Abstract
Marine diatoms, the major primary producer in ocean environment, are known to take up both CO2 and HCO3 − in seawater and efficiently concentrate them intracellularly, which enable diatom cells to perform high-affinity photosynthesis under limiting CO2. However, mechanisms so far proposed for the inorganic carbon acquisition in marine diatoms are significantly diverse despite that physiological studies on this aspect have been done with only limited number of species. There are two major hypotheses about this; that is, they take up and concentrate both CO2 and HCO3 − as inorganic forms, and efficiently supply CO2 to Rubisco by an aid of carbonic anhydrases (biophysical CO2-concentrating mechanism: CCM); and as the other hypothesis, biochemical conversion of HCO3 − into C4 compounds may play a major role to supply concentrated CO2 to Rubisco. At moment however, physiological evidence for these hypotheses were not related well to molecular level evidence. In this study, recent progresses in molecular studies on diatom-carbon-metabolism genes were related to the physiological aspects of carbon acquisition. Furthermore, we discussed the mechanisms regulating CO2 acquisition systems in response to changes in pCO2. Recent findings about the participation of cAMP in the signaling pathway of CO2 concentration strongly suggested the occurrences of mammalian-type-signaling pathways in diatoms to respond to changes in pCO2. In fact, there were considerable numbers of putative adenylyl cyclases, which may take part in the processes of CO2 signal capturing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine diatoms are the most successful group of algae and their photosynthesis in the oceans is known to be an extremely important component of global element cycles (Tréguer et al. 1995; Field et al. 1998; Falkowski et al. 1998, 2000, 2004). The mechanisms of uptake of dissolved inorganic carbon (DIC) and subsequent control of the intracellular flux of CO2 to the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) are the critical steps of algal primary production particularly in seawater conditions. Because the CO2-bicarbonate equilibrium favors hydration in the high salinity and pH of seawater, so that spontaneous CO2 formation occurs very slowly (one eighth to a quarter of that in freshwater at the same pH) (Matsuda et al. 2001; Chen et al. 2006). Moreover, the gaseous CO2 that can be dissolved in water is extremely limited (up to 15 μM at 20°C) and the diffusion rate of the dissolved molecule is about 10−4 of that of gaseous molecule (Raven 1994). The affinity of Rubisco protein in diatoms is known to be insufficient to support sustained high photosynthetic rates from dissolved CO2 at current atmospheric pressure. Marine diatoms as well as other microscopic algae have overcome these difficulties and minimize the occurrence of photorespiration under atmospheric level of pCO2 by concentrating saturating levels of CO2 in close proximity to Rubisco in the chloroplast even in a CO2-limited environment. These concentrating mechanisms are generally called CO2-concentrating mechanisms (CCMs), which are believed to include CO2 and HCO3 − transport systems (Colman and Rotatore 1995, 1988; Rotatore and Colman 1992; Rotatore et al. 1995; Johnston and Raven 1996; Matsuda et al. 2001), intra- and extracellular carbonic anhydrases (CAs) (Iglesias-Rodriguez and Merrett 1997; Satoh et al. 2001), and possibly C4 type biochemical pathways in diatoms (Reinfelder et al. 2000; Reinfelder et al. 2004; Roberts et al. 2007; McGinn and Morel 2008). Recently, algal type carbon-concentrating mechanism was expressed in a discriminable way, biophysical CCM to contrast to biochemical system of C4 (Kroth et al. 2008). However, CO2 acquisition mechanisms in marine photoautotrophs have not been defined at the molecular level and little is known so far about genetic control of the physiological aspects of photosynthetic activities in any diatom species. It should also be noted that the intracellular structure in diatoms is relatively complicated as compared to that in Chlorophyta due to the evolutionary history of multiple endosymbiosis (Gibbs 1981; Ludwig and Gibbs 1985; McFadden and Gilson 1995; Falkowski et al. 2004); that is, a 4-layered membrane system surrounds the diatom chloroplast and is a major structural obstacle for the movement of substrate. It is thus extremely important to relate any candidate molecules for the CO2 concentrating mechanism to physiological function with their topological properties.
