Abstract
The effects of trophic modes autotrophy, heterotrophy, and mixotrophy on growth, photosynthetic activity, lipid content, and fatty acids (FA) profile were investigated for the thermophilic Chlorophyta strain, Graesiella sp., isolated from a mats community colonizing geothermal springs in the north of Tunisia. Mixotrophic mode enhanced significantly both biomass productivity and lipid content compared to autotrophic and heterotrophic cultures. Indeed, the highest biomass productivity 0.17 gdw L−1 day−1 was obtained for mixotrophic cultures while it was 0.12 gdw L−1 day−1 in autotrophic cultures and did not exceed 0.05 gdw L−1 day−1 in heterotrophic ones. Furthermore, the maximum lipid productivity (0.068 gL L−1 day−1) was observed during mixotrophic stationary phase, and it was more than five and nine fold higher than that obtained in heterotrophic and autotrophic cultures. High lipid production was obtained in photoheterotrophic conditions since it was correlated concomitantly with the increase of the glucose uptake and the decrease of the maximal quantum yield of PSII. FA analysis revealed the predominance of unsaturated fatty acids (UFA) 16:1, 18:1n9, 18:2n6, and 18:3n3, which represented more than 55 % of the total FA. FA profile variation was observed with respect to trophic modes and growth phases. The ratio UFA to SFA (saturated FA) reached its highest level (3.5) in exponential growth phase for both heterotrophic and mixotrophic modes mainly due to the increase in the proportion of 16:1.The stationary growth phases of mixotrophic and heterotrophic cultures were particularly marked by a substantial increase of the 18 polyunsaturated carbon chains. In the first mode of culture, the proportion of 18:2n6 exceeded 14 % dw, while lipids produced in the second mode of cultures were particularly rich in 18:3n3, representing more than 3.5 % dw.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, interest in microalgal fatty acids has increased as a potential new source for bioactive and high value lipid-based chemical precursors (Barbosa and Wijffels 2013; Coates et al. 2013), including polyunsaturated fatty acids (PUFAs) which have applications in the pharmaceutical, nutraceutical, and food industries (Tseng 2001; Tan 2007; Hu et al. 2008). Microalgae exhibit competitive advantages to terrestrial crops and fish, as sources of PUFAs and in general display a higher concentration of 16:0 over 16:l fatty acids, and abundant C18 fatty acids (Mendoza Guzmán et al. 2011; Seyfabadi et al. 2011; Li et al. 2011; Guedes et al. 2011; Ngangkham et al. 2012), as well as of n-3 (ALA) and n-6 isomers. Two of the most abundant fatty acids in Chlorophyta strains are linoleic acid (18:2n6) and octadecatrienoic acid (18:3n3) (Mendoza Guzmán et al. 2011; Li et al. 2011), which are considered as essential for human development, since humans are able to synthesize long chain PUFAs from them (Hu et al. 2008; Rubio-Rodríguez et al. 2010). However, one of the major commercial drawbacks in cultivation of microalgae is their poor biomass growth rate, which consequently leads to low PUFA productivities, even though PUFA may account for as much as 10–20 % of their cell weight (Guedes et al. 2011).
To overcome these disadvantages, culture condition variation to increase lipid productivity (Rodolfi et al. 2009; Gouveia et al. 2009; Li et al. 2011; Cheirsilp and Torpee 2012; Li et al. 2013) and/or to modify fatty acid composition (Renaud et al. 1991; Dunstan et al. 1993; Otero et al. 1997; Guihéneuf et al. 2010; Li et al. 2013; Huang et al. 2014) have been under scrutiny.
The mode of cultivation could be decisive for the quantity as well the quality of fatty acids, since it significantly influences the growth and metabolic pathways in microalgae (Wang et al. 2014). Autotrophic cultivation is the most common procedure for microalgae culture. However, in autotrophic culture it is difficult to reach a high density of microalgae biomass since light penetration is inversely correlated to the cell concentration (Chen and Johns 1995; Liang et al. 2009), which leads to a very low microalgal lipid productivity resulting from low biomass productivity (Martinez and Orus 1991; Liang et al. 2009).
An alternative to photoautotrophy is the use of heterotrophic culture in which organic carbon is used as carbon source in the absence of light (Borowitzka 1999; Perez-Garcia et al. 2011; Cheirsilp and Torpee 2012). Heterotrophic cultivation could avoid the defects associated with photolimitation in photoautotrophy, thus, providing high biomass productivities (Miao and Wu 2006; Liu et al. 2011). Lipid content under heterotrophic cultivation is generally at par with or higher than that under photoautotrophic mode (Miao and Wu 2006; Xu et al. 2006), resulting in even higher lipid productivity.
