Abstract
Accumulation of the stress hormone abscisic acid (ABA) induces many cellular mechanisms associated with drought resistance. Recent years have seen a rapid advance in our knowledge of how increased ABA levels are perceived by ABA receptors, particularly the PYL/RCAR receptors, but there has been relatively less new information about how ABA accumulation is controlled and matched to stress severity. ABA synthesis and catabolism, conjugation and deconjugation to glucose, and ABA transport all are involved in controlling ABA levels. This highly buffered system of ABA metabolism represents both a challenge and opportunity in developing a mechanistic understanding of how plants detect and respond to drought. Recent data have also shown that direct manipulation of cytokinin levels in transgenic plants has dramatic effect on drought phenotypes and prompted new interest in the role of cytokinins and cytokinin signaling in drought. Both ABA and cytokinins will continue to be major foci of drought research but likely with different trajectories both in terms of basic research and in translational research aimed at increasing plant performance during drought.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plants must modulate their growth and metabolism to match many environmental inputs. One of the most pernicious environmental stresses facing plants is drought which leads to reduced soil water content and reduced water potential. Plants respond to water limitation through a series of mechanisms which contribute to avoidance and tolerance of low water potential. Mechanisms such as stomatal closure and increase in root-to-shoot ratio aim to conserve soil moisture or find additional water and thus avoid further decreases in water potential and dehydration of the plant tissue. Other mechanisms, such as accumulation of protective solutes and proteins and changes in redox-related metabolism serve mainly to allow continued plant function at reduced water potential and, to the extent possible, tolerate loss of water from the plant tissue. Further review of drought and the water potential concept as well as avoidance versus tolerance of drought and low water potential can be found in Kramer and Boyer (1995) and Verslues et al. (2006). Plant hormones are key messengers that integrate external signals with internal metabolic status and developmental state to determine the course of further growth and metabolism. Abscisic acid is a central regulator of responses to reduced water potential and dehydration of plant tissue that occur during periods of drought. The importance of ABA has been established by numerous observations of ABA accumulation in water stressed plants as well as impaired stress responses in ABA-deficient mutants (Cutler et al. 2010; Sharp 2002). While ABA interacts with several other hormones in mediating drought and low water potential response, there has been an upsurge of interest in cytokinins in recent plant stress research.
Different types of environmental stimuli elicit specific responses in the plant; however, some key questions are common and essential to understanding hormone-mediated responses to many environmental signals. First, how is the environmental factor initially detected by the plant? Then, how does the initial detection of the environmental signal change hormone metabolism and endogenous hormone concentrations at the relevant site(s) of action? How is the hormone signal perceived by the plant and what controls the sensitivity? What further signaling downstream of hormone perception is most critical for acclimation to the changing environment? How completely we can answer these questions differs dramatically between different types of environmental inputs. For example, the initial perception of light by phytochrome is understood in exquisite molecular detail. In contrast, we know little of how plants perceive a lack of water. There are several theories of how water loss or reduced turgor may be perceived via membrane-based mechanosensing mechanisms such as mechanosenstive channels or receptor kinases or by the cytoskeleton (Marshall et al. 2012; Yuan et al. 2014; Haswell and Verslues 2015; Verslues et al. 2013) but relatively little evidence to link specific sensing proteins to downstream responses such as ABA accumulation. Farther downstream, ABA-responsive transcriptional regulation and marker phenotypes amenable to genetic screening (for example seed germination and ABA-responsive promoter reporter constructs) have been analyzed in detail. But these may not capture all aspects of drought and low water potential response important for survival or productivity. For this author, it is interesting see how some aspects of ABA and cytokinin function in plant stress have advanced rapidly (for example study of ABA receptors) since last writing on this topic (Verslues and Zhu 2005, 2007). Meanwhile, other areas (for example sensing and signaling upstream of ABA; control of ABA metabolism) have seen less activity. A focus of this review is on processes that control low water potential-responsive ABA accumulation. ABA accumulation controls numerous downstream processes yet how it is itself controlled by stress is not as clearly understood. I also discuss some aspects of ABA perception and interesting new developments in the roles of cytokinins and cytokinin signaling in stress resistance.
ABA synthesis, catabolism, conjugation and transport match ABA accumulation to stress severity
ABA levels in unstressed plants are in the low nanogram per gram tissue fresh weight or dry weight range and reduced water potentials can lead to ABA accumulations more than 100-fold above the basal level (example data in Fig. 1a; Sharp and LeNoble 2002; Kumar and Verslues 2015; Verslues lab unpublished data). When plants are exposed to a controlled and constant low water potential stress, it can be seen that the level of ABA accumulation is closely linked to the severity of the stress (Fig. 1b). This fine control of ABA level is both a challenge and an opportunity in the broader context of plant stress research.
ABA accumulation of Arabidopsis seedlings in response to low water potential treatment. a Time course of ABA content after transfer of seedlings to low water potential (−1.2 MPa PEG-agar plates) at time 0. Data are for the Bensheim accession of Arabidopsis thaliana. ABA accumulates rapidly after transfer but then stabilizes by 96 h as the plants acclimate to the stress. ABA levels of mock treated plants (transferred to new plates of normal media) remain low through the time course (data not shown). Data are from Verslues and Bray (2004) and similar data for the Col-0 Arabidopsis accession is presented in Verslues and Bray (2006). b ABA measurements of seedlings collected at 96 h after transfer of seedlings to PEG-agar plates of a range of water potentials. ABA levels show a close correspondence to the stress severity (water potential) and also the seedling relative water content (RWC). Data are replotted from Verslues and Bray (2004)
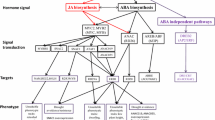
Classic studies indicated that reduced turgor pressure was the key factor needed for ABA accumulation (Creelman and Zeevaart 1985; Pierce and Raschke 1980, 1981; also discussed in Kumar et al. 2013). Interestingly, genetic alterations that upset the osmotic balance between cellular compartments can also activate ABA accumulation even in the absence of an externally imposed stress (Wilson et al. 2014). However, we still have not identified the stress sensing mechanisms responsible for the initial detection of water limitation that elicits ABA accumulation (Haswell and Verslues 2015; Verslues et al. 2013; Verslues and Zhu 2005). These unknown upstream signaling events can act on several pathways of ABA metabolism and transport that together determine the amount of ABA present at its sites of activity (Fig. 2).