Another intriguing aspect of CO2 acquisition system in diatoms is the mechanisms to regulate high-affinity photosynthesis in response to ambient CO2. Marine diatom species apparently possess systems to respond to environmental pCO2 to control the CO2 acquisition efficiency (Matsuda et al. 2001; Burkhardt et al. 2001). Such acclimating systems are commonly observed in freshwater microalgal species and have been extensively studied in cyanobacteria and the green alga, Chlamydomonas reinhardtii in which regulation takes place primarily at the transcriptional level (Marcus et al. 1983; Mayo et al. 1986; Miller et al. 1990; Colman and Rotatore 1995; Matsuda and Colman 1995a; Rawat and Moroney 1995; Matsuda et al. 2001; Kucho et al. 1999, 2003; Vance and Spalding 2005). However, the regulatory systems seem to vary considerably between species, which may indicate that they have been originated independently. Parts of the CO2 responsive signaling system have been elucidated in cyanobacteria (Omata et al. 2001; Wang et al. 2004; Woodger et al. 2007; Nishimura et al. 2008; Price et al. 2008) and in C. reinhardtii (Fukuzawa et al. 2001; Xiang et al. 2001; Miura et al. 2004; Wang et al. 2005; Kohinata et al. 2008; Yamano et al. 2008) but the mechanism for sensing ambient pCO2 remains totally unknown in all photoautotrophs. In the marine diatom, Phaeodactylum tricornutum, the participation of cAMP in the CO2 signaling process has been demonstrated (Harada et al. 2006), and resembled the CO2 response systems in animals (Buck et al. 1999; Chen et al. 2000), but the molecules mediating this signaling are yet to be determined. It is extremely important to know how photoautotrophs in the marine environment respond to increments of pCO2 and what the differences are from species to species in the mode of regulation.
In this review, recent progress in molecular studies on carbon acquisition, genetic regulation of putative CO2 acquisition systems and CO2 signal transduction system in marine diatoms will be related to the previously reported physiological data on photosynthetic carbon acquisitions and assimilations in marine diatoms.
Carbon acquisition in marine diatoms
A significant body of evidences has been accumulated about the occurrences of active uptake of HCO3 − and CO2 in both pennate and centric marine diatoms. The list in the Table 1 shows the results from the past literatures, all which employed relatively direct and thus reliable measures of DIC accumulation (silicon-oil centrifugation: SOC) or steady-state Ci flux (membrane-inlet mass spectrometry: MIMS; isotopic disequilibrium technique: IDT). Given the information in the Table 1, it is clear that these species of diatom can take up both CO2 and HCO3 − from the surrounding media, although there are significant species-dependent differences in preferences of the Ci species taken up. For example, P. tricornutum was shown to transport CO2 almost twice as fast as HCO3 − when cells were fully acclimated to atmospheric pCO2 whereas Thalassiosira weissflogii transported CO2 and HCO3 − at equivalent rates (Burkhardt et al. 2001). Examples of extreme substrate preference are given by Trimborn et al. (2008) who demonstrated that the marine diatom, Stellarima stellaris almost exclusively takes up HCO3 − whereas another, Nitzschia navis-varingica mainly takes up CO2. The mode of Ci use is thus quite diverse among diatoms and such diversity of Ci use is also observed in many microalgae (Colman et al. 2002). The selective use of CO2 may simply be an adaptive response to the energy cost required to transport HCO3 − across plasmalemma, which is much more expensive than that of CO2. For cells occurring in microenvironment relatively rich in CO2, it would be a significant advantage to develop mechanisms which enable an efficient utilization of CO2. Such environments can be expected in brackish water and shallow coastal waters for example. In contrast, HCO3 − users have adopted a high cost system to use of HCO3 −, which is much more abundant in seawater and for which there is less competition particularly under severe CO2 limitation, for instance during bloom.