Recently, mixotrophic culture regime has been adopted as an effective mode for producing microalgal lipids. In mixotophic cultivation, CO2 and organic carbon are simultaneously assimilated and both respiratory and photosynthetic metabolism operate concurrently (Lee 2004).
Complementing photoautotrophy with organic substrates, mixotrophic cultivation of microalgae can reduce biomass loss in dark hours due to respiration (Rym et al. 2010; Park et al. 2012). Moreover, the CO2 released by microalgae via aerobic respiration can be trapped and reused for photosynthesis under mixotrophic cultivation, which further enhances biomass, and then lipid, productivities (Mohan et al. 2014). Furthermore, mixotrophic cultivation might switch to photoheterotrophic growth, where organic carbon is the main source of carbon and where the energy is provided mainly by the photosynthetic apparatus (Wilken et al. 2014). This combination of photoautotrophic and organoheterotrophic growth should result in a highly efficient carbon conversion and leads to an even higher biomass and lipid productivity.
The production of microalgal lipids under mixotrophic cultivation has not been fully investigated, especially for thermophilic species. To our knowledge, no one has examined the effect of trophic mode and growth phases on the lipid productivity and fatty acid composition of species related to the genus Graesiella. According to Richmond (1986), organisms with high optimal growth temperature possess wider possibilities for adaptation, as they have a rapid growth rate and a flexible metabolism to deal with various environmental conditions.
This study was aimed at evaluating the effect of trophic modes (autotrophic, mixotrophic, and heterotrophic) on growth, lipid production, and fatty acid variations in a strain of Graesiella sp., isolated from Tunisian hot spring water. In this study, we evaluated at first the kinetics of lipid production, cell growth, photosynthesis activity, and glucose consumption in autotrophic, mixotrophic, and heterotrophic cultures. Secondly, we examined the effects of growth phase and trophic modes on fatty acid variation.
Materials and methods
Algal strain
Samples were taken from “Aïn Echfa”, a hot spring (60 °C) located in the northern part of Tunisia (36° 49′ N, 10° 34′ E). Sampling materials were composed of microbial mats anchored to submerged stones. Collected mats were treated by filtration, centrifugation, and dilution techniques according to standard microbiological protocols (Stanier et al. 1971; Rippka et al. 1979). The purified strain was identified by phylogenetic analysis as Graesiella sp. (Mezhoud et al. 2014).
The strain was initially grown under different temperature and light intensity conditions to define the optimal autotrophic growth conditions. These preliminary lab experiments indicated that the species had the highest growth rate at temperature of 30 °C and light intensity of 75 μmol photons m−2 s−1 (Mezhoud et al. 2014).
Culture conditions
All cultures were incubated, in triplicate, at 30 °C in a temperature-programmable chamber. Autotrophic and mixotrophic experimental cultures were exposed to halogen lamps providing 75 μmol photons m−2 s−1 and with light/dark period of 16:8 h. Phyto-Claude halogen lamps (400 W) were used to illuminate chambers. The intensity of incident light was measured using the silicon sensor HD 8366.
Bold’s Basal medium (BBM) (Bischoff and Bold 1963) was used in all experiments. It was modified according to the Elser concept for freshwater microalgae with C/N/P ratio equal to 166:20:1 (Elser et al. 2000). The components of modified basal culture medium are as follows: NaNO3 2.5 g L−1, CaCl2. 2H2O 0.025 g L−1, MgSO4. 7H2O 0.075 g L−1, KH2PO4 0.175 g L−1, K2HPO4 0.075 g L−1, NaCl 0.025 g L−1, EDTA 0.05 g L−1, KOH 0.031 g L−1, H3BO3 0.011 g L−1, FeSO4·7H2O 0.048 g L−1, H2SO4 0.001 mL, NaHCO3 16 g L−1, and trace mineral solution 1 mL L−1. The initial pH was adjusted to pH 6.8. The pH of each culture was measured every day and after 7 days of cultivation; the pH increased to 10 and 10.2.
For mixotrophic and heterotrophic cultures, the modified BBM medium was supplemented with 40 g L−1 glucose. Heterotrophic cultures were cultivated in flasks wrapped with aluminum foil.
