Abstract
Seasonal environmental changes may significantly influence macroalgal diversity and biomass. Cryptogam species richness increases towards the poles, especially in sub-Antarctic environments. Yet, subpolar seaweed biodiversity and ecophysiology remain understudied even though it is essential for the management and sustainability of endemic species of significant economic interest (e.g., Gigartina skottsbergii). We evaluate the seasonality and ecophysiology of the different life phases of the rhodophyte G. skottsbergii by analyzing variation in fluorescence yield and photosynthetic pigment composition. There were significant seasonal differences in maximum relative electron transport rate (rETRmax) between gametophyte and tetrasporophyte phase, and between reproductive and vegetative specimens. Photosynthetic efficiency (α) was not significantly different between reproductive states of G. skottsbergii. We found significant differences in mean concentrations of allophycocyanin (APC), phycocyanin (PC), and chlorophyll a (Chl a) between gametophyte and tetrasporophyte phases. Results obtained provide new insight into seasonal acclimation patterns of an ecologically important species, which can be used for the design of appropriate management and cultivation strategies of G. skottsbergii towards the restoration of natural populations in fragile, subpolar regions where some of the last, relatively undisturbed communities of G. skottsbergii still remain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Along the temperate American coastline of the South Pacific, cryptogam species richness increases towards the pole, especially in sub-Antarctic environments (Santelices and Marquet 1998). One of the most extensive coastal areas of the Pacific Ocean in South America is within the system of channels and fjords of the sub-Antarctic archipelagos of Chile (42° S–56° S). This territory is characterized by diverse environmental conditions that determine subareas of physiogeology and orography, geology, soils, and differentiated climates (Pisano 1977; Rosenfeld et al. 2015). The marine biota there forms a biogeographic Magellanic unit (Camus 2001), where the main phytogeographic component of macroalga species is sub-Antarctic (Meneses and Santelices 2000) as the kelp Lessonia flavicans, for example, and the red alga Gigartina skottsbergii. The macroalgal diversity in sub-Antarctic archipelago includes around 234 species, and the division of Rhodophyta exhibits the highest species richness (Ramirez 2010; Mansilla et al. 2013).
In the context of subpolar seaweed biodiversity, seaweed ecophysiology remains understudied though it is essential because (i) there are strong seasonal changes in variables of luminosity such as the irradiance of photosynthetically active radiation (PAR) and light period (Butorovic 2013). In turn, these seasonal changes may significantly influence regional macroalgal richness and biomass (Ojeda et al. 2014); and (ii) the importance of seaweed ecophysiology for the management and sustainability of endemic species of economic interest (e.g., G. skottsbergii) must not be underestimated. For example, in laboratory cultures of G. skottsbergii, growth rates of gametophytes and tetrasporophytes differed during early stages of development at similar light intensities (Marambio et al. 2014).
Gigartina skottsbergii is a carrageenan-producing species, endemic to the southern region of South America (Ramirez and Santelices 1991; Buschmann et al. 2008). Along the coast of Chile on the southern Pacific Ocean, G. skottsbergii is distributed from Corral, at latitude S 39° 88′ (Westermeier and Ramirez 1978; Ramirez and Santelices 1991) to the south of the Antarctic Peninsula at latitude S 63° 23′ (Bischoff- Bäsmann and Wiencke 1996). Along the southern Atlantic coast of Argentina, G. skottsbergii is distributed from Puerto Madryn at latitude S 42° 08′ to the southern coast of Argentina and sub-Antarctic islands (Piriz and Cerezo 1991). It was recently reported that the sub-Antarctic and Antarctic populations of G. skottsbergii are two different genetic units separated by 31 mutational steps (Billiard et al. 2015). Interestingly, the reproductive biological records for this Rhodophyte species indicate that it exhibits seasonality in its sexual reproduction (Zamorano and Westermeier 1996) and alternation in the dominance of reproductive stages similar to that described for other species of Gigartinales (Kim 1976). There are similar observations for Desmarestiales from Antarctica, where their reproductive cycle is dependent on light conditions, determining the survival or mortality of the gametophytes (Gómez and Wiencke 1996).