Overview of ABA and cytokinin metabolism, perception and downstream signalling. Both ABA and cytokinin metabolism are influenced by unknown upstream stress sensing and signalling mechanisms. These upstream mechanisms act on several coordinated processes which determine the hormone concentration at its site of action. Perception of ABA and cytokinin through specific receptors regulates downstream drought resistance phenotypes. Key genes affecting each process are listed and are discussed in the main text. Note that not all known genes of ABA and cytokinin metabolism or signalling are shown
ABA is synthesized via the cleavage of the carotenoids violaxanthin or neoxanthin into xanthoxin in the chloroplast followed by several subsequent steps in the cytoplasm (Cutler and Krochko 1999; Hauser et al. 2011; Nambara and Marion-Poll 2005; Schwartz et al. 2003). It is thought that the carotenoid cleavage reaction catalyzed by chloroplast localized 9-cis-epoxycarotenoid dioxygenases (NCEDs) is the rate limiting step in ABA synthesis (Endo et al. 2008; Qin and Zeevaart 1999; Schwartz et al. 1997). Arabidopsis NCED3, as well as orthologous NCEDs in other species, is induced by low water potential at both transcript and protein levels (Iuchi et al. 2001; Qin and Zeevaart 1999; Tan et al. 2003). Other enzymes of ABA synthesis may also exert some control on ABA accumulation (see for example Lin et al. 2007) but their effect on stress-induced ABA accumulation is more limited. Conversely, ABA is catabolized by a small group of cytochrome P450 type enzymes, the CYP707As, which convert ABA to phaseic acid or neophaseic acid (Krochko et al. 1998; Kushiro et al. 2004; Okamoto et al. 2011, 2006; Umezawa et al. 2006; Zhou et al. 2004).
ABA levels can also be modulated by the production of ABA conjugates. ABA conjugates, principally ABA-glucose ester (ABA-GE), had been thought to be a metabolic dead end that permanently inactivated ABA and targeted it for storage in the vacuole (Cutler and Krochko 1999). However, it is now clear that conjugation and deconjugation of ABA is a dynamic process used to adjust ABA levels. A number of glucosyltransferase genes able to produce ABA-GE have been identified (Dong et al. 2014; Liu et al. 2015; Priest et al. 2006; Xu et al. 2002) and are listed in Fig. 2. Increasing or decreasing the expression of these glucosyltransferases altered ABA levels, ABA-responsive phenotypes, and CYP707A expression, all indicative of disturbed ABA homeostasis (Dong et al. 2014; Liu et al. 2015). Conversely, two related β-glucosidases, BG1 and BG2, release free ABA from ABA-GE (Lee et al. 2006; Xu et al. 2012). BG1 is localized in the endoplasmic reticulum while BG2 is vacuolar, suggesting that both of these cellular compartments contain ABA-GE. The BG1 study is of special interest because they also demonstrated the polymerization of BG1 occurred rapidly in response to water limitation and increased the BG1 specific activity.
There have been recent advances on ABA transport as well (Boursiac et al. 2013). It had sometimes been thought that pH gradients were the driving force for distribution of ABA between apoplast and symplast and between cellular compartments. Such a mechanism was perhaps never completely satisfactory (Verslues and Zhu 2007) and indeed several ABC-type transporters which can move ABA have been identified. AtBCG25 and AtBCG40 are likely to be ABA importers which move ABA into guard cells (Kang et al. 2010; Kuromori et al. 2010). AtBCG22 is also an ABA transporter, possibly involved in ABA efflux, although this function is less clearly established (Kuromori et al. 2011). Several members of the NRT nitrate transporter family were identified as also having ABA import activity (Kanno et al. 2012). Interestingly, transporters for ABA-GE have not been identified even though movement of ABA-GE in the xylem and uptake into cells has been proposed (Hartung et al. 2002; Sauter et al. 2002) and such transporters may be necessary, for example, to move ABA-GE intracellularly into the ER where BG1 is located (Lee et al. 2006).
The extent of long distance ABA transport in the plant vascular system is an unclear area and is confounded with broader questions of what kind of signals (hydraulic, chemical or electrical) roots in drying soil may send to shoot and how necessary such signals are in controlling shoot responses to water limitation (Christmann et al. 2005, 2007; Davies et al. 2005; Hartung et al. 2002; Wilkinson and Davies 2002). Given their direct contact with drying soil, roots may be a logical site for drought perception. Split root experiments (where part of the root system is allowed to dry while another part is kept wet) and root pressurization (which allows the roots to dry but maintains turgor of the shoot) provided evidence that a non-hydraulic, chemical signal moved from root to shoot (see for example Davies et al. 2005; Holbrook et al. 2002; Saab and Sharp 1989). While such a chemical signal may exist, several studies suggest it may not be ABA as there is a relative lack of ABA synthesis in roots (Holbrook et al. 2002; Christmann et al. 2005, 2007). In the shoot, it has been observed that NCED3 expression and protein accumulation is mainly associated with the leaf vascular parenchyma cells between xylem and phloem bundles (Endo et al. 2008). Whether ABA (or ABA-GE) synthesized in these cells is loaded into xylem or phloem (and which transporters may be involved) for distribution has not been resolved. A presumed function of ABA transport, whether long distance through the vascular tissue or locally across the plasma membrane, is to regulate the concentration of ABA in stomatal guard cells and thus control stomatal aperture. However, it has been reported that ABA synthesis in the guard cell itself is sufficient for stomatal closure in response to low humidity (Bauer et al. 2013). Hopefully new tools (see below) can help resolve questions about the sites of ABA synthesis and how ABA may move from site of synthesis to site of action.
The highly buffered system controlling of ABA accumulation represents both a challenge and opportunity in understanding plant stress responses
A signaling intermediate such as ABA would be expected to be dynamic so that the ABA signal can be both rapidly generated and rapidly extinguished. Which of the processes described above are critical in the dynamic control of ABA accumulation? ABA synthesis is an obvious control point; however, NCED3 over expression leads to greater increases in the ABA catabolites phaseic acid and dehydrophaseic than increase in ABA itself (Priest et al. 2006; Qin and Zeevaart 2002) indicating that ABA catabolism was stimulated to match the increased ABA synthesis. The half-life of ABA in maize plants was found to be only 1–2 h under either unstressed or stressed conditions (Ren et al. 2007) implying that ABA synthesis and catabolism are in dynamic equilibrium. Consistent with such dynamic turnover, expression of both NCED3 and CYP707As can be elevated under low water potential stress (Fig. 3). Likewise, overexpression of an ABA glucosyltransferase resulted in dramatically increased levels of ABA-GE but little effect on ABA content (Priest et al. 2006). These data indicate the existence of ABA homeostasis mechanisms which are little disturbed by merely altering one branch of ABA metabolism. How the set-point level of ABA is determined and synthesis, catabolism and conjugation/deconjugation coordinated is not known.
