Abstract
Esterification of xanthophylls is important for improving their bioactive potency. The effects of metal cations on esterified xanthophyll biosynthesis and on the accumulation and composition of salt stress-induced carotenoids were investigated in the aerial microalga Coelastrella sp. KGU-Y002 (Chlorophyta, Scenedesmaceae). Under nitrogen-deficient and high-light conditions, salt-supplemented cultures showed enhanced synthesis of esterified astaxanthin, adonixanthin, and zeaxanthin. In particular, KCl supplementation caused a remarkable increase in esterification, such that 73 % of xanthophylls were esterified. After 1 day of KCl treatment, the concentration of endogenous abscisic acid had increased to 200-fold its initial concentration. In algal cells treated with abscisic acid in nitrogen-deficient and high-light conditions, the reactive oxygen species concentration was almost the same as that in the KCl-supplemented culture. In these algal cells, abscisic acid enhanced esterification without affecting total carotenoid accumulation. These findings suggested that xanthophyll esterification is regulated during the response to salt stress by abscisic acid, which has plant hormone-like bioactivity in this aerial microalga. Further studies have been designed to examine large-scale production of esterified xanthophylls by this aerial microalga under salt-stress conditions, and to assess the bioactivity of esterified xanthophylls extracted from algal cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotenoids are lipophilic pigments with an isoprenoid structure containing conjugated double bonds. Most carotenoids have antioxidant effects, and so they can protect organisms against oxidative stresses (Skibsted 2012). Carotenoids are divided into non-oxygenated and oxygenated species; carotenes and xanthophylls, respectively. Astaxanthin, with 13 conjugated double bonds, two hydroxyl groups, and two keto groups, is a xanthophyll with both lipophilic and hydrophilic properties. When the hydroxyl groups of xanthophylls react with fatty acids in the endoplasmic reticulum, mono and/or di-esterified derivatives are formed by enzymes in oil bodies.
A di-esterified astaxanthin was shown to have higher antioxidant activity than those of the mono-ester and free forms under hydrophobic solvent conditions (Kobayashi and Sakamoto 1999). Therefore, it is interesting to study the bioactive potency of esterified astaxanthins compared with free astaxanthins. In another study, the anticancer properties of a di-esterified astaxanthin from the microalga Haematococcus pluvialis were compared with those of its free form and other carotenoids using a skin cancer model in rats. The esterified xanthophylls showed threefold higher rates of skin cancer inhibition, and this efficacy was attributed to increased retinol in the serum of rats treated with the esterified form (Rao et al. 2013). Esterified astaxanthin was also shown to protect against ethanol-induced gastric ulcers in rats (Kamath et al. 2008). In another study, esterified astaxanthin was more effective than the free form in promoting antioxidant enzyme activity and in protecting against carbon tetrachloride-induced hepatotoxicity in rats (Rao et al. 2015). Together, the results of those studies showed that esterified xanthophylls are more potent bioactive compounds than are their free forms. Among such carotenoids, free adonixanthin was shown to have higher singlet oxygen (1O2) quenching activity than that of astaxanthin in aqueous solvents, because the xanthophyll structure is more polar than that of astaxanthin (Maoka et al. 2013). Esterified adonixanthin is expected to show greater bioactivity as a result of improvements in the bioavailability of key compounds.