Cultivation was conducted for 7 days, in sterilized 2-L Erlenmeyer flasks containing 1.8 L of culture and equipped with a device that enabled the aseptic removal of samples. Each batch culture was inoculated with an initial biomass concentration of 0.07 g L−1 from pre-cultures in autotrophic mode. Daily microscopic observations were undertaken and testing for bacterial contamination was also performed by inoculating the cultures into glucose peptone agar plates.
Analytical procedures
Determination of biomass concentration
Graesiella sp. biomass concentration was monitored daily by gravimetric determination of algal biomass dry weight (dw). Daily aliquots of 80 mL algal suspension were centrifuged at 4000 rpm for 10 min at 4 °C and washed with deionized water to remove the salt. The pellets were freeze-dried and subsequently weighed and stored at −20 °C until analysis.
Determination of glucose concentration
Glucose concentration in the supernatant was determined with dinitrosalicylic acid (DNS) method according to Adney and Baker (1996). Reaction mixture contained 1.5 mL of the relevant reducing sugar solution (culture medium) and 3 mL of DNS reagent. The mixture was boiled 5 min in a water bath; subsequently, 0.2-mL sample was withdrawn and diluted with 2.5 mL of distilled water. The absorbance was measured at 540 nm.
Lipid extraction
For all samples, lipid analysis was conducted in triplicate. Pellets of each sample (20–80 mg) were added to 5 mL of a methanol/water/HCl (30:3:1, by vol) mixture into clean Teflon-lined screw-capped glass test tubes and held at 55 °C for 6 h (Rezanka et al. 2003). A total of 15 mL of cold water/hexane (2:1, by vol) mixture was added to the sample and vortex mixed for 20 s. The hexane layer was filtered and concentrated to dryness under a stream of nitrogen at 10 °C. The residue was extracted three times with 5 mL chloroform, then filtered and concentrated to dryness under a stream of nitrogen at 10 °C. The dried hexane and chloroform extracts were combined. The solvent was removed by being flushed with nitrogen, and the total lipid content was estimated gravimetrically.
Fatty acid analysis
To convert fatty acids to methyl esters, 0.5 mL of 14 % boron trifluoride in methanol was added to the dried whole lipids, and the mixture was refluxed for 1 h at 100 °C (Morrison and Smith 1964; Medina et al. 1992). Distilled water (0.5 mL) was added and fatty acid methyl esters (FAME) were extracted three times with hexane. The hexane extracts were rinsed twice with distilled water and evaporated under a stream of nitrogen at 10 °C. FAMEs were detected using a Hewlett-Packard HP 5890, series II gas chromatograph fitted with a flame ionization detector (FID) and equipped with a 60 × 0.32-mm DB-23 polar column. High-purity nitrogen was used as the carrier gas. The initial column temperature was set at 150 °C and was subsequently raised to 250 °C at 2°C min−1. The FID temperature was kept at 280 °C. The fatty acid content was determined by comparison of their integrated peak areas with that of the nonadecanoic acid methyl ester (19:0) as internal standard. The structures of FAMEs were confirmed by comparison of retention times with those of standard FAMEs (PUFA-1 Marine Source, Supelco). We used a fatty acid shorthand notation of the form A:BnX, where A is the number of carbon atoms, B is the number of double bonds, and X is the position of the first double bond relative to the methyl end of the molecule.
Measurement of the polyphasic chlorophyll a fluorescence transient
Variable fluorescence of chlorophyll was measured using the plant efficiency analyzer (PEA-Hansatech) with fixed excitation wavelength at 650 nm. Emission was measured at wavelengths beyond 720 nm and initial time resolution was 50 μs. Before measurement, three replicates of each sample were maintained in the dark for 20 min and then illuminated with an intensity of 3000 μmol photons m−2 s−1. All fluorescence transients were recorded within a time scan of 10 μs to 1 s with a data acquisition rate of 103 readings per second.
Apparent PSII activity parameter were determined from the F0 (initial fluorescence when QA is fully oxidized) and Fm (maximum fluorescence when QA is transiently fully reduced) values of the variable chlorophyll fluorescence. The photosynthesis activity was derived by the maximum quantum yield (Fv/Fm) according to the formula Fv/Fm = (Fm−F0)/Fm, where F0 is the initial fluorescence and Fm is the maximum fluorescence (Krause and Weis 1991).
Statistical analysis
Biomass concentration, lipid concentration, and fatty acid percentage were compared using one-way ANOVA, followed by the Tukey’s multiple comparison test that estimated the significance of the differences (P < 0.05).