Gigartina skottsbergii forms dense sublittoral beds, reaching biomass levels of 1775–1780 g m−2 and densities of 15 individuals m−2 (Ávila et al. 2004). The extraction of this species in southern coastal regions provides the principal raw material for the production of carrageenan hydrocolloid (carrageenan), a gel with multiple applications in the food, cosmetic, and biomedicine industries (Romo et al. 2001; Pujol et al. 2006; Barahona et al. 2012; Mansilla et al. 2012). Due to the growing national and international demand for this raw material, algae beds have suffered significant losses and their restoration has been quite slow, showing largely damaged communities in beds of Puerto Montt (~41° S; Romo et al. 2001). Thus, a good share of the extractive pressure has moved towards the south, especially in the area of the Gulf of Penas (~47° S) as well as the Magellan region (~53° S; Romo et al. 2001; Mansilla et al. 2008).
Gigartina skottsbergii has a triphasic life cycle (Kim 1976). Each phase differs in the type of carrageenans it produces (Matulewicz et al. 1989), and the cultivation of different phases (carposporophyte and gametophyte) requires different light intensities in its early stages (Marambio et al. 2014). Considering the ecological importance (Rosenfeld et al. 2015), the ecophysiological and phenological characteristics (Marambio et al. 2014), and the economical potential value that G. skottsbergii have in the Magellan region, the study of their biology and ecophysiology of all the reproductive stages is essential to guide cultivation efforts for these species in Magallanes. Additionally, the high extractive pressure of this species and the region’s vulnerability to environmental changes (Mansilla et al. 2012) are pressing for the study of the ecophysiology of natural meadows of G. skottsbergii. Studying seaweed physiology, the photosynthetic performance and the concentration of pigments of different reproductive phases of G. skottsbergii will permit understanding of light acclimation strategies (morphological and physiological) in natural populations. In addition to its value as an artisanal extractive resource has relevant management benefits given this algae’s farming potential in sub-Antarctic fjords and channels.
This information is paramount for successful laboratory cultures and hatchery-scale cultivation of the species for its later transfer to the sea to repopulate and restore natural populations in the Magellan region of sub-Antarctic Chile, avoiding its complete extirpation.
In the present study, our objective was to evaluate the seasonality of the ecophysiological behavior of the different life cycle phases of G. skottsbergii by (a) analyzing variable chlorophyll fluorescence as a proxy for photosynthetic activity and (b) photosynthetic pigment composition in a natural meadow.
Materials and methods
Field sample collection
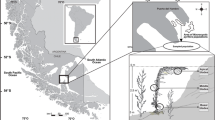
We randomly collected fronds of Gigartina skottsbergii by scuba diving from a natural population in Puerto del Hambre (53° 36 S–70° 55 W), Strait of Magellan, Chile (Fig. 1). We collected 7 gametophyte (young, non-fertile stage) fronds on each season in 2015 to assess seasonality, 7 carposporophytes, and 7 tetrasporophyte fronds were collected in the winter to compare different reproductive stages. We used the central and middle tissue of the fronds for pigment and photosynthetic activity analyses. Immediately upon collection in the field, we measured the relative electron transport rate (rETR) with a portable PAM chlorophyll fluorometer (Walz Diving-PAM). We then placed the samples in cooler containers to transport them to the Laboratory of Macroalgas Antarticas y Subantárticas (LMAS) at the University of Magallanes. Samples were kept in a walk-in culture chamber for 24 h for acclimatization at 8 ± 1 °C, and photoperiod was changed according to season (Table 1).
Photosynthetic activity
After the acclimatization period, we measured relative maximum photosynthetic electron transport rate (rETRmax), photosynthetic efficiency (α), and light saturation point (E k ). This information was processed with the software Kaleida Graph 4.0 (Synergy Software 1986–2005) and we applied Platt et al. (1980) Eq. (1), to analyze the photosynthesis irradiance data:
where P S is the light-saturated rate of photosynthesis, α is the light-limited rate of photosynthesis, and β describes the decrease in photosynthesis (Anderson 2005).