External CAs, located in a periplasmic space, have been thought to greatly enhance CO2 use. However, recent studies based upon gene silencing technology have revealed that external CAs are not essential. Despite the fact that the green alga C. reinhardtii uses CO2 preferentially and occurrence of external CA significantly stimulates photosynthetic affinity, a knock-out of the external CA, CAH1, did not confer on cells any high-CO2 requiring phenotype (Van and Spalding 1999). For massive HCO3 − users, it is also pointed out that the occurrences of external CAs might work rather to deplete HCO3 − from its transporter (Colman et al. 2002). There would be significant differences in requirement for fast inter-conversion of Ci species at the extracellular space between freshwater and marine species considering of the high salinity and alkalinity of seawater, and importance of extracellular CAs are still under controversy in marine system. Molecular-based evidence of localization and function are waited to be obtained as these diversities of preferences for Ci species and efficiency of Ci inter-conversion at extracellular space in diatom species would be factors govern their survivability under changing environment in the ocean.
Unicellular C4-like photosynthesis pathway, has been proposed in the marine diatom, T. weissflogii (Reinfelder et al. 2000; Reinfelder et al. 2004; Roberts et al. 2007; McGinn and Morel 2008) as another mechanism of CO2 acquisition. Since, the absence of a C4 pathway has been demonstrated in two other marine diatoms, P. tricornutum and Thalassiosira pseudonana (Holdsworth and Colbeck 1976; Roberts et al. 2007), the occurrence of C4-type photosynthesis might be limited to particular species. It is also interesting that T. weissflogii besides initially fixing CO2 to the C4 compound, still actively takes up both CO2 and HCO3 − at equal rates (Table 1) (Burkhardt et al. 2001). Similar physiological aspects of mixed C3 and C4 metabolism were also reported in diatoms, dinoflagellate and Chrysophyte (Beardall et al. 1976). Radio tracer experiment has been performed extensively to figure out the initial carboxylated compound in phytoplankton species including diatoms from late 1970s to early 1980s (Beardall et al. 1976; Holdsworth and Colbeck 1976; Coleman and Colman 1981). Coleman and Colman (1981) demonstrated that β-carboxyl of C4 acid always significantly radio labeled in sub-second period of labeling in cyanobacteria despite that the cells lack in efficient decarboxylation activity of C4 acid or regeneration of phosphoenolpyruvate and cyanobacteria revealed the massive biophysical CCM. These studies point out that the level of radio-labeled-C4 acids approached almost equivalent amount to 3-phosphoglyceric acid. This strongly suggests that radio tracer data need a prudent attention to interpret in this type of study. Probably, CO2 fixing system in marine phytoplanktons needs to be further studied with extensive support of molecular data on localization of carboxylation and decarboxylation enzymes together with localization data of a biophysical carbon flow controller, CAs.
Diatoms seem to have obtained a redundant set of carboxylation and decarboxylation enzymes, which could potentially constitute C4-type pathway when localized in the proper cellular loci and may have arisen from multiple origins during complicated endosymbiosis events, including lateral-gene transfer (LTG) (Montsant et al. 2005; Bowler et al. 2008; Moustafa et al. 2009). It may be possible that only cells which could not supply enough CO2 to Rubisco by a Ci flux control with biophysical CCM alone, underwent severe selection pressure to acquire additional processes for CO2 accumulation. In this respect, it is interesting to consider cell size and characteristics of the chloroplast in diatom cells; T. weissflogii is a relatively large centric cell (up to 40 μm diameter) whereas T. pseudonana and P. tricornutum are significantly smaller (up to 5–10 μm) (Fig. 1). Diatom cells sometimes reach an order of several hundred micrometers to millimeters in size and such a large cell size might be significant barrier for diffusion of DIC inside the cells, which is a function of square distance, and hence might require additional fixation step. The ellipsoidal shape of pennate diatoms might be advantageous to minimize the distance between the plasmalemma and the chloroplast and centric diatoms tend to possess numerous chloroplasts in one cell (Fig. 1), whereas pennate diatoms have mostly 1–2 chloroplasts and the shapes of diatom chloroplasts are very diverse. In the evolution of Chlorophyceae to higher plant, there seems to be a moderate correlation between the loss of the CCM with the large chloroplast and the acquiring of multiple small chloroplasts in relatively large mesophyll cells (Fig. 1). The reason why they lost the CCM is not known. However, land plants were exposed to much higher pCO2 at the begging of evolutional history and then start starving for CO2 by steep decrease of CO2 and increase of O2, which was a major drive force for land plants to develop C4 metabolism in requirement of suppression of photorespiration. Analogous evolutionary event might have taken place in the marine environment without losing biophysical CCM. However, there are no reports describing a “coevolution” of diatom chloroplast and metabolisms, which supported relationships between CO2 acquisition systems and cell size. Cell size evolution of diatoms are known to be significantly affected by nitrogen and phosphorous limitation, and mixing intensity of water (Litchman et al. 2009) although it is still a mystery why diatom cells had to undergo such an extreme range of diversification in cell size.