Microalgae that produce large amounts of carotenoids are good sources of commercially valuable materials because they grow faster than other photosynthetic organisms. Carotenoids accumulate in algal cells in response to several environmental stresses. Haematococcus pluvialis, Chlorococcum sp., and Scenedesmus sp. have been shown to accumulate esterified astaxanthin under nitrogen deficiency and high-light intensity (Yuan et al. 2002; Zhekisheva et al. 2002; Chu et al. 2011). Aeroterrestrial microalgae (hereafter referred to as aerial microalgae) isolated from rock surfaces in Japan were shown to accumulate large amounts and many types of xanthophylls as they adapted to nitrogen-deficient and high-light conditions (Abe et al. 2007; Aburai et al. 2013). The aerial microalga strain KGU-Y002 was isolated and identified as Scenedesmus sp. based on its 18S rDNA sequence (Aburai et al. 2013). As reported in 2013, this Scenedesmus sp. was found to belong to a species cluster dominated by sequences in the genus Coelastrella (Kaufnerová and Eliš 2013). Therefore, strain KGU-Y002 was formally transferred to the genus Coelastrella on the basis of the molecular evidence. Under certain conditions, cells of the aerial microalga Coelastrella sp. KGU-Y002 change from green to red, indicating the accumulation of large amounts of free and esterified astaxanthin and adonixanthin (Aburai et al. 2013). The putative role of esterification in carotenoid accumulation in photosynthetic microorganisms has not been effectively described. Among the few studies in this area, one reported that in the microalga H. pluvialis, astaxanthin accumulation was suppressed alongside a decrease in the production of the esterified form, and that a diacylglycerol acyltransferase inhibitor was responsible for this suppression (Chen et al. 2015). In our previous study, we showed that the synthesis of free and esterified astaxanthin and adonixanthin was regulated when strain KGU-Y002 was cultured under high-light and saline conditions (Aburai et al. 2015).
Abscisic acid (ABA) is a plant hormone with roles in many physiological processes in higher plants, including germination, stomatal movement, and responses to abiotic stresses (Mittler and Blumwald 2015). Endogenous ABA is also present in nearly 100 species of algae from different taxonomic groups (Hirsch et al. 1989). The endogenous ABA concentrations in members of the Chlorophyta appear to be influenced by environmental stresses such as nitrogen deficiency, heat, dryness, acidity, and light. Exogenous ABA also affects microalgae; for example, ABA treatment of H. pluvialis vegetative cells resulted in enhanced carotenogenesis and the formation of mature red cysts (Kobayashi et al. 1997). It is unclear whether ABA is involved in salt-stress tolerance in microalgae, as it is in higher plants.
The aim of this study was to investigate the effects of metal cations on esterified xanthophyll accumulation in strain KGU-Y002, and to explore the relationship between ABA and carotenogenic processes in this microalga. The results of this study will be useful for further research on producing esterified carotenoids with greater bioavailability and bioactive potency.
Materials and methods
Strain and culture conditions
Coelastrella sp. KGU-Y002 was precultured in Bold’s basal (BB) medium and maintained at 25 ± 2 °C and 40 μmol photons m−2 s−1 in a 500-mL Erlenmeyer flask with aeration for 21 days (Aburai et al. 2015). The BB medium contained 250 mg NaNO3, 175 mg KH2PO4, 75 mg K2HPO4, 25 mg MgSO4·7H2O, 25 mg NaCl, 50 mg EDTA, 30 mg KOH, 5 mg FeSO4·7H2O, and 11 mg H3BO3 per liter deionized water. The medium pH was adjusted to 8.0 with NaOH before autoclaving. Precultured cells were transferred to a 500-mL flat glass flask and further cultured at 40 μmol photons m−2 s−1 for 21 days. The green vegetative cells were then transferred to 100-mL Erlenmeyer flasks containing nitrogen-deficient medium and incubated in the presence or absence of 0.15 M NaCl, KCl, MgCl2, or CaCl2. Other conditions included 200 μmol photons m−2 s−1, 25 ± 2 °C, and reciprocal shaking at 120 rpm for 7 days, and initial absorbance at 750 nm (A750) = 0.5. The cultured algal cells were observed and photographed under a microscope (BX51, Olympus Corp., Japan).
Pigment extraction and analyses
Pigments were extracted from powdered samples with dichloromethane/methanol (25/75, v/v). Esterified xanthophylls were saponified by adding methanolic NaOH to extracts to a final concentration of 20 mM and then incubating the mixtures at 5 °C in darkness for 12 h. Total chlorophyll in samples was estimated from absorbance values, which were obtained using a spectrophotometer (V-730, JASCO Co., Ltd., Japan). Free and esterified xanthophylls in extracts were detected and quantified by high-performance liquid chromatography (HPLC). Carotenoids were analyzed by liquid chromatography-ion trap-time of flight/mass spectrometry (Shimadzu Corp., Japan) as described previously (Aburai et al. 2015).