Results
Biomass concentration, lipid content, and total glucose uptake
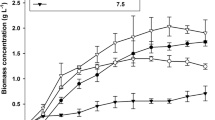
Graesiella sp. biomass and lipid production were evaluated by following the increase in biomass, lipid content, and glucose uptake kinetics of the batch cultures grown over 7 days under different trophic conditions: autotrophy (Fig. 1a), mixotrophy (Fig. 1b), and heterotrophy (Fig. 1c). Routine observation showed bacterial contamination at day 5 in heterotrophic cultures. Subsequently, these cultures were stopped.
Batch culture data were used for assessing two parameters describing maximum lipid productivity (gL L−1d−1) and maximum biomass productivity (gdw L−1d−1) (Table 1).
The highest biomass concentration (0.40 ± 0.01; 0.47 ± 0.04 gdw L−1) was observed under both mixotrophic and autotrophic cultures, and it was three times higher than the maximum biomass obtained in heterotrophic conditions. Nevertheless, mixotrophic conditions showed (Table 1) the highest biomass productivity (0.17 ± 0.03 gdw L−1 d−1) which was 4.47 and 1.42 higher than heterotrophic and autotrophic conditions, respectively.
The maximum lipid content (45.8 ± 1.9 % dw) was produced mixotrophically, and it was respectively 2.32 and 1.28 times higher than those achieved autotrophically and heterotrophically (Fig. 1). Furthermore, the highest lipid productivity (0.07 gL L−1d−1) was observed for mixotrophic cultures, representing respectively 10 and 5.83 times the lipid productivity obtained in autotrophic and heterotrophic ones (Table 1). For both mixotrophic and heterotrophic cultures, the lipid content increased substantially at the early stationary growth phase. Conversely, lipid content in autotrophic growth conditions decreased during the whole time course of culture.
Kinetics of glucose uptake (Fig. 1b, c) showed that for the mixotrophic cultures, glucose uptake was maintained at 9 to 11.6 g L−1 during the exponentially growth phase. Then, a steep rise to 32.2 g L−1 took place at the stationary phase. A similar trend was observed in the heterotrophic cultures with lower glucose uptake, not exceeding 11.6 g L−1 at day 4. During the whole time course of mixotrophic growth, 81 % of the initial glucose concentration was consumed while only 30 % was used during the dark heterotrophic growth conditions.
Photosynthesis activity
Chl-a fluorescence induction curves (OJIP) enable calculation of several phenomenological and biophysical expressions for quantifying the energy fluxes and energy ratios through photosystem II (Mehta et al. 2010; Strasser et al. 2010; Mathur et al. 2011). Ting and Owens (1992) and Schreiber et al. (1995) suggested that chlorophyll fluorescence estimation is not an accurate method for the determination of absolute PSII activity; we have used in our study the maximum efficiency of PSII photochemistry assessed by (Fv/Fm) ratios as a tool only to confirm active photon capture in the light-harvesting antenna complexes of PSII.
At the beginning of the experiments (Fig. 2), mixotrophic and heterotrophic cultures acted similarly as autotrophic cultures and have Fv/Fm above 0.70 ± 0.08 at days 1 and 2. But, at the stationary stage, mixotrophic and heterotrophic maximal quantum yields of PSII dropped significantly to 0.09 ± 0.04, respectively, at day 5. The autotrophic cultures were not significantly affected by growth phase and the Fv/Fm remained steady at 0.77 ± 0.08.
Fatty acid profile
Independent of the nutrition mode and growth phase, the fatty acid profile (Table 2) of this species was characterized by the predominance of unsaturated fatty acids (UFA): palmitoleic acid (16:1), linolenic acid (18:3n3), oleic acid (18:1), and linoleic acid (18:2n6) which represented more than 54 to 77 % of total fatty acids. Saturated carbon chain fatty acids (SFA) (23 to 46 % FA) were also identified with palmitic acid (16:0) and myristic acid (14:0) being the main components. Results also showed that Graesiella sp. synthesize 13:0, 15:1, 17:0, 18:0, 20:1n9, and 22:0 FAs whose content was smaller and did not exceed 10 %.
For all nutritional modes of cultures, a substantial variation in the fatty acid proportions (Table 2) was observed with respect to the growth phases. The exponentially growth phase was mainly characterized by a considerable increase in the proportion of palmitoleic acid (16:1), (from 1.84 at the initial to 2.38–8.27 % dw) while palmitic acid (from 2.29 % dw at the initial to 0.89–1.82 % dw) and the subtotal of unsaturated C18 fatty acids (e.g., linolenic, oleic and linoleic acids) (from 2.85 to 0.84–2.24 % dw) decreased substantially.