Correlations of cell size and number of chloroplast per cell in centric diatoms. Maximum diameters of valve and number of chloroplast per cell of 25 centric diatoms were correlated. Filled black diamond, Actinocyclus octonarius; open square, Aulacoseira sp.; open triangle, Chaetoceros sp.; filled black circle, 2 Coscinodiscus sp.; filled black triangle, 6 Cyclotella sp.; open diamond, Ditylum brightwelli; filled grey square, Hydrosera whampoensis; filled grey diamond, Melosira varians; filled grey triangle, Pleurosira laevis; filled grey circle, Skeletonema sp.; open circle, 4 Stephanodiscus sp.; filled black square, 4 Thalassiosira sp.. For comparison, roughly estimated cell diameters of open inverted triangle, Chloroella sp.; plus, Chlamydomonas reinhardtii; asterisk, Nicotiana tabacum (protoplast of mesophyll cell); times, Arabidopsis thaliana (protoplast of mesophyll cell) were also plotted
It is important to consider the essential differences between biophysical CCM and C4. Both can work for concentrating CO2, but the biophysical CCM maintains carbon in inorganic state all way from uptake to fixation by Rubisco. This process is supported by one of the fastest enzymes, CA, and DIC is highly permeable molecule in a state of CO2. These characteristics indicate that the CCM can be a highly reversible process which works for both concentrating and efflux of inorganic carbon. Probably these two states could be switched almost instantaneously depending on the physiological state of cells. As most biophysical CCMs require light energy to operate, such reversible control would be useful for energy dissipation as previously pointed out by Tchernov et al. (1997). In contrast, the C4 system would be much slower system, in which, once HCO3 − was fixed within oxaloacetate, it would stay stable until consumption in the TCA cycle in mitochondria or decarboxylation in the chloroplast, processes which might be suitable for large sized cells.