Abscisic acid extraction and analyses
Endogenous ABA was extracted with methanol and quantified by HPLC analysis, as described by Hou et al. (2008), with some modifications. Powdered cells (5 mg) were extracted with 2 mL 80 % aqueous methanol at 4 °C for 16 h, and the extract was then centrifuged at 4 °C at 2380×g for 15 min. The supernatant was passed through an InertSep C18 cartridge (GL Sciences Inc., Japan) that was preconditioned with 3 mL deionized water followed by 3 mL methanol. The cartridge was washed with 1 mL 20 % aqueous methanol containing 0.1 % (by vol) formic acid, and the retained phytohormones were then eluted with 1 mL 80 % aqueous methanol. Chromatographic analysis was performed on an HPLC system equipped with an Inertsil ODS-3 column (4.6 × 150 mm i.d., C18, 3 μm, GL Sciences Inc., Japan). The mobile phase was (A) methanol and (B) aqueous phosphoric acid solution (pH 2.5). The eluent gradient program was 95 % B for 5 min, a linear gradient from 95 to 30 % over 15 min, 30 % B for 10 min, and then 100 % B for 5 min, at a flow rate of 1.0 mL min−1 and a column temperature 40 °C. Elution was monitored at 265 nm. Injection volumes were 20 μL. A standard solution of ABA (Tokyo Chemical Industry Co., Ltd., Japan) was prepared in methanol and chromatographed separately to determine the standard retention time. A calibration curve constructed from the data of matrix-matched calibration standards was used to quantify ABA in the samples.
Measurement of intracellular reactive oxygen species
The dichloro-dihydro-fluorescein-diacetate (DCFH-DA) method was used to quantify intracellular reactive oxygen species (ROS) levels (Rosenkranz et al. 1992). After cell disruption by an ultrasonic disrupter (UD-211, Tomy Seiko Co., Ltd., Japan), 25-μM DCFH-DA solution was added, and DCFH fluorescence was detected by spectrofluorometry (FP-770, JASCO Co., Ltd., Japan).
Statistical analyses
Means and standard deviations were calculated for each treatment. Data were subjected to one-way analysis of variance, and differences were considered statistically significant at p < 0.05.
Results and discussion
Effects of metal cations on carotenoid accumulation in Coelastrella sp. KGU-Y002
In our previous study, carotenogenesis in Coelastrella sp. KGU-Y002 was specifically activated by 0.3 M NaCl when the algal cells were grown for 7 days under a high initial cell density (A750 of 2.0) and high-light intensity (200 μmol photons m−2 s−1). The carotenoid content was significantly higher in the algal cells cultured at an initial A750 of 0.5 than in those cultured at an initial A750 of 2.0 (Aburai et al. 2015). Therefore, we further analyzed the effects of initial cell density and salt concentration on carotenogenesis for the selective production of esterified carotenoids. In the present study, Coelastrella sp. KGU-Y002 cells were cultured in nitrogen-deficient medium at an initial A750 of 0.5 with 0.15 M added salt (NaCl, KCl, MgCl2, or CaCl2) or without added salt at 200 μmol photons m−2 s−1. The algal cells, which were approximately 10 μm in diameter, changed from green to reddish orange under control and salt-stress conditions and often underwent expansion and plasmolysis in 0.15 M NaCl (Fig. 1). After 7 days of salt stress, carotenoids had accumulated to 6.15, 7.50, 7.58, 10.20, and 3.93 mg g−1 dry weight cells (dwc) in the control and NaCl-, KCl-, MgCl2-, and CaCl2-supplemented cultures, respectively (Fig. 2). The effects of salts on carotenogenesis differed from those observed in our previous study, because of the higher light intensity conditions in the present study (Aburai et al. 2015).
