans sp. nov., a marine member of the family Flavobacteriaceae that degrades ulvan from green algae. Int J Syst Evol Microbiol 61:1899–1905
Cabrita ARJ, Maia MRG, Oliveira HM, Sousa-Pinto I, Almeida AA, Pinto E, Fonseca AJM (2016) Tracing seaweeds as mineral sources for farm-animals. J Appl Phycol 28:3135–3150
Cao YZ, Maddox JF, Mastro AM, Scholz RW, Hildenbrandt G, Reddy CC (1992) Selenium deficiency alters the lipoxygenase pathway and mitogenic response in bovine lymphocytes. J Nutr 122:2121–2127
Caroprese M, Ciliberti MG, Annicchiarico G, Albenzio M, Muscio A, Sevi A (2014) Hypothalamic-pituitary-adrenal axis activation and immune regulation in heat-stressed sheep after supplementation with polyunsaturated fatty acids. J Dairy Sci 97:4247–4258
Carvalho LPF, Melo DSP, Pereira CRM, Rodrigues MAM, Cabrita ARJ, Fonseca AJM (2005) Chemical composition, in vivo digestibility, N degradability and enzymatic intestinal digestibility of five protein supplements. Anim Feed Sci Technol 119:171–178
Ciliberti MG, Albenzio M, Annicchiarico G, Sevi A, Muscio A, Caroprese M (2015) Alterations in sheep peripheral blood mononuclear cell proliferation and cytokine release by polyunsaturated fatty acid supplementation in the diet under high ambient temperature. J Dairy Sci 98:872–879
Collén PN, Sassi J-F, Rogniaux H, Marfaing H, Helbert W (2011) Ulvan lyases isolated from the Flavobacteria Persicivirga ulvanivorans are the first members of a new polysaccharide lyase family. Journal Biol Chem 286:42063–42071
Correc G, Hehemann J-H, Czjzek M, Helbert W (2011) Structural analysis of the degradation products of porphyran digested by Zobellia galactanivorans β-porphyranase a. Carbohydr Polym 83:277–283
Cronin G, Hay M (1996) Within-plant variation in seaweed palatability and chemical defenses: optimal defense theory versus the growth-differentiation balance hypothesis. Oecologia 105:361–368
de Paula Silva PH, McBride S, de Nys R, Paul NA (2008) Integrating filamentous ‘green tide’ algae into tropical pond-based aquaculture. Aquaculture 284:74–80
Domingues B, Abreu M, Sousa-Pinto I (2015) On the bioremediation efficiency of Mastocarpus stellatus (Stackhouse) Guiry, in an integrated multi-trophic aquaculture system. J Appl Phycol 27:1289–1295
Domozych DS, Ciancia M, Fangel JU, Mikkelsen MD, Ulvskov P, Willats WGT (2012) The cell walls of green algae: a journey through evolution and diversity. Front Plant Sci 3:82
Droke EA, Spears JW (1993) In vitro and in vivo immunological measurements in growing lambs fed diets deficient, marginal or adequate in zinc. J Nutr Immunol 2:71–90
Esteban-Redondo I, Innes EA (1997) Toxoplasma gondii infection in sheep and cattle. Comp Immunol Microbiol Infect Dis 20:191–196
Farenzena R, Kozloski GV, Gindri M, Stefanello S (2016) Minimum length of the adaptation and collection period in digestibility trials with sheep fed ad libitum only forage or forage plus concentrate. J Anim Physiol Anim Nutr. doi:10.1111/jpn.12550
FEDNA (2010) Tablas FEDNA de composición y valor nutritivo de alimentos para la fabricación de piensos compuestos, 3ª edición edn. Fundación Española para el Desarrollo de la Nutrición Animal, Madrid
Felix N, Brindo RA (2014) Evaluation of raw and fermented seaweed, Ulva lactuca as feed ingredient in giant freshwater prawn Macrobrachium rosenbergii. International J Fish Aquat Stud 1:199–204
Fonseca AJM, Dias-da-Silva AA, Ørskov ER (1998) In sacco degradation characteristics as predictors of digestibility and voluntary intake of roughages by mature ewes. Anim Feed Sci Technol 72:205–219
Graham SP, Jones GE, MacLean M, Livingstone M, Entrican G (1995) Recombinant ovine interferon gamma inhibits the multiplication of Chlamydia psittaci in ovine cells. J Comp Pathol 112:185–195
Hansen HR, Hector BL, Feldmann J (2003) A qualitative and quantitative evaluation of the seaweed diet of north Ronaldsay sheep. Anim Feed Sci Technol 105:21–28
Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G (2010) Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 464:908–912