This trend was inversed in the stationary growth phase compared to the exponential growth stage. Indeed, palmitoleic acid fraction decreased to 0.20–3.42 % dw, while unsaturated 18 carbon fatty acids became predominant and reached, particularly in heterotrophic and mixotrophic cultures, 6.84 and 16.98 % dw, respectively. The palmitic acid also showed a significant increase from 0.89 to 1.82 % dw at exponential stage to 0.99–7.9 % dw in stationary growth phase.
Comparing the different nutrition modes of the experimental cultures, the unsaturation degree of 18 carbon fatty acids in the stationary growth phase was more pronounced in mixotrophic and heterotrophic modes than it was in the autotrophic cultures. Indeed, the autotrophic mode was characterized by the predominance of 18:1n9 (0.75 % dw) and 18:2n6 (1.27 % dw) fatty acids, while in the mixotrophic and the heterotrophic cultures, the 18:2n6 (14.10 and 2.21 % dw, respectively) and the 18:3 n3 (2.56 and 3.62%dw, respectively) fatty acids became predominant.
Discussion
The current study revealed the extent to which Graesiella sp. had the capacity to grow and to produce lipids phototrophically and by utilizing glucose. The results clearly showed that supplementation of glucose led to a significant improvement of both growth and lipid production when the microalga was grown mixotrophically. The major feature of mixotrophic culture is the presence of two energy sources, organic carbon sources and light, so microalgae may benefit from both photoautotrophic and heterotrophic growth. In this study, in mixotrophic cultures, photoautotrophic and heterotrophic growth proceeds independently and in an additive manner, such that the maximum biomass productivity was about equal to the sum of the photoautotrophic and heterotrophic modes. These results are consistent with those obtained for several microalgae, including Chlorella vulgaris and Chlorella sp. (Ogawa and Aiba 1981; Orús et al. 1991, Ratha et al. 2012) and for several cyanobacteria (Marquez et al. 1993).
Conversely, previous reports showed that high glucose concentrations can reduce growth rates (Wan et al. 2011) and/or the photosynthetic efficiency (Liu et al. 2009) in several microalgae strains which was attributed to the presence of substrate inhibition (Liang et al. 2009; Pagnanelli et al. 2014). The inhibitory glucose concentration varies between 5 and 166 g L−1 depending on microalgal species and environmental conditions (Perez-Garcia et al. 2011). Interestingly, although large amounts of glucose were used in this work (40 g L−1), the photosynthetic efficiency was not affected during the exponential growth phase of mixotrophic cultures. Indeed, the rate of the photochemical efficiency of photosystem II was at the same level to autotrophic mode during the correspondent growth phase.
Negative effect of the glucose concentration on photosynthetic activity was observed during the mixotrophic stationary phase of culture. Indeed, the photosynthetic activity declined strongly after the first 2 days of culture, as shown by the rapid decrease of the maximal quantum yields of PSII in both mixotrophic and heterotrophic modes compared to autotrophic mode. This trend confirmed that Graesiella sp. was then undergoing completely heterotrophic metabolism. The substantial increase of the glucose uptake during the stationary growth phase under mixotrophic cultures supported this hypothesis. Thus, as shown in many eukaryotic microalgae (Wilken et al. 2014), besides dark respiration organic carbon can also be metabolized using energy derived from light which is converted to ATP via the cyclic electron transport around PSI (photoheterotrophy).
Compared to autotrophic cultures, the highest lipid productivity was reported for mixotrophic conditions. Under mixotrophic condition, the lipid productivity reached its maximum level at the stationary growth phase. Since glucose uptake in these conditions was also highly enhanced and since photosynthesis was suppressed, this leads us to suppose that the carbon excess under mixotrophic condition was then mainly used into photoheterotrophic lipid biosynthesis. Hence, in heterotrophic conditions, the consumption of sugars at a rate higher than the rate of cell generation could promote conversion of excess sugar into lipids (Chen and Johns 1991; Ratledge and Wynn 2002; De Swaaf et al. 2003).
This trend was not confirmed under dark heterotrophic cultures which showed very low growth capacity and where the lipid biosynthesis declined even when the growth declined and the glucose uptake was enhanced. This may suggests that the operation of enzymes related to growth and lipid synthesis by Graesiella sp. is light dependent. The effect of light on synthesis and accumulation of enzymes catalyzing lipid synthesis was not well investigated, especially in microalgae species. Nevertheless, light has been shown to induce the increase of MGD transcripts followed by the activation of the monogalactosyldiacylglycerol (MGDG) synthase in terrestrial plants (Yamaryo et al. 2000).