Pigment analysis
We macerated 300 mg of fresh biomass, with liquid nitrogen to a fine powder, which was diluted in 50 mM phosphate buffer, pH 5.5 at 4 °C. The resulting solution was centrifuged (Genesys 10 centrifuge) for 20 min at 44,000×g and 4 °C, obtaining a phycobiliprotein supernatant (Plastino and Guimarães 2001). The remaining pellet was diluted in 4 mL of 90 % acetone, which was centrifuged at 12.000 rpm and 4 °C for 10 min to obtain a chlorophyll supernatant. Absorption was measured in the Hewlett Packard 8452A spectrophotometer. The concentration of phycobiliproteins (allophycocyanin (APC), phycocyanin (PC), and phycoerythrin (PE)) was quantified using wavelengths of 498.5, 614, and 651 nm to quantify each phycobiliprotein pigment, and applying the specific absorption coefficients 630, 647, and 664 nm, respectively, in Kursar et al.’s (1983) protocol and Eqs. (2), (3), and (4):
We followed Jeffrey and Humphrey’s (1975) equation to determine the concentrations of chlorophyll a (Eq. (5)):
The pigment proportions were analyzed on a seasonal base and for the various reproductive stages of G. skottsbergii.
Data analyses
Initial diagnosis of the data for photosynthetic metabolism showed normal distribution. We applied one-way ANOVA (with a Tukey post hoc test) and applied a Kruskal-Wallis non-parametric test with 95 % confidence level of (p = 0.05) to analyze the data on pigment concentration (phycobiliproteins and chlorophyll a). All statistical analyses were performed using Statistica v7.1 (2004) software.
Results
Photosynthetic activity
We found significant differences in rETRmax (Table 2) between the winter and spring specimens of gametophytes (p = 0.002) and between autumn (was the highest: 7.26 ± 3.03 r.u.) and winter (p = 0.042; ANOVA Tukey’s HSD test). The photosynthetic efficiency (α; Fig. 2a) in spring and summer ranged from 0.11 to 0.16 μmol e− m−2 s−1, respectively, with the saturation point (E k ) being significantly different (p = 0.002; ANOVA; Tukey’s HSD test) between the lowest observed in spring (39.64 ± 29.36 μmol e− m−2 s−1) and the highest in autumn (69.49 ± 27.06 μmol e− m−2 s−1), though both are still low.
The rETRmax was higher in the tetrasporophytic phase (13.86 ± 8.41 r.u.; Table 3), with significant differences gametophyte and carposporophyte (p = 0.033) and between gametophyte and tetrasporophyte (p = 0.015). Photosynthetic efficiency (α) (Fig. 2b) was not significantly different between reproductive states of G. skottsbergii. The irradiance or saturation point (E k ) presented significant marginal differences between gametophyte and carposporophyte (0.047), with the highest value in tetrasporophyte (107.64 ± 103.66 μmol e− m−2 s−1).
Pigment analysis
No significant differences were observed in the mean concentration of APC, PC, and PE (Fig. 3a), but Chl a was significantly different between spring and summer (p = 0.015; Kruskal-Wallis), with the highest mean concentration during summer (7.64 ± 4.36 μg g−1 DW; Table 4) and the lowest during the spring (3.97 ± 1.50 μg g−1 DW). On the seasonal comparison, the highest pigment concentration was PE, though the PE/Chl a ratio was high too (Fig. 3b; Table 6), in all seasons.