Upregulated expression of both ABA synthesis and catabolism genes under stress shows the potential for rapid ABA turnover. Gene expression data are from microarray analysis of Col-0 seedlings transferred to either control or low water potential media (−1.2 MPa) for 96 h (the same treatment shown in Fig. 1a). Data are from Bhaskara et al. (2012)
Which aspect of ABA metabolism responds directly to stress sensing to initiate ABA accumulation? NCED3 expression is rapidly induced by stress, yet it has been shown that stress induction of NCED3 is reduced in ABA-deficient mutants such as aba3 or aba2-1 (Sharma and Verslues 2010; Xiong et al. 2002). Thus, there is a chicken and egg conundrum that if increased NCED3 expression is required for ABA accumulation yet ABA accumulation is required to fully induce NCED3 expression, what gets the whole process started? One likely explanation is that the conjugation and deconjugation of ABA-GE plays a larger role than previously suspected. Polymerization and activation of BG1 (and perhaps BG2) can be a more rapid way to release active ABA than de novo ABA synthesis (Lee et al. 2006). What kind of stress signal and post-translational modification prompts BG1 to polymerize is not known and would seem to be a particularly promising area for further research. NCEDs also undergo poorly understood post-translational processing which is thought to affect NCED association with thylakoid membrane and access to its lipid-soluble substrate (Endo et al. 2008; Tan et al. 2001).
The above discussion illustrates the complexity of ABA metabolism and its regulation. But ABA accumulation can also be used to understand stress sensing and signalling. A limitation in genetic studies of plant stress is the lack of outputs (phenotypes) that provide a clear readout of stress signalling activation yet can be measured in a rapid and precise enough manner for genetic studies. While still challenging, ABA is becoming more accessible as an output factor that can hopefully lead us back to upstream steps of stress sensing/signalling which control ABA accumulation and the processes that distribute ABA to its sites of action in the plant.
One significant new front in ABA research is the development of ABA-biosensors. Two groups independently constructed genetically encoded Förster resonance energy transfer (FRET) sensors that respond to changes in ABA content (Jones et al. 2014; Waadt et al. 2014). For both groups, the basic strategy was to construct a hybrid protein consisting of the ABA binding pocket of the PYL/RCAR type ABA receptors along with the portion of a protein phosphatase 2C (PP2C) that contacts the ABA binding pocket and acts as a co-receptor (see below for description of the PYL/RCAR/PP2C ABA sensing system). While the basic strategy used by both groups was similar, the sensors they produced have different characteristics. The sensor produced by Waadt et al. (2014) has high ABA affinity, a decreased FRET signal in response to ABA binding and its expression in planta makes the plant less sensitive to ABA, perhaps by sequestering ABA. The sensors produced by Jones et al. (2014) had somewhat lower ABA affinity, an increased FRET signal in response to ABA binding, and their expression in planta led to ABA hypersensitivity. Waadt et al. (2014) showed evidence that their sensors could detect stress-induced changes in endogenous ABA while this was less clear for Jones et al. (2014). Waadt et al. (2014) used their sensor to investigate long distance ABA movement and found that shoot to root ABA movement could be detected while root to shoot ABA movement could not be detected (perhaps because of limited transpiration in their experimental conditions). In both studies, the change in ABA sensitivity caused by sensor expression is not ideal and for Jones et al. (2014) their ABA sensors needed to be expressed in a mutant defective in RNA-silencing to prevent silencing of sensor expression. Thus, the use of ABA sensors is promising, although further experimentation is needed to optimize ABA sensors for in planta use. As envisioned by both groups, optimized ABA sensors could be used in stress experiments and combined with various genetic backgrounds (mutants) to investigate factors controlling local ABA concentrations (Jones 2015).
Forward genetic screening based on promoter:reporter constructs has been effective in identifying stress and ABA-related loci (Ishitani et al. 1997). A newer genetic screen has used the NCED3 promoter driving a Luciferase reporter as the basis for isolating mutants with altered stress response. Use of the NCED3 promoter was based at least in part on the idea that if NCED3 expression has a role in controlling ABA content, its expression may respond to signalling mechanisms upstream of ABA. Two mutants from this screen have been reported in detail. In one report, mutation of the cutin biosynthesis gene BODYGAURD, as well as other cutin synthesis mutants, led to reduced NCED3 expression and reduced ABA content as well as increased damage of seedlings exposed to low water potential stress (Wang et al. 2011b). How decreased cutin formation is mechanistically related to NCED3 expression and ABA accumulation is unclear. Another mutant with reduced NCED3 expression and reduced ABA content was found to be affected in Vacuolar Sorting Receptor1 (VSR1) (Wang et al. 2015). Further analysis indicated that this mutant was impaired in expression of several ABA synthesis genes including NCED3 and that the reduced ABA of vsr1 may be related to changes in intracellular pH regulation. It would also seem possible that vsr phenotypes may be related to the uptake or release of ABA or ABA-GE from the vacuole or ER. Identification of additional mutants from this screen is of substantial interest; although, interpretation is complicated by the fact mentioned above that NCED3 expression is affected not only by upstream signals but also by ABA as part of feedback regulation. Since the NCED3 promoter screen was initiated, new loci have been identified that may be even more promising for mutant screening. For example, PYL4, PYL5 and PYL6 expression was downregulated dramatically by low water potential and this down regulation did not require ABA accumulation (Bhaskara et al. 2012). Thus, the PYL5 and PYL8 promoters may respond to unknown signalling involved in controlling ABA sensitivity (see below for further discussion of PYL expression and ABA sensitivity).
In our laboratory, we have observed substantial natural variation in low water potential-induced ABA accumulation among Arabidopsis accessions (R. Kalladan, J.R. Lasky, S. Sharma, T.E. Juenger, P.E. Verslues; unpublished observations). Currently we are investigating whether genome wide association mapping and QTL mapping based on these ABA differences can identify new loci involved in controlling ABA accumulation.