Under different trophic modes and different growth phases, the predominant Graesiella sp. fatty acids were 14:0, 16:0, 16:1, 18:0, 18:1n9, 18:2n6, and 18:3n3. In relative terms of the fatty acid composition of Graesiella sp., our results are in agreement with the earlier studies of Chlorella species, mainly concerning the predominance of UFA (Mendoza Guzmán et al. 2011; Seyfabadi et al. 2011; Li et al. 2011; Guedes et al. 2011; Ngangkham et al. 2012). Little variations could be recorded due to different growing conditions and the methods of extraction of lipids and fatty acids.
The ratio of UFA to SFA has been used as indirect indicator of membrane fluidity (Casadei et al. 2002). In this work, this ratio was unaffected under different growth phases for autotrophic mode. It reaches its highest level in exponential growth phase for both heterotrophic and mixotrophic modes. Thus, we can conclude that Graesiella sp. cells increase their membrane fluidity when the culture medium was supplemented with glucose which could be related to the glucose uptake efficiency. Moreover, at high growth rates, the demand for structural components increases, consequently increasing the proportion of UFAs (Kates and Volcani 1966). At the stationary stage for both mixotrophic and heterotrophic modes, the UFA/SFA ratio decreased mainly due to the high increase of 16:0. This was also observed by Fisher and Schwarzenbach (1978) for other microalgae, supporting the idea that 16:0 could represent an energy storage linked to extra production when the cell division is altered (Siron et al. 1989). Moreover, for all tested trophic modes in this work, the PUFA/MUFA ratio increased substantially in the stationary phase. This increase was essentially due to the reduction of 16:1 and the increase of 18:2 and 18:3 fatty acids, although biosynthesis of PUFA was observed mainly during intense cellular activity in a few microalgae species (Brown et al. 1996; Liang et al. 2006). Bigogno et al. (2002) and Mansour et al. (2005) reported that PUFA could be accumulated as reserve building blocks under high culture density.
In conclusion it was found that the addition of glucose in mixotrophic culture was able, compared to autotrophic mode, to enhance more than 1.4-fold the biomass productivity and more than 9-fold the lipid productivity for this thermophilic Graesiella strain. Moreover, mixotrophic mode showed also high content of polyunsaturated 18:2n6 and 18:3n3 fatty acids, exceeding 16.6 % dw with 14.1 % dw of 18:2n6. In dark heterotrophic mode, high level of 18:3n3 (3.2 % dw) was observed. Nevertheless, high lipid contents were associated with relatively low biomass productivity and, consequently, low overall lipid productivity. Hence, a two-phase cultivation strategy in mixotrophic mode can be suggested to promote the lipid productivity of this strain of Graesiella. A first phase could be conducted in reactor to optimize growth and biomass production followed by a second lipid induction phase, under a high biomass concentration, to promote linoleic acid (18:2n6) and linolenic acid (18:3n3).
References
Adney, B., Baker, J. (1996) Measurement of cellulase activities: chemical analysis and testing task. Laboratory analytical procedure. Technical report NREL/TP-510-42628
Barbosa, M.J., Wijffels, R.H. (2013) Biofuels from microalgae. In Richmond A, Hu Q (eds) Handbook of microalgal culture: Applied phycology and biotechnology. Blackwell, Oxford, pp 566–577
Bigogno C, Khozin-Goldberg I, Cohen Z (2002) Accumulation of arachidonic acid-rich triacylglycerols in the microalga Parietochloris incisa (Trebuxiophyceae, Chlorophyta). Phytochemistry 60:135–143
Bischoff HW, Bold HC (1963) Phycological studies. IV. Some soil algae from Enchanted Rock and related algal species. Univ Texas Publ 6318:1–95
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Brown MR, Dunstan GA, Norwood SJ, Miller KA (1996) Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J Phycol 32:64–73
Casadei MA, Mañas P, Niven G, Needs E, Mackey BM (2002) Role of membrane fluidity in pressure resistance of Escherichia coli NCTC 8164. Appl Environ Microbiol 68:5965–5972
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Chen F, Johns MR (1991) Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana. J Appl Phycol 3:203–209
Chen F, Johns M (1995) A strategy for high cell density culture of heterotrophic microalgae with inhibitory substrates. J Appl Phycol 7:43–46
Coates RC, E. T, Gerwick WH (2013) Bioactive and novel chemicals from microalgae. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: Applied Phycology and biotechnology. Blackwell, Oxford, pp 504-531.