Concentration of chlorophyll (Chl a), allophycocyanin (APC), phycocyanin (PC), and phycoerythrin (PE), and of pigment ratio in G. skottsbergii. a Pigment concentration in μg g−1 DW in seasonal comparisons of gametophytes. b Pigment ratios APC/Chl a, PC/Chl a, PE/Chl a, PE/PC, and PC/APC in seasonal comparisons of gametophytes. c Pigment concentration in μg g−1 DW in gametophyte, tetrasporophyte, and carposporophyte phases, during winter. d Pigment ratios APC/Chl a, PC/Chl a, PE/Chl a, PE/PC, and PC/APC in gametophyte, tetrasporophyte, and carposporophyte phases
We observed significant differences in mean concentrations of APC (p = 0.003) and PC (p = 0.015) (p < 0.05; Table 5) between gametophyte and tetrasporophyte phases (Fig. 3c). The highest mean concentration was measured in PE (78.52 ± 38.98 μg g−1 DW), but this shows no significant differences between reproductive phases, and again the ratio of PE/Chl a was the highest (Fig. 3d; Table 6).
Discussion
Photosynthetic activity
This study shows that the species G. skottsbergii exhibits low levels both in the photosynthetic rate rETR and the saturation point (E k ) when comparing among different seasons. Gómez et al. (2004) report that the low rates of these factors are related to the subtidal habitats in which some of the species occur. But in addition to being a subtidal species (5–20 m deep), G. skottsbergii lives in the understorey, which is formed by the canopy of Macrocystis pyrifera (Mansilla and Ávila 2011; Rosenfeld et al. 2015). Algae inhabiting the intertidal and upper subtidal show higher light saturation points of photosynthesis (E k ; Wiencke et al. 2007). The saturation point (E k ) in G. skottsbergii has been reported to be in the similar low range as in Callophyllis variegata, and in general as in algae that live below a depth of 10 meters (Gómez et al. 2004), and which are known as shade-adapted species (Wiencke et al. 2007).
Antarctic species such as Palmaria decipiens and Iridaea cordata present seasonal differences in the photosynthetic capacity over the course of a year, with higher photosynthetic efficiency in autumn, winter, and spring (Lüder et al. 2001). In contrast, G. skottsbergii, a species characteristic of high latitudes, displays the high levels of maximum photosynthesis (rETRmax) in summer, autumn, and winter, and the lowest levels in spring. This parameter is associated with the ability of the enzyme rubisco to fix CO2. This rises under high irradiance because the photon flow is not limiting, resulting in a high rETRmax (Beer et al. 2014). Therefore, rETRmax is low in spring, but this begins to increase towards summer and autumn, declining towards the winter. This decrease may relate to variations in temperature, changes in the photoperiod, and the start of the mature reproductive stages (tetrasporophyte and carposporophyte). These processes occur simultaneously during this period and become more noticeable in winter and spring. The same performance has been observed by Wiencke et al. (2007) in Ascoseira mirabilis. The decrease in rETRmax, similar to the one happening in P. decipiens, could be associated with a reduction in the size of the light harvesting antennae. This could be an adaptive advantage, which facilitates a rapid acclimation to changes in the environment, particularly in regard to light and the photoperiod (Lüder et al. 2002). Seasonal variations in rETR were not observed during the present study, possibly due to G. skottsbergii’s metabolism (e.g., low growth rate; Ávila et al. 2003; Marambio et al. 2014).
The small differences we observed in both E k and rETRmax showed the various reproductive stages of G. skottsbergii, with the low saturation point being in accordance with the results obtained by Marambio et al. (2014). In this study, the authors assess the cultivation of different reproductive stages of G. skottsbergii in its first growth phase; they came to the conclusion that G. skottsbergii required low irradiance of PAR and different requirements between reproductive phases. This differed from the results gained by Ávila et al. (1996, 1999, 2003) in experiments cultivating G. skottsbergii. They observed that this species showed different requirements in terms of light, photoperiod, and temperature depending on the latitude. The rETRmax of other species of Gigartinales (e.g., Mazzaella laminarioides) agrees with the results of the present study, where the physiological requirements of the alga depend on the different reproductive stages and the latitude in which the populations occur (Varela et al. 2006).