ABA perception and downstream signaling
In contrast, to ABA metabolism, the perception of ABA and immediate downstream signaling has seen rapid progress (for review see Cutler et al. 2010; Hubbard et al. 2010; Raghavendra et al. 2010). For many years, genetic screens of ABA sensitivity mutants and other analyses had failed to uncover ABA receptors. This situation then changed dramatically with reports of multiple ABA receptors including GCR2 (Liu et al. 2007), Mg-chelatase H subunit ABAR (Shen et al. 2006), GTG1 and GTG2 (Pandey et al. 2009) and the PYR-PYL/RCAR proteins (Ma et al. 2009; Nishimura et al. 2010; Park et al. 2009). Whether GCR2 is truly an ABA receptor was subsequently questioned based on concerns about ABA binding (Risk et al. 2009), description of the protein function and homology (Johnston et al. 2007), and reproducibility of phenotypic assays which could link the putative receptor to ABA sensitivity (Gao et al. 2007; Guo et al. 2008). For ABAR, there is some follow-up experimentation which support its role in ABA perception and signaling (Du et al. 2012; Shang et al. 2010; Wu et al. 2009), but its role remains unclear in part because of conflicting data about its ABA binding (Tsuzuki et al. 2011). For GTG1 and GTG2, there has been relatively little in the way of follow up experiments which could verify its ABA binding and provide a stronger mechanistic link to other ABA signaling components (but see Alvarez et al. 2013). Thus the role of GTG proteins in ABA perception and signaling also remains uncertain.
Progress has been much more rapid for the PYR-PYL/RCAR proteins (hereafter referred to as PYLs for convenience). The PYLs are a 14-member gene family in Arabidopsis which interact directly with the nine members of the Clade A protein PP2Cs. The formation of a ternary complex of PYL-ABA-PP2C was an immediately compelling mechanism because it linked the newly identified ABA receptor to the Clade A PP2Cs which were some of the earliest identified ABA signaling proteins. This interaction was structurally analyzed and the existence of a complete pathway from ABA perception to phosphorylation and activation of ABA regulated transcripton factors and ion channels demonstrated (Brandt et al. 2012; Dupeux et al. 2011; Fujii et al. 2009; Melcher et al. 2009; Nishimura et al. 2009; Peterson et al. 2010; Soon et al. 2012). With 14 PYLs and 9 Clade A PP2Cs, the number of combinations is large and it has been shown that different PYL-ABA-PP2C complexes have different ABA-binding affinities (Szostkiewicz et al. 2010) and thus may respond differently to ABA accumulation. Interestingly, a number of PYL-PP2C interactions are not dependent on ABA (Bhaskara et al. 2012; Hao et al. 2011) and the signaling function of these interactions is not well understood. A major effect of PYL-PP2C binding is to release repression of downstream SNF-related2 (SnRK2) kinases. Identification of additional SnRK2 phosphorylation targets is also an area of active research in ABA signaling (Umezawa et al. 2013; Wang et al. 2013).
Some authors have suggested that the PYL-PP2C system is the dominant pathway of perception and signaling with other ABA receptors playing minor roles (Gonzalez-Guzman et al. 2012). This is a broad assertion and fully addressing it requires molecular genetic experiments to directly compare the different ABA signaling systems. For example construction of mutant lines where both PYLs and GTGs or ABAR are knocked out to see if there is an additive effect on ABA response (which, to the knowledge of this author has not been done). Another factor to consider is changes in ABA sensitivity under different conditions. The concentrations of exogenous ABA applied in many experiments (10–100 μM) produce tissue ABA concentrations orders of magnitude greater than the amount of ABA that accumulates during stress (Verslues and Bray 2006). Thus, quantitatively, unstressed plants are less sensitive to ABA than stressed plants for many phenotypes (for example, proline accumulation; Sharma and Verslues 2010; Verslues and Bray 2006) or have different responses than stressed plants (Sharp and LeNoble 2002). What we know of the core PYL-PP2C pathway so far does not explain such an increased competence/sensitivity of stressed plants to respond to ABA. Expression of several PYLs is repressed by low water potential while Clade A PP2C expression is increased (see for example Bhaskara et al. 2012). This suggests that during stress there are fewer PYLs available to inhibit relatively more PP2Cs. This would make the plant less sensitive to ABA rather than more sensitive as observed in physiology experiments. Downregulation of PYLs during stress has been proposed to be a feedback regulatory mechanism. However, how stress changes actual protein levels of the PYLs is not known and understanding how the PYL regulatory system functions in planta is still a work in progress. Clearly, there are other inputs that control ABA sensitivity. Whether these are from other ABA receptors or other environmental stress sensing pathways is of interest for placing ABA signaling in the broader context of stress biology.
Cytokinins in abiotic stress resistance
Cytokinins are N 6-substituted adenine derivatives which have numerous roles in plant development and interaction with other hormones (Hwang et al. 2012; Kieber and Schaller 2010). Cytokinin metabolism is more complex than that of ABA, but overall cytokinin levels are controlled by a similar range of processes as ABA (synthesis, catabolism, conjugation and transport; Frébort et al. 2011) (Fig. 2). Cytokinin metabolism genes are stress regulated in a manner consistent with reduced cytokinin levels under many types of abiotic stress (Brenner et al. 2012). Direct measurements have confirmed that cytokinin levels decrease during low water potential and salinity stress (Havlova et al. 2008).
While stress decreases cytokinins, transgenic plants with elevated cytokinin levels exhibited delayed leaf senescence (Gan and Amasino 1995). Blumwald and co-workers (Rivero et al. 2007) made the connection between these sets of observations. They reported that transgenic plants with a regulated increase in cytokinin production at the onset of stress [by the Senescence inducible Receptor Kinase (SARK) promoter driving expression of isopentenyltransferase (IPT)] had increased drought tolerance. This was because of delayed leaf senescence and cytokinin-regulated changes in metabolism (Rivero et al. 2010, 2009). A crucial aspect of their study was that correctly regulating the increased cytokinin synthesis to occur only in response to water limitation circumvented the inhibition of shoot growth and delay in defense activation seen when cytokinins are constitutively elevated. This strategy, or modifications of it, has subsequently been tested in a range of other species (see for example Kant et al. 2015; Kuppu et al. 2013; Mackova et al. 2013; Merewitz et al. 2012; Peleg et al. 2011).