CAs in diatom and their localization
Carbonic anhydrases are categorized into 6 subtypes from α to ζ (Tripp et al. 2001; So et al. 2004; Lane et al. 2005). The α-CAs are found predominantly in animals but also occur in bacteria, higher plants and green algae, the β-CAs are known to be abundant in plants, green algae, eubacteria and archaea, and the γ-CAs may be the most ancient form of carbonic anhydrases, having evolved long before the α class and predominant in bacteria (Tripp et al. 2001). Eukaryotic algae have been reported to possess all these three “traditional” CAs (Satoh et al. 2001). Interestingly, among diatoms, β-type CA has only been reported in the pennate diatom P. tricornutum but there is no homologue in the genome of the centric diatom, T. pseudonana (Montsant et al. 2005; Tachibana et al. 2011). Although, molecular studies on CAs in marine diatoms are, as yet, quite limited, some very interesting characteristics have been reported. In the centric marine diatom, T. weissflogii, two unique types of CA have been reported; that is, δ- and ζ-type CAs, respectively, designated TWCA1 and CDCA1, were identified as CAs (Roberts et al. 1997; Lane and Morel 2000, Lane et al. 2005; Xu et al. 2008). ζ-type CA was already identified at the molecular level and crystal structure was defined (Xu et al. 2008), on the other hand direct quantitative evidence of δ-CA activity is not obtained clearly yet. ε-CA was firstly identified in the chemolithotrophic bacterium Halothiobacillus neapolitanus as CsoS3/CsoSCA as a component of the carboxysomal shell and csoS3 homologue genes were also found in cyanobacteria and chemolithotrophic bacteria (So et al. 2004). The crystal structure of ε-CA is similar to β-type CAs despite its unique primary amino acid sequence (Sawaya et al. 2006). CsoS3/CsoSCA is essential for the operation of the CCM in H. neapolitanus because of its role in dehydrating HCO3 − at the carboxysomal shell to supply CO2 to Rubisco within the carboxysome (Dou et al. 2008). Discovery of these novel examples of convergent evolution revealed strong possibilities of CAs that have evolved from an unexpectedly wide variety of origins and function in marine autotrophs. Moreover, cofactor of diatoms’ ζ-CAs was reported to be substituted by Cd when Zn is not available (Xu et al. 2008), strongly suggesting their competence under metal depleted environment.
As described in the previous section, the operation of DIC transport as a basal system to ensure CO2 supply to photosynthesis in marine environment in diatoms seemed to be almost unequivocal and CAs must be the critical component to control inorganic carbon fluxes. In contrast to the uncertainty about the role of external CAs, the importance of intracellular CAs has been demonstrated at molecular levels in C. reinhardtii; a lumenal-type α-CA, CAH3 was shown to be a critical factor for cells to survive under atmospheric CO2 condition (Funke et al. 1997; Karlsson et al. 1998) and this CA is believed to supply CO2 to Rubisco in the thylakoids penetrating the pyrenoid structure, using the lumenal acidity during active photosynthesis (Raven 1997). The importance of internal CAs for the operation of high-affinity photosynthesis in diatoms was also demonstrated previously at physiological levels in P. tricornutum; that is, highly permeable CA inhibitor, ethoxyzolamide (EZA) severely suppressed the operation of high-affinity photosynthesis, whereas weakly permeable inhibitor, acetazolamide (AZA) had little effect on photosynthetic affinity (Satoh et al. 2001). Since CA catalyzes the fairly simple hydration/dehydration reactions between CO2 and H2CO3, depending on their location and pH of their occurrence, they could catalyze the reaction in either direction, so that it is crucial to know the localization of CAs to estimate their functions.
The localization and functional aspects of diatom CAs have been most extensively investigated in the pennate diatom, P. tricornutum (Tanaka et al. 2005; Kitao et al. 2008; Kitao and Matsuda 2009; Tachibana et al. 2011) and the centric diatom, T. pseudonana (Tachibana et al. 2011). It is interesting that there are five α-, two β-, and two γ-type CA candidates in the genome of P. tricornutum and these subclasses are localized in specific organelles depending on the subtype (Tachibana et al. 2011). That is, all the putative α-CAs were localized in the four-layered chloroplast envelopes, two β-CAs were within the stroma, and two putative γ-CAs were localized (or predicted to be localized) in the mitochondrion (Tanaka et al. 2005; Kitao et al. 2008; Tachibana et al. 2011). It is of particular interest that the four-layered chloroplast membrane system held all five putative α-CAs, and that these genes were expressed independently of CO2 concentration and light, except that one putative CA gene was repressed in the dark (Tachibana et al. 2011). This strongly suggests that CAs in the complicated membrane systems may work constitutively to maintain efficient permeation of Ci across the cytoplasm and the stroma, and in efflux direction of CO2 from the stroma this membrane system could be an efficient leakage proof by recapturing the leaking CO2 by CAs. The only CO2 responsive CAs were two β-type CAs, PtCA1, and PtCA2 (Tachibana et al. 2011), both of which were derepressed under low CO2 conditions (Harada and Matsuda 2005; Harada et al. 2005) and PtCA1 which turned out to be a pyrenoidal CA (Tachibana et al. 2011). Our preliminary study indicated that these β-CAs are under redox regulation mediated by thioredoxins (in preparation), indicating the operation of these CAs under low CO2 and light in the pyrenoid. In the genome of T. pseudonana, there were 13 CA candidates (3 α-, 5 γ-, 4 δ-, 1 ζ-types), but most of them are yet to be localized. At present, only two CA candidates, an α- and a ζ-type, have been localized, at the stroma and the cell surface, respectively, (presumably in the periplasmic space). Detailed data for localizations and transcriptional controls of the diatom CAs are available in Tachibana et al. (2011).