Pigment analysis
Our pigment analysis in G. skottsbergii showed the highest concentration of pigments measured in summer and autumn, leaving a critical period between winter and spring. The photoperiod and the intensity of the solar radiation vary significantly throughout the seasons, leading to changes in the amount of pigments in macroalgae (e.g., Gunnarsson and Ingólfsonn 1995; Ojeda et al. 2014). Moreover, these seasonal changes cause variations in size and number of the antennae, mainly in the phycobilisome, over the course of a year (Lüder 2003). These variations in the concentration of pigment have been linked to other members of the order of Gigartinales, like I. cordata, which is found in Antarctica and there is affected by several months of darkness (Weykam et al. 1997; Lüder 2003).
The highest level of Chl a in G. skottsbergii was measured in summer. This might indicate better conditions for the alga regarding temperature, irradiance, and photoperiod, in contrast to periods like spring, when the alga lives in a state of recovery after the winter months. In this period, Chl a is reduced, similar to the phycobilisome, before then being rapidly synthesized in summer, which improves the photosynthetic performance (Lüder et al. 2002). While PE showed higher concentrations, it is common in red algae, particularly in Gigartinales (Gantt 1990; Navarro 2004).
The Chl a, PE, and APC showed higher concentrations in the gametophytes and the PE decreased drastically in both the carposporophyte and tetrasporophyte stages. This is related to these two stages being purely reproductive periods. We observed an increase in the accessory PC in G. skottsbergii tetrasporophytes phase. These pigment concentration increases have been registered for Gracilaria bursa-pastoris, which increases the concentrations of PC and APC in the photosynthetic process, decreasing the concentration of PE, achieving successful power transfer to the Chl a (Marinho-Soriano 2012). Conversely, fronds of G. skottsbergii in their reproductive phase and optimal maturity stage are able to absorb certain phycobiliproteins, which correspond to nitrogen compounds functioning as nutrient reserves for the cell (Werlinger 2004). Variations in the concentration of pigments have been measured for different stages of the life cycle of the brown algal order Laminariales, with the concentration of photosynthetic pigment being influenced by growth, development, reproduction, and environmental factors (Lüning and Neushul 1978). In Desmarestia, the concentration of photosynthetic pigments changes significantly between juvenile and adult thalli (Thomas and Wiencke 1991; Gómez and Wiencke 1996). In red algae, the main pigments of the photosynthetic units are the phycobiliproteins, and PE, especially, is present in relatively higher levels in red algae (Beer et al. 2014). Gigartina skottsbergii in all life cycle stages presents a high ratio of Chl a/PE. This alga inhabits the subtidal zone, under the “shadow” created by the canopy of Macrocystis pyrifera, and under such condition, blue light is stronger than it is in higher zones of the intertidal slope. This kind of light stimulates the accumulation of pigments that efficiently absorb the broadband wavelengths (Chl a and PE; Figueroa et al. 1995). The phycobiliproteins, especially PE, are closely related to environmental factors such as levels of light and photosynthetic efficiency (Yokoya et al. 2007). Therefore, both the concentration and ratio of pigments not only depend on seasonal or environmental changes but also on the respective period in the life cycle of the alga such as the reproductive state (Dieckmann et al. 1985).
Conclusion
There are variations in the photosynthetic rate of G. skottsbergii that are directly linked to the concentration of photosynthetic pigments, as well as the seasonal variation in gametophytes and the changes between different reproductive stages. These variations are likely determined by the significant seasonal changes over the course of a year predominating in the sub-Antarctic region. The changes in the photosynthetic parameters and the pigment composition in different reproductive stages of G. skottsbergii are subject to the requirements of each one of these stages and the inherent biological and ecophysiological characteristics of the species.
It is worth pointing out that there exist only a few studies of this kind, on macroalgae of commercial importance and extracted from natural populations. These studies provide key knowledge to understand the responses by natural meadows to commercial extraction and their behavior under controlled laboratory and hatchery conditions for future restoration of natural populations in the sub-Antarctic Chilean region of Magallanes and those facing similar threats of algal overexploitation.