Recently, cytokinin transporters likely involved in root to shoot cytokinin movement were discovered (Ko et al. 2014; Zhang et al. 2014). Also, several glucosyltransferases mediating either O-glycosylation of the cytokinin side chain or N-glycosylation of the purine ring have been described (Martin et al. 1999; Hou et al. 2004; Jin et al. 2013; Wang et al. 2011a). Also, new side chain modifications which affect cytokinin activity in controlling shoot growth have been reported (Kiba et al. 2013). Whether or not these new aspects of cytokinin biology affect low water potential response, or can be altered to promote drought resistance, is of interest. Tissue specific alterations in cytokinin content are also of interest but less clear as Werner et al. (2010) found that a root specific reduction in cytokinin content led to increased root system size and better survival of severe water limitation while Ghanem et al. (2011) found that increase in root-synthesized cytokinin increased shoot growth and yield of tomato under salt stress.
The mechanisms of cytokinin perception and immediate downstream signaling have been the subject of numerous studies (Hwang et al. 2012; Kieber and Schaller 2010) and consist (in Arabidopsis) of a bacterial type histidine phosphorelay system with Histidine Kinase (AHK) cytokinin receptors signaling through downstream Histidine Phosphotransfer proteins (AHPs) and Response Regulators (ARRs) (Fig. 2). Note however that AHK1, which has attracted much interest in plant stress research (Tran et al. 2007; Urao et al. 1999; Kumar et al. 2013), is not a cytokinin receptor. Tran et al. (2007) proposed that AHK2, AHK3 and AHK4 function as negative regulators of dehydration and salt tolerance. Kang et al. (2012) also found that ahk2 and ahk3 mutants had a higher survival rate after rapid severe dehydration stress (plants were removed from growth media and allowed to dehydrate then returned to growth media and survival scored). Conversely, Kumar and Verslues (2015) found AHK-specific phenotypes with ahk3 having increased root growth at low water potential while ahk2 mutants were specifically sensitive to salt stress but similar to wild type in low water potential sensitivity. Kumar and Verslues (2015) also found that ahk double mutants had reduced proline accumulation, suggesting a more general function of cytokinin signaling in regulating proline and consistent with reports that increased cytokinin levels stimulated proline accumulation (Merewitz et al. 2012). The differing conclusions about the stress roles of AHKs likely lie in whether stress tolerance is defined as being able to survive severe stress [as in Tran et al. (2007) and Kang et al. (2012)] or as being able to maintain higher growth rates under less severe, non-lethal, stress treatments (as in Kumar and Verslues 2015). Other studies have found that AHKs affect stomatal regulation (Marchadier and Hetherington 2014) and cold stress (Jeon et al. 2010). Another study (Nishiyama et al. 2013) reported that mutant of Arabidopsis Histidine Phosphotransfer proteins (ahp2, ahp3 and ahp5) had increased tolerance to water limitation. The same group (Nishiyama et al. 2011) also reported that cytokinin deficient plants had increased dehydration tolerance (in contrast to the above studies where increased cytokinin promoted drought tolerance). Here again, they defined “drought tolerance” as the ability to survive severe dehydration and the results were likely influenced by different sizes of the plants leading to different rates of soil water depletion. Interestingly though, they also reported that the cytokinin deficient plants were ABA hypersensitive and had altered ABA content. Thus, there may be crossregulation between ABA and cytokinin via yet unknown mechanisms. ABA-cytokinin ratio may affect various processes such as growth and stomatal regulation.
Perspective
ABA and cytokinin are two hormones that feature prominently in current plant stress research. However, research on the two is moving in different directions. ABA research has largely put aside efforts to directly manipulate ABA levels as a way to engineer drought tolerance. One reason for this is that ABA metabolism is so highly buffered that it is difficult to change ABA accumulation unless the (still unknown) overriding regulatory factors matching ABA content to environmental inputs are identified. Another reason is that aside from promoting stomatal closure to conserve water it is not clear that accumulating more ABA is actually beneficial to increasing plant growth and productivity under drought. Many of the most widely reported ABA responses occur under severe stress or a more prevalent in mature tissue (Claeys and Inze 2013). While the low basal level of ABA present in unstressed plants has important physiological functions, the accumulation of high ABA levels is in many ways an emergency signal. Having the plant declare a bigger emergency (by accumulating more ABA) and halting growth and development is likely not to be beneficial in terms of plant productivity under moderate levels of drought stress. A new focus of ABA research is to use knowledge of the PYL-PP2C signalling system as a basis for the design of small molecules that can selectively activate (or repress) ABA signalling (Cao et al. 2013; Park et al. 2015; Takeuchi et al. 2014). How this line of research develops both in terms of new tools for basic science as well as field application will be of interest. In terms of basic science, our understanding of stress signalling will be without firm foundation until we understand the cellular mechanisms plants use to detect water limitation and control the amount and activity of second messengers such as ABA.
For cytokinins, in contrast, compelling evidence shows that the direct manipulation of cytokinin levels is an effective way to alter drought tolerance. The challenges are to understand why and to sort through conflicting results that make it hard to generalize what the effect of cytokinin manipulation is and what could be the best strategies for altering cytokinins to improve drought tolerance (Zwack and Rashotte 2015). The strongest evidence is that preventing stress-induced decrease in cytokinin levels in the shoot has a positive effect on abiotic stress tolerance. This seems to be largely due to delaying or blocking the activation of senescence pathways, although other metabolic changes are also involved. Further understanding of the types of environments where cytokinin engineered plants may be of practical value as well as to what extent altering cytokinin levels changes ABA levels or response are all topics of future interest. Our understanding of how cytokinin signalling components affect plant response to water limitation is also somewhat confused (Zwack and Rashotte 2015). Experiments such as determining if the stress resistance phenotypes associated with increased cytokinin levels are dependent on specific combinations of AHKs and downstream AHPs and ARRs would seem promising.