Microscopic observations of Coelastrella sp. KGU-Y002 cells with no added salt (control) (a), and with added salt: NaCl (b), KCl (c), MgCl2 (d), or CaCl2 (e). After precultivation in BB medium at 40 μmol photons m−2 s−1 for 21 days, algal cells were cultured for 7 days in nitrogen-deficient medium with 0.15 M added salt and 200 μmol photons m−2 s−1. Scale bars = 10 μm
Carotenoid content in Coelastrella sp. KGU-Y002 cells. After precultivation in BB medium at 40 μmol photons m−2 s−1 for 21 days, algal cells were transferred and cultured for 7 days in nitrogen-deficient medium with 0.15 M salt (NaCl, KCl, MgCl2, or CaCl2; gray bars) and 200 μmol photons m−2 s−1. Asterisk indicates statistically significant difference (p < 0.05) compared with control. Values are average of triplicate experiments
The carotenoid content in cells was 1.7-fold higher in the MgCl2-supplemented culture than that in the control (Table 1). Microalgae require sufficient Mg2+ to grow; for example, increased Mg2+ concentrations were shown to increase algal growth (Gorain et al. 2013). Chlorophyll, the main molecule for trapping light energy in photosynthesis, requires one Mg2+ ion for each molecule. High doses of Mg2+ result in high biomass and lipid production, and this ion also affects chlorophyll biosynthesis (Gorain et al. 2013). The addition of Mg2+ under NH4 +-deficient conditions was shown to promote the biosynthesis of organic compounds, including pyruvate and α-ketoglutarate, in the aerial microalga Trentepohlia aurea (Abe et al. 2014).
The addition of NaCl, KCl, MgCl2, or CaCl2 reduced the final violaxanthin content from 0.31 (control) to 0.05, 0.11, 0.09, and 0.05 μmol g−1 dwc (from 186 to 30, 66, 54, and 30 μg g−1 dwc), respectively. These algal cells showed approximately fourfold increases in zeaxanthin content with a concurrent one-quarter reduction in violaxanthin content under nitrogen-deficient and high-light stress as a control culture for 7 days (Table 1). The violaxanthin cycle represents an important photoprotection mechanism in photosynthetic microorganisms (Goss and Jakob 2010). Abscisic acid, which is produced by carotenoid degradation via intermediate violaxanthin, has been detected in many algae from different taxonomic groups (Cutler and Krochko 1999). Thus, the violaxanthin content can be reduced not only via consumption in the violaxanthin cycle but also by ABA biosynthesis. In algal cells, astaxanthin is synthesized from β-carotene through two hydroxylation reactions at the C-3 and 3′ positions, and oxidation of ketone groups at the C-4 and 4′ positions. Figure 3 shows a proposed model for the astaxanthin biosynthetic pathway in Coelastrella sp. KGU-Y002. The different metal cations (NaCl, KCl, and MgCl2) had different effects on astaxanthin biosynthetic pathways in KGU-Y002. Algal cells cultured with NaCl accumulated zeaxanthin and/or lutein, those cultured with KCl accumulated canthaxanthin, and those cultured with MgCl2 accumulated zeaxanthin and canthaxanthin (Fig. 3 and Table 1).
Effects of metal cations on esterified xanthophyll accumulation in Coelastrella sp. KGU-Y002
Addition of NaCl or MgCl2 increased the esterified astaxanthin and adonixanthin contents in algal cells, and addition of KCl markedly increased xanthophyll esterification, such that 73 % of xanthophylls were esterified with fatty acids (palmitic, palmitoleic, oleic, linoleic, and α-linolenic acids) in these cells (Fig. 4) (Aburai et al. 2015). In plants, K+ is an essential nutrient and also the most abundant cation. Cytosolic K+ concentrations are consistently maintained at 0.1–0.2 M (Shabala and Pottosin 2010). In higher plants, K+ is required for many aspects of growth and metabolism, such as osmoregulation, stomatal movement, and enzyme activation (Wang et al. 2013); for example, KCl was shown to activate a recombinant acyltransferase in vitro, but the mechanism of this activation was unclear (Chang et al. 2010).