References
Anderson R (ed) (2005) Algal culturing techniques. Academic Press, NY 596 pp
Ávila M, Candia A, Romo H, Pavez H, Torrijos C (2003) Exploitation and cultivation of Gigartina skottsbergii in southern Chile. Proc 17th International Seaweed Symposium, pp. 137–143
Ávila M, Candía A, Nuñez M, Romo H (1999) Reproductive biology of Gigartina skottsbergii (Gigartinaceae, Rhodophyta) from Chile. Hydrobiologia 398/399:149–157
Ávila M, Cáceres J, Nuñez M, Camus P, Pavez H, Cortes H, González J, Tapia C, Mejias P, Cornejo S, Romo H, Candia A (2004) Investigación y manejo de praderas de luga roja en la XII Región. Informe final proyecto FIP-IFOP 2002–27, 417 pp.
Ávila M, Otaiza R, Norambuena R, Nuñez M (1996) Biological basis for the management of “Luga Negra” Sarcothalia crispata (Gigartinales, Rhodophyta) in southern Chile. Hydrobiologia 326/327:245–252
Barahona T, Encinas MV, Mansilla A, Matsuhiro B, Zúñiga EA (2012) A sulfated galactan with antioxidant capacity from the green variant of tetrasporic Gigartina skottsbergii (Gigartinales, Rhodophyta). Carbohydr Res 347:114–120
Beer S, Björk M, Beardall J (2014) Photosynthesis in the marine enviroment. John Wiley & Sons, Ltd., Oxford, pp. 61–64
Billiard S, Husse L, Lepercq P, Godé C, Bourceaux A, Jacque L, Vernet P, Saumitou-Laprade P (2015) Selfish male-determining element favors the transition from hermaphroditism to androdioecy. Evolution 69:683–693
Bischoff- Bäsmann B, Wiencke C (1996) Temperature requirements for growth and survival of Antarctic Rhodophyta. J Phycol 32:525–535
Buschmann AH, Varela DA, Hernandez-González MC, Huovinen P (2008) Opportunities and challenges for the development of an integrated seaweed-based aquaculture activity in Chile: determining the physiological capabilities of Macrocystis and Gracilaria as biofilters. J Appl Phycol 20:571–577
Butorovic N (2013) Resumen Meteorológico año 2013. Estación Jorge C. Schythe. A Inst Pat 42(1), 89–99.
Camus PA (2001) Biogeografía marina de Chile. Rev Chil Hist Nat 74:587–617
Dieckmann G, Reichardt W, Zielinski K (1985) Growth and production of the seaweed, Himantothallus grandifolius, at King George Island. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp. 104–108
Figueroa F, Aguilera J, Xavier F (1995) Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). Eur J Phycol 30:11–18
Gantt E (1990) Pigmentation and photoacclimation. In: Cole KM, Sneath RG (eds) Biology of the red seaweeds. Cambridge University Press, Cambridge, pp. 203–219
Gómez I, Figueroa FL, Ulloa N, Morales V, Lovengreen C, Huovinen P, Hess S (2004) Patterns of photosynthesis in 18 species of intertidal macroalgae from southern Chile. Mar Ecol Prog Ser 207:103–116
Gómez I, Wiencke C (1996) Seasonal growth and photosynthetic performance of the Antarctic macroalga Desmarestia menziesii (Phaeophyceae) cultivated under fluctuating Antarctic day lengths. Bot Acta 110:25–31
Gunnarsson K, Ingólfsonn A (1995) Seasonal changes in the abundance of intertidal algae in southwestern Iceland. Bot Mar 38:69–77
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kim DH (1976) A study of the development of cystocarps and tetrasporangial sori in the taxonomy of the Gigartinaceae (Rhodophyta, Gigartinales). Nova Hedwigia 26:1–237
Kursar TA, van der Meer JP, Aberte RS (1983) Light harvesting system of the red alga Gracilaria tikvahiae. I. Biochemical analyses of pigment mutations. Plant Physiol 73:353–360
Lüder, UH (2003) Acclimation of the photosynthetic apparatus of the endemic Antarctic red macroalga Palmaria decipiens to seasonally changing light conditions. Publishing Alfred Wegener InstitutWeb https://epic.awi.de/26648/1/BerPolarforsch2003469.pdf