References
Alvarez S, Choudhury SR, Hicks LM, Pandey S (2013) Quantitative proteomics-based analysis supports a significant role of GTG proteins in regulation of ABA response in Arabidopsis roots. J Proteome Res 12:1487–1501. doi:10.1021/pr301159u
Bauer H et al (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23:53–57. doi:10.1016/j.cub.2012.11.022
Bhaskara GB, Thao Thi N, Verslues PE (2012) Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol 160:379–395. doi:10.1104/pp.112.202408
Boursiac Y, Leran S, Corratge-Faillie C, Gojon A, Krouk G, Lacombe B (2013) ABA transport and transporters. Trends Plant Sci 18:325–333. doi:10.1016/j.tplants.2013.01.007
Brandt B et al (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109:10593–10598. doi:10.1073/pnas.1116590109
Brenner WG, Ramireddy E, Heyl A, Schmülling T (2012) Gene regulation by cytokinin. Front Plant Sci. doi:10.3389/fpls.2012.00008
Cao MJ et al (2013) An ABA-mimicking ligand that reduces water loss and promotes drought resistance in plants. Cell Res 23:1043–1054. doi:10.1038/cr.2013.95
Christmann A, Hoffmann T, Teplova I, Grill E, Muller A (2005) Generation of active pools of abscisic acid revealed by in vivo Imaging of water-stressed Arabidopsis. Plant Physiol 137:209–219. doi:10.1104/pp.104.053082
Christmann A, Weiler EW, Steudle E, Grill E (2007) A hydraulic signal in root-to-shoot signalling of water shortage. Plant J 52:167–174. doi:10.1111/j.1365-313X.2007.03234.x
Claeys H, Inze D (2013) The agony of choice: how plants balance growth and survival under water-limiting conditions. Plant Physiol 162:1768–1779. doi:10.1104/pp.113.220921
Creelman RA, Zeevaart JAD (1985) Abscisic acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol 77:25–28. doi:10.1104/pp.77.1.25
Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4:472–478. doi:10.1016/s1360-1385(99)01497-1
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Ann Rev Plant Biol 61:651–679. doi:10.1146/annurev-arplant-042809-112122
Davies WJ, Kudoyarova G, Hartung W (2005) Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J Plant Growth Regul 24:285–295. doi:10.1007/s00344-005-0103-1
Dong T, Xu ZY, Park Y, Kim DH, Lee Y, Hwang I (2014) Abscisic acid uridine diphosphate glucosyltransferases play a crucial role in abscisic acid homeostasis in Arabidopsis. Plant Physiol 165:277–289. doi:10.1104/pp.114.239210
Du S-Y et al (2012) Roles of the different components of magnesium chelatase in abscisic acid signal transduction. Plant Mol Biol 80:519–537. doi:10.1007/s11103-012-9965-3
Dupeux F et al (2011) A thermodynamic switch modulates abscisic acid receptor sensitivity. EMBO J 30:4171–4184. doi:10.1038/emboj.2011.294
Endo A et al (2008) Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol 147:1984–1993. doi:10.1104/pp.108.116632
Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka P (2011) Evolution of cytokinin biosynthesis and degradation. J Exp Bot 62:2431–2452. doi:10.1093/jxb/err004
Fujii H et al (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462:660–664. doi:10.1038/nature08599
Gan SS, Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270:1986–1988. doi:10.1126/science.270.5244.1986
Gao YJ, Zeng QN, Guo JJ, Cheng J, Ellis BE, Chen JG (2007) Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Plant J 52:1001–1013. doi:10.1111/j.1365-313X.2007.03291.x
Ghanem ME et al (2011) Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 62:125–140. doi:10.1093/jxb/erq266
Gonzalez-Guzman M et al (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24:2483–2496. doi:10.1105/tpc.112.098574
Guo JJ, Zeng QN, Emami M, Ellis BE, Chen JG (2008) The GCR2 gene family is not required for aba control of seed germination and early seedling development in Arabidopsis. PLoS One. doi:10.1371/journal.pone.0002982
Hao Q et al (2011) The molecular basis of ABA-independent inhibition of PP2Cs by a subclass of PYL proteins. Mol Cell 42:662–672. doi:10.1016/j.molcel.2011.05.011
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53:27–32. doi:10.1093/jexbot/53.366.27
Haswell ES, Verslues PE (2015) The ongoing search for the molecular basis of plant osmosensing. J Gen Physiol 145:389–394. doi:10.1085/jgp.201411295
Hauser F, Waadtl R, Schroeder JI (2011) Evolution of abscisic acid synthesis and signaling mechanisms. Curr Biol 21:R346–R355. doi:10.1016/j.cub.2011.03.015
Havlova M et al (2008) The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ 31:341–353. doi:10.1111/j.1365-3040.2007.01766.x
Holbrook NM, Shashidhar VR, James RA, Munns R (2002) Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot 53:1503–1514. doi:10.1093/jexbot/53.373.1503
Hou BK, Lim EK, Higgins GS, Bowles DJ (2004) N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J Biol Chem 279:47822–47832. doi:10.1074/jbc.M409569200
Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24:1695–1708. doi:10.1101/gad.1953910
Hwang I, Sheen J, Mueller B (2012) Cytokinin signaling networks. Ann Rev Plant Biol 63:353–380
Ishitani M, Xiong LM, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: Interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9:1935–1949. doi:10.1105/tpc.9.11.1935
Iuchi S et al (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333. doi:10.1046/j.1365-313x.2001.01096.x
Jeon J et al (2010) A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J Biol Chem 285:23369–23384. doi:10.1074/jbc.M109.096644
Jin SH, Ma XM, Kojima M, Sakakibara H, Wang YW, Hou BK (2013) Overexpression of glucosyltransferase UGT85A1 influences trans-zeatin homeostasis and trans-zeatin responses likely through O-glucosylation. Planta 237:991–999. doi:10.1007/s00425-012-1818-4
Johnston CA et al (2007) Comment on “A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid”. Science. doi:10.1126/science.1143230
Jones AM (2015) A new look at stress: abscisic acid patterns and dynamics at high-resolution. New Phytol. doi:10.1111/nph.13552
Jones AM, Danielson JA, ManojKumar SN, Lanquar V, Grossmann G, Frommer WB (2014) Abscisic acid dynamics in roots detected with genetically encoded FRET sensors. Elife. doi:10.7554/eLife.01741
Kang J, Hwang JU, Lee M, Kim YY, Assmann SM, Martinoia E, Lee Y (2010) PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc Natl Acad Sci USA 107:2355–2360. doi:10.1073/pnas.0909222107
Kang NY, Cho C, Kim NY, Kim J (2012) Cytokinin receptor-dependent and receptor-independent pathways in the dehydration response of Arabidopsis thaliana. J Plant Physiol 169:1382–1391. doi:10.1016/j.jplph.2012.05.007
Kanno Y et al (2012) Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci USA 109:9653–9658. doi:10.1073/pnas.1203567109