CAs as pyrenoid forming factor and flux control of Ci in the chloroplast
As described above, two β-type CAs, PtCA1, and PtCA2, in P. tricornutum were likely the CAs most closely localized with Rubisco in the pyrenoid and most probably function under low CO2 in the light (Tachibana et al. 2011). This is the first piece of molecular evidence to show the occurrence of CA in the pyrenoid, although this localization has been assumed for long time (Pronina and Semenenko 1984; Kuchitsu et al. 1991). Our previous studies demonstrated that both PtCA1 and 2 were localized in the stroma forming large clumped particles (Tanaka et al. 2005; Kitao et al. 2008). This clumped particle formation was clearly demonstrated to be driven by the function of an amphipathic helix in the C-terminal ends of PtCA1 and 2 in which 5 hydrophobic residues appeared every 3 amino acids and thus cluster at one side of the helix (Kitao and Matsuda 2009). The same structural motif also exists in PtCA2, the other clump forming chloroplastic β-CA (Kitao and Matsuda 2009). Presumably, these two paralogous CAs form complex together in the pyrenoid. In fact, it is known that purified PtCAs formed large fragile complex in vitro (Kitao and Matsuda 2009). However, other interaction partners of PtCAs in vivo are not known at any level. Also, there is no information on whether or not PtCAs interact with Rubisco protein in the pyrenoid of eukaryotes, in contrast to the information about the cyanobacterial carboxysome, in which CA and Rubisco directly interact to form the carboxysome shell (So et al. 2004). It is noteworthy that the location of PtCA1 was likely limited to the central part of the lens-shaped pyrenoid (Tachibana et al. 2011). This may suggest that there is a layered structure consisting of different protein components in the diatom pyrenoid. Relating to this, it should be noted that two low-CO2 inducible proteins LCIB and LCIC form heteromeric hexamer and are localized apparently surrounding the pyrenoid structure in C. reinhardtii (Yamano et al. 2010). It was pointed out by Yamano et al. (2010) that LCIB homologues are widespread among some cyanobacteria, the Chlorophyta, and Heterokontophyta, and they concluded that LCIB/C are part of a general mechanism to supply CO2 inside the pyrenoid under moderately limited CO2 conditions. The proposed mechanism of the LCIB/C complex in C. reinhardtii was to shield the pyrenoid from CO2 leakage or was a part of recapturing system for leaking CO2 from the pyrenoid in cooperation with stromal CA, CAH6 (Mitra et al. 2004; Yamano et al. 2010). The model needs to be modified if it is applied to diatoms since there is neither stromal CA outside the pyrenoid nor lumenal CA in the thylakoid in P. tricornutum. Presumably, DIC taken up by transporters is kept predominantly as HCO3 − in the cytosol due to the absence of cytosolic CA. HCO3 − would then pass through the 4-layered membrane of the chloroplast with the aid of CAs in the membrane matrix and enter into the stroma as HCO3 − due to alkaline pH there in the light. HCO3 − might be captured by an LCIB-family protein and converted to CO2 only at the central part of the pyrenoid. Although our preliminary study revealed the occurrence of a HCO3 − transporter on plasmalemma (in preparation), transport systems for HCO3 − and CO2 which are the counterpart of CAs and ensure the flow of Ci as described above in diatoms are not studied. Also pHs inside the chloroplastic membrane system are unknown but are critical factors in this model.