Proportions of esterified xanthophylls in Coelastrella sp. KGU-Y002 cells. Percentages represent esterified xanthophylls out of total cellular xanthophylls. After precultivation for 21 days in BB medium at 40 μmol photons m−2 s−1, algal cells were transferred and cultured for 7 days in nitrogen-deficient medium with 0.15 M added salt (NaCl, KCl, MgCl2, or CaCl2; gray bars) at 200 μmol photons m−2 s−1. Asterisk indicates statistically significant difference (p < 0.05) compared with control. Values are average of triplicate experiments
Zeaxanthin and lutein are xanthophylls that are widely distributed in terrestrial plants, especially in fruits and vegetables. Esterified zeaxanthin and lutein have been extracted from marigold flowers and Chinese wolfberries (Sajilata et al. 2008), but such esterified forms have not been identified in algae so far. It is noteworthy that in the present study, esterified forms of zeaxanthin and lutein accumulated to 0.46 or 0.48 mg g−1 dwc (esterification rates of 20 and 49 %, respectively) in cells of an aerial microalga cultured with added NaCl or KCl.
Effects of metal cations on abscisic acid accumulation in Coelastrella sp. KGU-Y002
Abscisic acid, which is the universal stress hormone in terrestrial plants, has been detected at very low concentrations in various other organisms, including cyanobacteria, algae, bryophytes, fungi, and lichens (Hirsch et al. 1989; Mittler and Blumwald 2015). When strain Coelastrella sp. KGU-Y002 was cultivated in nitrogen-deficient medium with 0.15 M added salt (NaCl, KCl, MgCl2, or CaCl2) under high-light conditions for 7 days, the ABA contents increased to 57, 57, 53, and 32 nmol g−1 dwc (15, 15, 14, and 9 μg g−1 dwc), equivalent to 2.6, 2.7, 2.4, and 1.5 nmol g−1 fresh weight cells (fwc) (0.7, 0.7, 0.6, and 0.4 μg g−1 fwc), respectively (Fig. 5a). The ABA content in the control was 20.0 nmol g−1 dwc (5.3 μg g−1 dwc), equivalent to 0.9 nmol g−1 fwc (0.2 μg g−1 fwc). These values were significantly higher than those in microalgae under normal conditions (3–34 pmol g−1 fwc (0.8–8.9 μg g−1 fwc)) (Hirsch et al. 1989). In photosynthetic organisms, the ROS hydrogen peroxide functions as an intermediate in ABA-signaling transduction in photosynthetic organisms (Hirsch et al. 1989; Zhang et al. 2001; Yoshida et al. 2004). The ROS produced under oxidative stress in KGU-Y002 cells were measured using the DCFH-DA method (Rosenkranz et al. 1992). Under high-light and nitrogen-deficient conditions, NaCl, KCl, and MgCl2 significantly reduced ROS generation from 7.9 (control) to 3.3, 2.9, and 3.8 μmol g−1 dwc (from 269 to 112, 99, and 129 μg g−1 dwc), respectively (Fig. 5b). Abscisic acid was shown to reduce ROS generation and increase antioxidant biosynthesis and the expression of genes encoding antioxidant enzymes (Pancha et al. 2015).
Effects of metal cations on abscisic acid accumulation (a) and reactive oxygen species generation in Coelastrella sp. KGU-Y002 cells (b). Algae were precultured in a flat glass flask in BB medium at 40 μmol photons m−2 s−1 for 21 days, and then transferred to 100-mL Erlenmeyer flasks and cultured for 7 days under high-light conditions (HL, 200 μmol photons m−2 s−1) and 0.15 M added salt (NaCl, KCl, MgCl2, or CaCl2; gray bars). Asterisk indicates statistically significant difference (p < 0.05) compared with control. Data are means ± SD of at least three replicate cultures
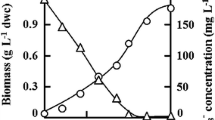
The ABA and ROS contents were monitored in Coelastrella sp. KGU-Y002 cells cultured in nitrogen-deficient medium, at 200 μmol photons m−2 s−1 and with or without 0.15 M KCl for 7 days (Fig. 6). The ABA contents increased dramatically from 2.9 to 652.4 nmol g−1 dwc (0.8–172.0 μg g−1 dwc), equivalent to 0.1–22.5 nmol g−1 fwc (0.03–5.95 μg g−1 fwc) within 1 day of salt stress and then decreased gradually to 294.5 nmol g−1 dwc (77.7 μg g−1 dwc) after 3 days of salt stress.