Hind Z, Rabah A, Christelle B, Hacène B, Yves B (2014) Chemical and biological evaluation of the nutritive value of Algerian green seaweed Ulva lactuca using in vitro gas production technique for ruminant animals. Int J Adv Res 2:916–925
Imbs AB, Latyshev NA, Svetashev VI, Skriptsova AV, Le TT, Pham MQ, Nguyen VS, Pham LQ (2012) Distribution of polyunsaturated fatty acids in red algae of the genus Gracilaria, a promising source of prostaglandins. Russ J Mar Biol 38:339–345
Ito K, Hori K (1989) Seaweed: chemical composition and potential foods uses. Food Rev Int 5:101–144
Jeon HJ, Choi HS, Lee OH, Jeon YJ, Lee BY (2012) Inhibition of reactive oxygen species (ROS) and nitric oxide (NO) by Gelidium elegans using alternative drying and extraction conditions in 3 T3-L1 and RAW 264.7 cells. Prev Nutr Food Sci 17:122–128
Jeon HJ, Seo MJ, Choi HS, Lee OH, Lee BY (2014) Gelidium elegans, an edible red seaweed, and hesperidin inhibit lipid accumulation and production of reactive oxygen species and reactive nitrogen species in 3 T3-L1 and RAW264.7 cells. Phytother Res 28:1701–1709
Jiménez-Escrig A, Sánchez-Muniz F (2000) Dietary fibre from edible seaweeds: chemical structure, physicochemical properties and effects on cholesterol metabolism. J Nutr Res 20:585–598
Kannan G, Saker KE, Terrill TH, Kouakou B, Galipalli S, Gelaye S (2007) Effect of seaweed extract supplementation in goats exposed to simulated preslaughter stress. Small Ruminant Res 73:221–227
Khairy HM, El-Shafay SM (2013) Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 55:435–452
Kumar CS, Ganesan P, Suresh PV, Bhaskar N (2008) Seaweeds as a source of nutritionally beneficial compounds - a review. J Food Sci Tech Mys 45:1–13
Lawton RJ, Mata L, de Nys R, Paul NA (2013) Algal bioremediation of waste waters from land-based aquaculture using Ulva: selecting target species and strains. PLoS One 8(10):e77344
Makkar HPS, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P (2016) Seaweeds for livestock diets: a review. Anim Feed Sci Technol 212:1–17
Mata L, Schuenhoff A, Santos R (2010) A direct comparison of the performance of the seaweed biofilters, Asparagopsis armata and Ulva rigida. J Appl Phycol 22:639–644
Michel G, Nyval-Collen P, Barbeyron T, Czjzek M, Helbert W (2006) Bioconversion of red seaweed galactans: a focus on bacterial agarases and carrageenases. Appl Microbiol Biot 71:23–33
Mišurcová L (2011) Chemical composition of seaweeds. In: Kim S-K (ed) Handbook of marine macroalgae: biotechnology and applied phycology. John Wiley, NY, pp. 171–192
Mouritsen OG, Dawczynski C, Duelund L, Jahreis G, Vetter W, Schröder M (2013) On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). J Appl Phycol 25:1777–1791
Nagalakshmi D, Dhanalakshmi K, Himabindu D (2009) Effect of dose and source of supplemental zinc on immune response and oxidative enzymes in lambs. Vet Res Commun 33:631–644
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391
Oltenacu PA, Algers B (2005) Selection for increased production and the welfare of dairy cows: are new breeding goals needed? Ambio 34:311–315
Orpin CG, Greenwood Y, Hall FJ, Paterson IW (1985) The rumen microbiology of seaweed digestion in Orkney sheep. J Appl Bacteriol 58:585–596
Peña-Rodríguez A, Mawhinney TP, Ricque-Marie D, Cruz-Suárez LE (2011) Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem 129:491–498
Pereira L, Amado AM, Critchley AT, van de Velde F, Ribeiro-Claro PJA (2009) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocolloid 23:1903–1909
Ramnani P, Chitarrari R, Tuohy K, Grant J, Hotchkiss S, Philp K, Campbell R, Gill C, Rowland I (2012) In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 18:1–6
Rey-Crespo F, López-Alonso M, Miranda M (2014) The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Animal 8:580–586
Robertson JB, Van Soest PJ (1981) The detergent system of analysis and its application to human foods. In: James WPT, Theander O (eds) The analysis of dietary fiber in food. Marcel Dekker Inc., New York, NY, pp. 123–158
Rupérez P (2002) Mineral content of edible marine seaweeds. Food Chem 79:23–26