Transcriptional control in response to CO2 in diatoms
An intriguing aspect of algal CO2 acquisition systems is their transcriptional control in response to changes in pCO2. Physiological evidence of CO2 response of the CCM has been reported in cyanobacteria and green algae since the early 1980s (Marcus et al. 1983; Mayo et al. 1986; Sültemeyer et al. 1991; Matsuda and Colman 1995a, b; Dionisio-Sese et al. 1990). In cyanobacteria, the critical controlling factor for overall CCM activity has been shown to be the CO2/O2 ratio (Marcus et al. 1983) and total DIC rather than pH or individual DIC species in the medium (Mayo et al. 1986). In contrast, in the green algae, Chlorella ellipsoidea and C. reinhardtii, it was demonstrated quantitatively that the critical Ci species for CCM regulation was CO2 in the medium (Matsuda and Colman 1995b; Bozzo and Colman 2000). Bicarbonate and total DIC in the medium were not critical determinants for the extent of CCM expression in these studies, and neither internal DIC concentration nor light was correlated with CCM expression levels in C. ellipsoidea (Matsuda and Colman 1995b). The results of these investigations strongly suggested the accumulation of some metabolites, upon exposure of high-CO2 grown cells to air, would be a primary mediator of the CO2 signal in cyanobacteria and 2-phosphoglycolate (2-PG) was proposed to be the candidate molecule (Nishimura et al. 2008). The CO2 response mechanism in eukaryotic algae is not necessarily dependent on metabolite feedback but also includes more direct mechanisms (Matsuda and Colman 1995b).
In cyanobacteria, in fact, a transcription factor of Synechocystis sp. PCC 6803, CmpR, which is the LysR family inducing factor of HCO3 − transporter operon cmpABCD, was demonstrated to bind directly to the promoter region of the cmpABCD operon and binding was significantly stimulated in the presence of a physiological level 2-PG (Omata et al. 2001; Nishimura et al. 2008). It has also been suggested recently that other light-dependent carbon metabolites such as RuBP are also possible candidates for the CmpR activation factor (from the discussion in the CCM7 Symposium). In the marine cyanobacterium, Synechococcus sp. PCC7002, the CO2-responsive genes, sbtA, ndhF3/ndhD3/chpY/orf133, bicA/nhaS3/mnhD1/mnhD2/mnhB, and porB, are also repressed by binding of CcmR/NdhR to these promoters under high CO2 conditions (Woodger et al. 2007; Price et al. 2008). These data indicate that there is both low-CO2 inducible and high-CO2 repressive regulations in CCM-related gene transcription in cyanobacteria.
In eukaryotes on the other hand, most of the molecular work on transcriptional controls of putative CCM components have been done with C. reinhardtii. The promoter region of the periplasmic CA, Cah1 was regulated bidirectionally in response to changing CO2 concentrations and regulation was done by tandemly aligned enhancer and silencer regions on the promoter (Kucho et al. 1999). This regulation was known to be under the control of a zinc finger protein, CCM1/CIA5 and this protein was found to govern the upregulation of most of the low CO2-inducible genes and thus considered to be a master regulator of CO2 responsive gene expressions (Fukuzawa et al. 2001; Xiang et al. 2001; Miura et al. 2002, 2004; Wang et al. 2005; Yamano et al. 2008). However, the key factor, which modulate the activity of such regulator, by capturing the signal of CO2 is not known in eukaryotic algae.