De Swaaf ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Dunstan GA, Volkman JK, Barrett SM, Garland CD (1993) Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J Appl Phycol 5:71–83
Elser JJ, Sterner RW, Galford AE, Chrzanowski TH, Findlay DL, Mills KH, Paterson MJ, Stainton MP, Schindler DW (2000) Pelagic C:N:P stoichiometry in a eutrophied lake: responses to a whole-lake food-web manipulation. Ecosystems 3:293–307
Fisher NS, Schwarzenbach RP (1978) Fatty acid dynamics in Thalassiosira pseudonana (Bacillariophyceae): implications for physiological ecology. J Phycol 14:143–150
Gouveia L, Marques AE, da Silva TL, Reis A (2009) Neochloris oleabundans UTEX #1185: a suitable renewable lipid source for biofuel production. J Ind Microbiol Biotechnol 36:821–826
Guedes AC, Amaro HM, Barbosa CR, Pereita RD, Malcata X (2011) Fatty acid composition of several wild microalgae and cyanobacteria, with a focus on eicosapentaenoic, docosahexaenoic and α-linolenic acids for eventual dietary uses. Food Res Int 44:2721–2729
Guihéneuf F, Fouqueray M, Mimouni V, Ulmann L, Jacquette B, Tremblin G (2010) Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J Appl Phycol 22:629–638
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang X, Wei L, Huang Z, Yan J (2014) Effect of high ferric ion concentrations on total lipids and lipid characteristics of Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis. J Appl Phycol 26:105–114
Kates M, Volcani BE (1966) Lipid components of diatoms. Biochim Biophys Acta - Lipids Lipid Metab 116:264–278
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Lee Y-K (2004) Algal nutrition: heterotrophic carbon nutrition. In: Richmond A (ed) Microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp. 116–124
Li Z, Yuan H, Yang J, Li B (2011) Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341. Bioresour Technol 102:9128–9134
Li T, Wan L, Li A, Zhang C (2013) Responses in growth, lipid accumulation, and fatty acid composition of four oleaginous microalgae to different nitrogen sources and concentrations. Chin J Oceanol Limnol 31:1306–1314
Liang Y, Beardall J, Heraud P (2006) Changes in growth, chlorophyll fluorescence and fatty acid composition with culture age in batch cultures of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Bot Mar 49:165–173
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liu X, Duan S, Li A, Xu N (2009) Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J Appl Phycol 21:239–246
Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 102:106–110
Mansour MP, Frampton DMF, Nichols PD, Volkman JK, Blackburn SI (2005) Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24-C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300
Marquez FJ, Sasaki K, Kakizono T, Nishio N, Nagai S (1993) Growth characteristics of Spirulina platensis in mixotrophic and heterotrophic conditions. J Ferment Bioeng 76:408–410
Martinez F, Orus MI (1991) Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM 101. Plant Physiol 95:1150–1155
Mathur S, Allakhverdiev SI, Jajoo A (2011) Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of photosystem II in wheat leaves (Triticum aestivum). Biochim Biophys Acta - Bioenerg 1807:22–29
Medina I, Aubourg S, Gallardo JM, Pérez-Martin R (1992) Comparison of six methylation methods for analysis of the fatty acid composition of albacore lipid. Int J Food Sci Technol 27:597–601
Mehta P, Jajoo A, Mathur S, Bharti S (2010) Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol Biochem 48:16–20
Mendoza Guzmán H, de la Jara Valido A, Carmona Duarte L, Freijanes Presmanes K (2011) Analysis of interspecific variation in relative fatty acid composition: use of flow cytometry to estimate unsaturation index and relative polyunsaturated fatty acid content in microalgae. J Appl Phycol 23:7–15
Mezhoud N, Zili F, Bouzidi N, Helaoui F, Ammar J, Ouada HB (2014) The effects of temperature and light intensity on growth, reproduction and EPS synthesis of a thermophilic strain related to the genus Graesiella. Bioprocess Biosyst Eng 37:2271–2280
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Mohan, S.V. Devi, M.P. Subhash, G.V. Chandra, R. (2014) Chapter 8 - Algae oils as fuels. In: Pandey, A. Lee, D-J. Chisti, Y. (eds) Biofuels from algae. Elsevier, Amsterdam pp 155–187
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 5:600–608