Rymer C (2000) The measurement of forage digestibility in vivo. In: Givens DI, Owen E, Omed HM, Axford RFE (eds) Forage evaluation in ruminant nutrition. CAB International, Wallingford, p. 113
Saker KE, Fike JH, Veit H, Ward DL (2004) Brown seaweed - (Tasco) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J Anim Physiol Anim Nutr 88:122–130
Santos SA, Vilela C, Freire CS, Abreu MH, Rocha SM, Silvestre AJ (2015) Chlorophyta and Rhodophyta macroalgae: a source of health promoting phytochemicals. Food Chem 183:122–128
Schuenhoff A, Shpigel M, Lupatsch I, Ashkenazi A, Msuya FE, Neori A (2003) A semi-recirculating, integrated system for the culture of fish and seaweed. Aquaculture 221:167–181
Shields RJ, Lupatsch I (2012) Algae for aquaculture and animal feeds. Technikfolgenabschätzung-Theorie und Praxis 21:23–37
Silva DM, Valente LMP, Sousa-Pinto I, Pereira R, Pires MA, Seixas F, Rema P (2015) Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J Appl Phycol 27:1671–1680
Spears JW, Kegley EB (2002) Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J Anim Sci 80:2747–2752
Steinfeld H, Gerber P, Wassenaar T, Castel V, Rosales M, de Haan C (2006) Livestock’s long shadow: environmental issues and options. Food and Agriculture Organization of the United Nations, Rome
Turner RJ, Finch JM (1990) Immunological malfunctions associated with low selenium-vitamin E diets in lambs. J Comp Pathol 102:99–109
van Ginneken VJ, Helsper JP, de Visser W, van Keulen H, Brandenburg WA (2011) Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids Health Dis 10:104
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Ventura MR, Castañón JIR (1998) The nutritive value of seaweed (Ulva lactuca) for goats. Small Ruminant Res 29:325–327
Wattegedera S, Sills K, Howard CJ, Hope JC, McInnes CJ, Entrican G (2004) Variability in cytokine production and cell proliferation by mitogen-activated ovine peripheral blood mononuclear cells: modulation by interleukin (IL)-10 and IL-12. Vet Immunol Immunopathol 102:67–76
Williams AG, Withers S, Sutherland AD (2013) The potential of bacteria isolated from ruminal contents of seaweed-eating North Ronaldsay sheep to hydrolyse seaweed components and produce methane by anaerobic digestion in vitro. Microb Biotech 6:45–52
Yarish C, Pereira R (2008) Mass production of marine macroalgae. In: Jørgensen SE, Fath BD (eds) Ecological engineering. Encyclopedia of Ecology, vol 3. Elsevier, Oxford, pp. 2236–2247
Acknowledgments
This work received financial support from the European Union (FEDER funds through COMPETE) and National Funds (FCT) through projects EXPL/CVT-NUT/0286/2013 - FCOMP-01-0124-FEDER-041111 and UID/QUI/50006/2013 - POCI/01/0145/FERDER/007265 (LAQV). To all financing sources, the authors are greatly indebted. The authors also acknowledge Sílvia Azevedo (ICBAS-UP) for the valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present experiment was conducted at the Clinical and Veterinary Investigation Center of Vairão (Vila do Conde, Portugal) from the Institute of Biomedical Sciences Abel Salazar, University of Porto (ICBAS-UP), in strict accordance with good animal practice as defined by national authorities and European Union Directive 2010/63/EU. The experimental animal procedures were approved by the Local Animal Ethics Committee of ICBAS-UP, licensed by the Portuguese Directorate-General of Food and Veterinary Medicine (Direção Geral de Alimentação e Veterinária) of the Ministry for Agriculture and Sea (Ministério da Agricultura e do Mar), and conducted by trained scientists following FELASA category C recommendations. All methods and procedures were performed following the established guidelines from these institutions.
Rights and permissions
About this article
Cite this article
Cabrita, A.R.J., Correia, A., Rodrigues, A.R. et al. Assessing in vivo digestibility and effects on immune system of sheep fed alfalfa hay supplemented with a fixed amount of Ulva rigida and Gracilaria vermiculophylla . J Appl Phycol 29, 1057–1067 (2017). https://doi.org/10.1007/s10811-016-0999-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0999-9