Lüder UH, Knoetzel J, Wiencke C (2001) Two forms of phycobilisomes in the Antarctic red macroalga Palmaria decipiens (Palmariales, Florideophyceae). Physiol Plant 112:572–581
Lüder UH, Knoetzel J, Wiencke C (2002) Acclimation of photosynthesis and pigments during and after six months of darkness in Palmaria decipiens (Rhodophyta): a study to simulate Antarctic winter sea ice cover. J Phycol 38:904–913
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of Laminarian gametophytes in southern and central California. Mar Biol 45:297–309
Mansilla A, Ávila M, Ramirez MA, Rodriguez JP, Rosenfeld S, Marambio J (2013) Macroalgas marinas bentónicas del submareal somero de la ecoregión subantártica de Magallanes, Chile. A Inst Pat 41(2):49–62
Mansilla A, Ávila M, Yokoya N (2012) Current knowledge on biotechnological interesting seaweeds from the Magellan region, Chile. Rev Bras 22:760–767
Mansilla A, Ávila M (2011) Using Macrocystis pyrifera (L.) C. Agardh from southern Chile as a source of applied biological compounds. Rev Bras 21:262–267
Mansilla A, Palacios M, Navarro N, Ávila M (2008) Growth and survival performance of the gametophyte of Gigartina skottsbergii (Rhodophyta, Gigartinales) under defined nutrient conditions in laboratory culture. J Appl Phycol 20:439–446
Marambio J, Mansilla A, Ávila M, Rosenfeld S, Ojeda J (2014) The effects of different light intensities on the culture of Gigartina skottsbergii (Rhodophyta, Gigartinales) tetrasporophytes and gametophytes in the Magellan region, Chile. J Appl Phycol 26:1963–1969
Marinho-Soriano E (2012) Effect of depth on growth and pigment contents of the macroalgae Gracilaria bursapastoris. Rev Bras 22:730–735
Matulewicz MC, Ciancia M, Noseda MD, Cerezo AS (1989) The carrageenan system from tetrasporic and cystocarpic stages of Gigartina skottsbergii. Phytochemistry 28:2937–2941
Meneses I, Santelices B (2000) Patterns and breaking points in the distribution of benthic algae along the temperate Pacific coasts of South America. Rev Chil Hist Nat 73:615–623
Navarro N (2004) Efeitos da radiação UVB em Iridaea cordata (Gigartinales, Rhodophyta). Disertation Universidade de São Paulo
Ojeda J, Rosenfeld S, Marambio J, Rozzi R, Mansilla A (2014) Patrones estacionales y espaciales de la diversidad de moluscos intermareales de bahía Róbalo, canal Beagle, Reserva de la Biosfera Cabo de Hornos, Chile. Rev Biol Mar Oceanogr 49:493–509
Piriz M, Cerezo A (1991) Seasonal variation of carrageenans in tetrasporic, cystocarpic and “sterile” stages of Gigartina skottsbergii S. et G. (Rhodophyta, Gigartinales). Hydrobiologia 226:65–69
Pisano EV (1977) Fitogeografía de Fuego-Patagonia chilena comunidades vegetales entre las latitudes 52 y 56°S. An Inst Pat 8:122–250
Plastino EM, Guimarães M (2001) Diversidad intraespecífica. In: Alveal KV, Antezana TJ (eds) Sustentabilidad de la Biodiversidad. Universidad de Concepción, Concepción, pp. 19–27
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Pujol CA, Scolaro LA, Ciancia M, Matulewicz MC, Cerezo AS, Damonte EB (2006) Antiviral activity of a carrageenan from Gigartina skottsbergii against intraperitoneal murine herpes simplex virus infection. Planta Med 72:121–125
Ramirez ME, Santelices B (1991) Catálogo de las algas marinas bentónicas de la costa temperada del Pacífico de Sudamérica. Monografías Biológicas 5. Ediciones Universidad Católica de Chile, Santiago 437 pp
Ramirez ME (2010) Benthic marine flora from southern South America and Antarctica. A Inst Pat (Chile) 38(1):57–71
Romo H, Avila M, Candía A (2001) Manual de técnicas de cultivo y repoblación de “Luga Roja” (Gigartina skottsbergii). Proyecto FONDEF D97I1064 y D00I1109. Universidad de Concepción–IFOP, Chile.