Time courses of abscisic acid (ABA) accumulation (a) and reactive oxygen species generation (b) in Coelastrella sp. KGU-Y002 cells cultured with metal cations. Algae were precultured in a flat glass flask in BB medium at 40 μmol photons m−2 s−1 for 21 days, and then transferred to 100-mL Erlenmeyer flasks and cultured for 7 days at 200 μmol photons m−2 s−1 with 0.15 M KCl (triangles) or 7.4 nM ABA (squares) or without ABA (circles). Data are means ± SD of at least three replicate cultures
The ABA content increased to more than 200-fold its initial concentration with the addition of KCl. This stress-dependent ABA increase in Coelastrella sp. KGU-Y002 cells was almost the same magnitude as those reported in terrestrial plants (Verslues 2016). In terrestrial plants, the acquisition and conservation of ABA-mediated responses to drought stress was a critical evolutionary event for adaptation to harsh environments. It was presumed here that aerial microalgae also acquired ABA biosynthesis. The ROS content in control cells gradually rose from 6.3 to 8.9 μmol g−1 dwc (214–303 μg g−1 dwc) over 1 day and then remained constant (Fig. 6b). In KCl-supplemented cultures, the ROS content reached a transient maximum of 8.7 μmol g−1 dwc (296 μg g−1 dwc) after 1 day, and then decreased to 2.9 μmol g−1 dwc (99 μg g−1 dwc) after 3 days. In algal cells treated with 7.4 nM ABA in nitrogen-deficient and high-light conditions, the ROS concentration was almost the same as that in the KCl-supplemented culture. In these algal cells, ABA enhanced esterification without affecting total carotenoid accumulation, and 35 % of constituent xanthophylls were esterified with fatty acids (data not shown). Esterified xanthophylls have been suggested to be powerful ROS scavengers in algal cells. The ABA-signaling pathway was shown to regulate acyltransferase transcription in Arabidopsis seedlings (Yang et al. 2011). The findings of the present study suggested that ABA has plant hormone-like bioactivity that could have activated xanthophyll esterification in the aerial microalga Coelastrella sp. KGU-Y002.
Conclusions
The accumulation of esterified xanthophylls was characterized in the aerial microalga Coelastrella sp. KGU-Y002 cultured in nitrogen-deficient, high-light conditions, with or without certain metal cations. The addition of salts (NaCl, KCl, or MgCl2) activated the synthesis of esterified xanthophylls in these algal cells. In the cells grown with KCl, 73 % of xanthophylls were esterified with fatty acids. Furthermore, the cellular ABA content increased to 200-fold its initial concentrations within 1 day after KCl addition. These results indicated that xanthophyll esterification in these algal cells was modulated under salt stress and was physiologically regulated by endogenous ABA. Further studies have been designed to examine large-scale production of esterified xanthophylls by this aerial microalga under salt-stress conditions, and to assess the bioactivity of esterified xanthophylls extracted from algal cells.