Ngangkham M, Ratha SK, Prasanna R, Saxena AK, Dhar DW, Saika C, Prasad RBN (2012) Biochemical modulation of growth, lipid quality and productivity in mixotrophic cultures of Chlorella sorokiniana. Springerplus 1:33. doi:10.1186/2193-1801-1-33
Ogawa T, Aiba S (1981) Bioenergetic analysis of mixotrophic growth in Chlorella vulgaris and Scenedesmus acutus. Biotechnol Bioeng 23:1121–1132
Orús MI, Marco E, Martínez F (1991) Suitability of Chlorella vulgaris UAM 101 for heterotrophic biomass production. Bioresource Technol 38:179–184
Otero A, García D, Fábregas J (1997) Factors controlling eicosapentaenoic acid production in semicontinuous cultures of marine microalgae. J Appl Phycol 9:465–469
Pagnanelli F, Altimari P, Trabucco F, Toro L (2014) Mixotrophic growth of Chlorella vulgaris and Nannochloropsis oculata: interaction between glucose and nitrate. J Chem Technol Biotechnol 89:652–661
Park KC, Whitney C, McNichol JC, Dickinson KE, MacQuarrie S, Skrupski BP, Zou J, Wilson KE, O’Leary SJB, McGinn PJ (2012) Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada: potential applications for wastewater remediation for biofuel production. J Appl Phycol 24:339–348
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Ratha SK, Babu S, Renuka N, Prasanna R, Prasad RB, Saxena AK (2012) Exploring nutritional modes of cultivation for enhancing lipid accumulation in microalgae. J Basic Microbiol 53:440–450
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Renaud SM, Parry DL, Thinh LV, Kuo C, Padovan A, Sammy N (1991) Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp and Nannochloropsis oculata for use in tropical aquaculture. J Appl Phycol 3:43–53
Rezanka T, Dor I, Prell A, Dembitsky VM (2003) Fatty acid composition of six freshwater wild cyanobacterial species. Folia Microbiol 48:71–75
Richmond A (1986) Cell response to environmental factors. In: Richmond A (ed) Handbook of microalgal mass culture, 1st edn. CRC Press, Florida, pp. 69–101
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici M (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low cost photobioreactor. Biotechnol Bioeng 102:100–112
Rubio-Rodríguez N, Beltrán S, Jaime I, de Diego SM, Sanz MT, Carballido JR (2010) Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innov Food Sci Emerg Technol 11:1–12
Rym BD, Nejeh G, Lamia T, Ali Y, Rafika C, Khemissa G, Jihene A, Hela O, Hatem BO (2010) Modeling growth and photosynthetic response in Arthrospira platensis as function of light intensity and glucose concentration using factorial design. J Appl Phycol 22:745–752
Schreiber U, Hormann H, Neubauer C, Klughammer C (1995) Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Funct Plant Biol 22:209–220
Seyfabadi J, Ramezanpour Z, Khoeyi ZA (2011) Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol 23:721–726
Siron R, Giusti G, Berland B (1989) Changes in the fatty acid composition of Phaeodactylum tricornutum and Dunaliella tertiolecta during growth and under phosphorus deficiency. Mar Ecol Prog Ser 55:95–100
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta - Bioenerg 1797:1313–1326
Tan LT (2007) Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 68:954–979
Ting CS, Owens TG (1992) Limitations of the pulse-modulated technique for measuring the fluorescence characteristics of algae. Plant Physiol 100:367–373
Tseng CK (2001) Algal biotechnology industries and research activities in China. J Appl Phycol 13:375–380
Wan M, Liu P, Xia J, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie Z, Qiu G (2011) The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl Microbiol Biotechnol 91:835–844
Wang J, Yang H, Wang F (2014) Mixotrophic cultivation of microalgae for biodiesel production: status and prospects. Appl Biochem Biotechnol 172:3307–3329
Wilken S, Schuurmans JM, Matthijs HCP (2014) Do mixotrophs grow as photoheterotrophs? Photophysiological acclimation of the chrysophyte Ochromonas danica after feeding. New Phytol 204:882–889
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Yamaryo Y, Kanai D, Awai K, Masuda T, Shimada H, Takamiya K, Ohta H (2000) Transcriptional regulation by light and phytohormones of the MGD gene in cucumber. Biochem Soc Trans 28:738–740
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zili, F., Bouzidi, N., Ammar, J. et al. Mixotrophic cultivation promotes growth, lipid productivity, and PUFA production of a thermophilic Chlorophyta strain related to the genus Graesiella . J Appl Phycol 29, 35–43 (2017). https://doi.org/10.1007/s10811-016-0941-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0941-1