Kant S, Burch D, Badenhorst P, Palanisamy R, Mason J, Spangenberg G (2015) Regulated expression of a cytokinin biosynthesis gene ipt delays leaf senescence and improves yield under rainfed and irrigated conditions in canola (Brassica napus L.). PLoS One. doi:10.1371/journal.pone.0116349
Kiba T, Takei K, Kojima M, Sakakibara H (2013) Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Dev Cell 27:452–461. doi:10.1016/j.devcel.2013.10.004
Kieber JJ, Schaller GE (2010) The perception of cytokinin: a story 50 years in the making. Plant Physiol 154:487–492. doi:10.1104/pp.110.161596
Ko D et al (2014) Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc Natl Acad Sci USA 111:7150–7155. doi:10.1073/pnas.1321519111
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic, San Diego
Krochko JE, Abrams GD, Loewen MK, Abrams SR, Cutler AJ (1998) (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol 118:849–860. doi:10.1104/pp.118.3.849
Kumar MN, Verslues PE (2015) Stress physiology functions of the Arabidopsis histidine kinase cytokinin receptors. Physiol Plant 154:369–380. doi:10.1111/ppl.12290
Kumar MN, Jane W-N, Verslues PE (2013) Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol 161:942–953. doi:10.1104/pp.112.209791
Kuppu S et al (2013) Water-deficit inducible expression of a cytokinin biosynthetic gene IPT Improves drought tolerance in cotton. PLoS One. doi:10.1371/journal.pone.0064190
Kuromori T et al (2010) ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci USA 107:2361–2366. doi:10.1073/pnas.0912516107
Kuromori T, Sugimoto E, Shinozaki K (2011) Arabidopsis mutants of AtABCG22, an ABC transporter gene, increase water transpiration and drought susceptibility. Plant J 67:885–894. doi:10.1111/j.1365-313X.2011.04641.x
Kushiro T et al (2004) The Arabidopsis cytochrome P450CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J 23:1647–1656. doi:10.1038/sj.emboj.7600121
Lee KH et al (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126:1109–1120. doi:10.1016/j.cell.2006.07.034
Lin PC, Hwang SG, Endo A, Okamoto M, Koshiba T, Cheng WH (2007) Ectopic expression of ABSCISIC ACID 2/GLUCOSE INSENSITIVE 1 in Arabidopsis promotes seed dormancy and stress tolerance. Plant Physiol 143:745–758. doi:10.1104/pp.106.084103
Liu XG, Yue YL, Li B, Nie YL, Li W, Wu WH, Ma LG (2007) A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science 315:1712–1716. doi:10.1126/science.1135882
Liu Z et al (2015) UDP-glucosyltransferase71c5, a major glucosyltransferase, mediates abscisic acid homeostasis in Arabidopsis. Plant Physiol 167:1659–1670. doi:10.1104/pp.15.00053
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324:1064–1068. doi:10.1126/science.1172408
Mackova H et al (2013) Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J Exp Bot 64:2805–2815. doi:10.1093/jxb/ert131
Marchadier E, Hetherington AM (2014) Involvement of two-component signalling systems in the regulation of stomatal aperture by light in Arabidopsis thaliana. New Phytol 203:462–468. doi:10.1111/nph.12813
Marshall A et al (2012) Tackling drought stress: receptor-like kinases present new approaches. Plant Cell 24:2262–2278. doi:10.1105/tpc.112.096677
Martin RC, Mok MC, Mok DWS (1999) Isolation of a cytokinin gene, ZOG1, encoding zeatin O-glucosyltransferase from Phaseolus lunatus. Proc Natl Acad Sci USA 96:284–289. doi:10.1073/pnas.96.1.284
Melcher K et al (2009) A gate–latch–lock mechanism for hormone signalling by abscisic acid receptors. Nature 462:602–608. doi:10.1038/nature08613
Merewitz EB, Du H, Yu W, Liu Y, Gianfagna T, Huang B (2012) Elevated cytokinin content in IPT transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. J Exp Bot 63:1315–1328. doi:10.1093/jxb/err372
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Ann Rev Plant Biol 56:165–185. doi:10.1146/annurev.arplant.56.032604.144046
Nishimura N et al (2009) Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science 326:1373–1379. doi:10.1126/science.1181829
Nishimura N et al (2010) PYR/PYL/RCAR family members are major in vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J 61:290–299. doi:10.1111/j.1365-313X.2009.04054.x
Nishiyama R et al (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183. doi:10.1105/tpc.111.087395
Nishiyama R et al (2013) Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc Natl Acad Sci USA 110:4840–4845. doi:10.1073/pnas.1302265110
Okamoto M et al (2006) CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol 141:97–107. doi:10.1104/pp.106.079475
Okamoto M, Kushiro T, Jikumaru Y, Abrams SR, Kamiya Y, Seki M, Nambara E (2011) ABA 9′-hydroxylation is catalyzed by CYP707A in Arabidopsis. Phytochemistry 72:717–722. doi:10.1016/j.phytochem.2011.02.004
Pandey S, Nelson DC, Assmann SM (2009) Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell 136:136–148. doi:10.1016/j.cell.2008.12.026
Park SY et al (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324:1068–1071. doi:10.1126/science.1173041
Park SY, Peterson FC, Mosquna A, Yao J, Volkman BF, Cutler SR (2015) Agrochemical control of plant water use using engineered abscisic acid receptors. Nature 520:545–548
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J 9:747–758. doi:10.1111/j.1467-7652.2010.00584.x
Peterson FC et al (2010) Structural basis for selective activation of ABA receptors. Nat Struct Mol Biol 17:1109–1113. doi:10.1038/nsmb.1898
Pierce M, Raschke K (1980) Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 148:174–182. doi:10.1007/bf00386419
Pierce M, Raschke K (1981) Synthesis and metabolism of abscisic acid in detached leaves of Phaseolus vulgaris L. after loss and recovery of turgor. Planta 153:156–165. doi:10.1007/bf00384097
Priest DM et al (2006) Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J 46:492–502. doi:10.1111/j.1365-313X.2006.02701.x
Qin XQ, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA 96:15354–15361. doi:10.1073/pnas.96.26.15354
Qin XQ, Zeevaart JAD (2002) Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol 128:544–551. doi:10.1104/pp.010663
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15:395–401. doi:10.1016/j.tplants.2010.04.006
Ren H et al (2007) Dynamic analysis of ABA accumulation in relation to the rate of ABA catabolism in maize tissues under water deficit. J Exp Bot 58:211–219. doi:10.1093/jxb/erl117
Risk JM, Day CL, Macknight RC (2009) Reevaluation of abscisic acid-binding assays shows that G-protein-coupled receptor2 does not bind abscisic acid. Plant Physiol 150:6–11. doi:10.1104/pp.109.135749
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104:19631–19636. doi:10.1073/pnas.0709453104