References
Abe K, Hattori H, Hirano M (2007) Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem 100:656–661
Abe K, Bito T, Sato A, Aburai N (2014) Effects of light intensity and magnesium supplementation in pretreatment cycle on ammonium removal from wastewater of photobioreactor using a biofilter composed of the aerial microalga Trentephohlia aurea. J Appl Phycol 26:341–347
Aburai N, Ohkubo S, Miyashita H, Abe K (2013) Composition of carotenoids and identification of aerial microalgae isolated from the surface of rocks in mountainous districts of Japan. Algal Res 2:237–243
Aburai N, Sumida D, Abe K (2015) Effect of light level and salinity on the composition and accumulation of free and ester-type carotenoids in the aerial microalga Scenedesmus sp. (Chlorophyceae). Algal Res 8:30–36
Chang CCY, Miyazaki A, Dong R, Kheirollah A, Yu C, Geng Y, Higgs HN, Chang TY (2010) Purification of recombinant acyl-coenzyme A: cholesterol acyltransferase 1 (ACAT1) from H293 cells and binding studies between the enzyme and substrates using difference intrinsic fluorescence spectroscopy. Biochemistry 49:9957–9963
Chen G, Wang B, Han D, Sommerfeld M, Lu Y, Chen F, Hu Q (2015) Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J 81:95–107
Chu FL, Pirastru L, Popovic R, Sleno L (2011) Carotenogenesis up-regulation in Scenedesmus sp. using a targeted metabolomics approach by liquid chromatography-high-resolution mass spectrometry. J Agric Food Chem 59:3004–3013
Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4:472–478
Gorain PC, Bagchi SK, Mallick N (2013) Effects of calcium, magnesium and sodium chloride in enhancing liquid accumulation in two green microalgae. Environ Technol 34:1887–1894
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Hirsch R, Hartung W, Gimmler H (1989) Abscisic acid content of algae under stress. Bot Acta 102:326–334
Hou S, Zhu J, Ding M, Lv G (2008) Simultaneous determination of gibberellic acid, indole-3-acetic acid and abscisic acid in wheat extracts by solid-phase extraction and liquid chromatography-electrospray tandem mass spectrometry. Talanta 76:798–802
Kamath BS, Srikanta BM, Dharmesh SM, Sarada R, Ravishankar GA (2008) Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur J Pharmacol 590:387–395
Kaufnerová V, Eliáš M (2013) The demise of the genus Scotiellopsis Vinatzer (Chlorophyta). Nova Hedwigia 97:415–428
Kobayashi M, Sakamoto Y (1999) Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol Lett 21:265–269
Kobayashi M, Hirai N, Kurimura Y, Ohigashi H, Tsuji Y (1997) Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regul 22:79–85
Maoka T, Yasui H, Ohmori A, Tokuda H, Suzuki N, Osawa A, Shindo K, Ishibashi T (2013) Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J Oleo Sci 62:181–186
Mittler R, Blumwald E (2015) The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27:64–70
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348
Rao AR, Sindhuja HN, Dharmesh SM, Sankar KU, Sarada R, Ravishankar GA (2013) Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J Agric Food Chem 61:3842–3851
Rao AR, Sarada R, Shylaja MD, Ravishankar GA (2015) Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J Food Sci Technol 52:6703–6710
Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ (1992) A microplate assay for the detection of oxidative products using 2′, 7′ –dichlorofluorescin-diacetate. J Immunol Methods 156:39–45
Sajilata MG, Singhal RS, Kamat MY (2008) The carotenoid pigment zeaxanthin—a review. Compr Rev Food Sci Food Saf 7:29–49
Shabala S, Pottosin II (2010) Potassium and potassium-permeable channels in plant salt tolerance. In: Demidchik V, Maathuis F (eds) Ion channels and plant stress responses. Springer, Berlin, pp. 87–110
Skibsted LH (2012) Carotenoids in antioxidant networks. Colorants or radical scavengers. J Agric Food Chem 60:2409–2417
Verslues PE (2016) ABA and cytokinins: challenge and opportunity for plant stress research. Plant Mol Biol 91:629–640
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390
Yang Y, Yu X, Song L, An C (2011) ABI4 activates DGAT1 expression in Arabidopsis seedlings during nitrogen deficiency. Plant Physiol 156:873–883
Yoshida K, Igarashi E, Wakatsuki E, Miyamoto K, Hirata K (2004) Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci 167:1335–1341
Yuan JP, Chen F, Liu X, Li XZ (2002) Carotenoid composition in the green microalga Chlorococcum. Food Chem 76:319–325
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation of high light is correlated with that of astaxanthin esters. J Phycol 38:325–331
Acknowledgments
This study was supported by a grant from the Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sport, Science, and Technology, Japan (S1411005). This work was supported in part by the Research Institute for Science and Technology of Kogakuin University for a special Grant-in-Aid to earn KAKENHI by the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeki, K., Aburai, N., Aratani, S. et al. Salt-stress and plant hormone-like responses for selective reactions of esterified xanthophylls in the aerial microalga Coelastrella sp. KGU-Y002. J Appl Phycol 29, 115–122 (2017). https://doi.org/10.1007/s10811-016-0911-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0911-7