Rosenfeld S, Aldea C, Mansilla A, Marambio J, Ojeda J (2015) Richness, systematics, and distribution of molluscs associated with the macroalga Gigartina skottsbergii in the Strait of Magellan, Chile: a biogeographic affinity study. ZooKeys 519:49–100
Santelices B, Marquet P (1998) Seaweeds, latitudinal diversity patterns, and Rapoport’s rule. J Cons Biogeogr. doi:10.1046/j.1472-4642.1998.00005.x.
Thomas DN, Wiencke C (1991) Photosynthesis, dark respiration and light independent carbon fixation of endemic Antarctic macroalgae. Polar Biol 11:329–337
Varela DA, Santelices B, Correa JK, Arroyo MK (2006) Spatial and temporal variation of photosynthesis in intertidal Mazzaella laminarioides (Bory) Fredericq (Rhodophyta, Gigartinales). J Appl Phycol 18:827–838
Werlinger C (2004) (ed) Biología Marina y Oceanografía, Conceptos y Procesos, Consejo Nacional del Libro y la Literatura – Universidad de Concepción. Trama Impresores S. A., Chile 700 pp.
Westermeier R, Ramirez C (1978) Algas marinas de Niebla y Mehuín (Valdivia-Chile). Med Amb 3:44–49
Weykam G, Thomas DN, Wiencke C (1997) Growth and photosynthesis of the Antarctic red algae Palmaria decipiens (Palmariales) and Iridaea cordata (Gigartinales) during and following extended periods of darkness. Phycologia 36:395–405
Wiencke C, Clayton MN, Gómez I, Iken K, Lüder UH, Amsler CD, Karsten U, Hanelt D, Bischof K, Dunton K (2007) Life strategy, ecophysiology and ecology of seaweeds in polar waters. Rev Environ Sci Biotechnol 6:95–126
Yokoya NS, Necchi Júnior O, Martins AP, Gonzalez SF, Plastino EM (2007) Growth responses and photosynthetic characteristics of wild and phycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta). J Appl Phycol 19:197–205
Zamorano J, Westermeier R (1996) Phenology of Gigartina skottsbergii (Gigartinaceae, Rhodophyta) in Ancud Bay, southern Chile. Hydrobiologia 326/327:253–225
Acknowledgments
This research was funded by Chile’s National Council for Research in Science and Technology—CONICYT, FONDECYT grant 1140940 to A. Mansilla, the Millennium Scientific Initiative (grant P05-002 ICM, Chile), and the Basal Financing Program (grant PFB-23, Chile). Thank you for the graduate scholarships by the Institute of Ecology and Biodiversity granted to F. Méndez (ICM, P05-002), J.P. Rodríguez (ICM, P05-002), J. Marambio (PFB-23-2008), and S. Rosenfeld (ICM P05-002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marambio, J., Mendez, F., Ocaranza, P. et al. Seasonal variations of the photosynthetic activity and pigment concentrations in different reproductive phases of Gigartina skottsbergii (Rhodophyta, Gigartinales) in the Magellan region, sub-Antarctic Chile. J Appl Phycol 29, 721–729 (2017). https://doi.org/10.1007/s10811-016-0913-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0913-5