Rivero RM, Shulaev V, Blumwald E (2009) Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 150:1530–1540. doi:10.1104/pp.109.139378
Rivero RM, Gimeno J, Van Deynze A, Walia H, Blumwald E (2010) Enhanced cytokinin synthesis in tobacco plants expressing P-SARK:IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol 51:1929–1941. doi:10.1093/pcp/pcq143
Saab IN, Sharp RE (1989) Non-hydraulic signals from maize roots in drying soil—inhibition of leaf elongation but not stomatal conductance. Planta 179:466–474. doi:10.1007/bf00397586
Sauter A, Dietz KJ, Hartung W (2002) A possible stress physiological role of abscisic acid conjugates in root-to-shoot signalling. Plant Cell Env 25:223–228. doi:10.1046/j.1365-3040.2002.00747.x
Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874. doi:10.1126/science.276.5320.1872
Schwartz SH, Qin XQ, Zeevaart JAD (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131:1591–1601. doi:10.1104/pp.102.017921
Shang Y et al (2010) The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 22:1909–1935. doi:10.1105/tpc.110.073874
Sharma S, Verslues PE (2010) Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ 33:1838–1851. doi:10.1111/j.1365-3040.2010.02188.x
Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25:211–222. doi:10.1046/j.1365-3040.2002.00798.x
Sharp RE, LeNoble ME (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53:33–37. doi:10.1093/jexbot/53.366.33
Shen YY et al (2006) The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443:823–826. doi:10.1038/nature05176
Soon FF et al (2012) Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 335:85–88. doi:10.1126/science.1215106
Szostkiewicz I et al (2010) Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J 61:25–35. doi:10.1111/j.1365-313X.2009.04025.x
Takeuchi J et al (2014) Designed abscisic acid analogs as antagonists of PYL-PP2C receptor interactions. Nat Chem Biol 10:477–482. doi:10.1038/nchembio.1524
Tan BC, Cline K, McCarty DR (2001) Localization and targeting of the VP14 epoxy-carotenoid dioxygenase to chloroplast membranes. Plant J 27:373–382. doi:10.1046/j.1365-313X.2001.01102.x
Tan BC, Joseph LM, Deng WT, Liu LJ, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56. doi:10.1046/j.1365-313X.2003.01786.x
Tran LSP, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA 104:20623
Tsuzuki T et al (2011) Mg-chelatase H subunit affects ABA signaling in stomatal guard cells, but is not an ABA receptor in Arabidopsis thaliana. J Plant Res 124:527–538. doi:10.1007/s10265-011-0426-x
Umezawa T et al (2006) CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J 46:171–182. doi:10.1111/j.1365-313X.2006.02683.X
Umezawa T, Sugiyama N, Takahashi F, Anderson JC, Ishihama Y, Peck SC, Shinozaki K (2013) Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci Signal. doi:10.1126/scisignal.2003509
Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, Shinozaki K (1999) A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell 11:1743
Verslues PE, Bray EA (2004) LWR1 and LWR2 are required for osmoregulation and osmotic adjustment in Arabidopsis. Plant Physiol 136:2831–2842. doi:10.1104/pp.104.045856
Verslues PE, Bray EA (2006) Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J Exp Bot 57:201–212. doi:10.1093/jxb/erj026
Verslues PE, Zhu JK (2005) Before and beyond ABA: upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem Soc Trans 33:375–379
Verslues PE, Zhu JK (2007) New developments in abscisic acid perception and metabolism. Curr Opin Plant Biol 10:447–452. doi:10.1016/j.pbi.2007.08.004
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu JH, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539. doi:10.1111/j.1365-313X.2005.02593.x
Verslues PE, Bhaskara GB, Kesari R, Kumar MN (2013) Drought tolerance mechanisms and their molecular basis. In: Jenks MA, Hasegawa PM (eds) Plant abiotic stress, 2nd edn. Wiley, Ames, pp 15–46
Waadt R, Hitomi K, Nishimura N, Hitomi C, Adams SR, Getzoff ED, Schroeder JI (2014) FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. Elife. doi:10.7554/eLife.01739
Wang J, Ma XM, Kojima M, Sakakibara H, Hou BK (2011a) N-glucosyltransferase UGT76C2 is involved in cytokinin homeostasis and cytokinin response in Arabidopsis thaliana. Plant Cell Physiol 52:2200–2213. doi:10.1093/pcp/pcr152
Wang ZY, Xiong LM, Li WB, Zhu JK, Zhu JH (2011b) The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 23:1971–1984. doi:10.1105/tpc.110.081943
Wang PC et al (2013) Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc Natl Acad Sci USA 110:11205–11210. doi:10.1073/pnas.1308974110
Wang ZY, Gehring C, Zhu JH, Li FM, Zhu JK, Xiong LM (2015) The Arabidopsis vacuolar sorting receptor1 is required for osmotic stress-induced abscisic acid biosynthesis. Plant Physiol 167:137–152. doi:10.1104/pp.114.249268
Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmulling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22:3905–3920. doi:10.1105/tpc.109.072694
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210. doi:10.1046/j.0016-8025.2001.00824.x
Wilson ME, Basu MR, Bhaskara GB, Verslues PE, Haswell ES (2014) Plastid osmotic stress activates cellular stress responses in Arabidopsis. Plant Physiol 165:119–128. doi:10.1104/pp.114.236620
Wu FQ et al (2009) The magnesium-chelatase H subunit binds abscisic acid and functions in abscisic acid signaling: new evidence in Arabidopsis. Plant Physiol 150:1940–1954. doi:10.1104/pp.109.140731
Xiong L, Lee H, Ishitani M, Zhu J-K (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277:8588–8596. doi:10.1074/jbc.M109275200
Xu Z-J, Nakajima M, Suzuki Y, Yamaguchi I (2002) Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol 129:1285–1295. doi:10.1104/pp.001784
Xu ZY et al (2012) A vacuolar beta-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 24:2184–2199. doi:10.1105/tpc.112.095935
Yuan F et al (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514:367–371. doi:10.1038/nature13593
Zhang K et al (2014) Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat Commun. doi:10.1038/ncomms4274
Zhou R et al (2004) A new abscisic acid catabolic pathway. Plant Physiol 134:361–369. doi:10.1104/pp.103.030734
Zwack PJ, Rashotte AM (2015) Interactions between cytokinin signalling and abiotic stress responses. J Exp Bot. doi:10.1093/jxb/erv172
Acknowledgments
Work in the Verslues laboratory is supported by funding from Academia Sinica and the Taiwan Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verslues, P.E. ABA and cytokinins: challenge and opportunity for plant stress research. Plant Mol Biol 91, 629–640 (2016). https://doi.org/10.1007/s11103-016-0458-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0458-7