The control of the expression of high-affinity photosynthesis in response to changes in ambient [CO2] are also observed in many marine eukaryotic photoautotrophs (Colman and Rotatore 1995; Matsuda and Colman 1995a; Johnston and Raven 1996; Badger et al. 1998; Sültemeyer 1998; Matsuda et al. 2001; Colman et al. 2002). In P. tricornutum, it has been shown that CO2 concentration in the medium is correlated with the degree of expression of high-affinity photosynthesis (Matsuda et al. 2001). Molecular studies on the mechanisms of CO2 response in marine diatoms has been done most extensively with P. tricornutum by using the ptca1 gene, which encodes the pyrenoidal CA (Tachibana et al. 2011), PtCA1 and is known to be markedly derepressed at the transcriptional level in response to a decrease in [CO2] (Harada et al. 2005, 2006). Interestingly, GUS reporter assay of the promoter region of the ptca1 (Pptca1) revealed that a relatively short sequence up to 100 bp upstream the transcription-start site played a key role in CO2-responsive regulation of transcription and that this region was found to be rich in animal-type cAMP-response elements, CREs, p300-binding element (Harada et al. 2006), and additionally a putative Skn-1 binding element (unpublished data). Indeed, it was shown that the Pptca1 was never derepressed under CO2-limiting conditions in the presence of the cAMP analogue, dibutyryl cAMP (dbcAMP) (Harada et al. 2006) but this effect of dbcAMP disappeared by impairing the sequences around CREs and the p300-binding element (Harada et al. 2006). These results strongly suggest that the CO2 signal is mediated by cAMP as a second messenger in marine diatoms.
CO2 sensing in diatoms
Perception of the inorganic carbon signal via cAMP is likely a widespread system in living organisms from cyanobacteria and fungi to mammals, except that there is no direct evidence for function of cAMP in higher plants. It was demonstrated in rat testis that the activity of soluble adenylyl cyclase (sAC) is regulated by HCO3 − and pH, functioning as a HCO3 − sensor and this sAC was evolutionarily close to bacterial-type sAC (Chen et al. 2000). Similarly in the pathogenic fungus, Candida albicans, filamentation of hyphae was found to be directly regulated by the activity of sAC in response to CO2 or HCO3 − (Klengel et al. 2005). The same group of sACs was also found in cyanobacteria. In Anabaena sp. PCC7120, the activity of sAC, CyaB1, was shown to be stimulated by HCO3 − (Cann et al. 2003). It was also shown in Synechocystis sp. PCC6803, that sAC, Cya1, was regulated directly by CO2 rather than HCO3 − (Hammer et al. 2006). However, sACs have not so far been related to CCM regulation in photoautotrophs, but rather has been shown to be related to blue light responses (Iseki et al. 2002; Terauchi and Ohmori 2004), mating system (Quarmby 1994), and phasing of cell division (Carré and Edmunds 1993) in cyanobacteria and green algae.
In the genome of P. tricornutum, there are three candidates for CO2/HCO3 −-activated ACs; one as soluble AC, which possess two catalytic domains, C1 and C2, and two as transmembrane-type ACs (tmAC1 and tmAC2) (Fig. 2), which we designated, respectively, as PtsAC (C1 and C2), PttmAC1 and PttmAC2. Phylogenetic analysis by the Neighbor-Joining method and sequence alignment of putative catalytic domains of various CO2/HCO3 −-activated/inactivated ACs from other origins are shown, respectively, in Fig. 2. PtsAC and PttmAC2 were grouped with ACs, which have been demonstrated to be activated by CO2/HCO3 − in mammals, bacteria, and fungi (Fig. 2a). PttmAC1 were independently grouped together with two tmAC candidates from T. pseudonana, TptmAC1 and TptmAC2 (Fig. 2a), all of which possessed reasonably conserved active site sequences (Fig. 2b). A ClustalW alignment of the catalytic domains of five putative diatom ACs with those of a number of prokaryotic and eukaryotic ACs showed that the active site amino acids involved in substrate definition for ATP (Lys646; numbering as for Anabaena CyaB1) (Fig. 2b, asterisk), divalent metal ion coordination (Asp650) (Fig. 2b, solid wedge), and transition state stabilization (Asn728, Arg732) (Fig. 2b, closed circles) (Cann et al. 2003; Masuda and Ono 2005) were reasonably conserved although some amino acids were substituted (Fig. 2b). Thr721, a residue essential for ATP binding and full catalysis in Anabaena CyaB1 (Kanacher et al. 2002) was conserved in PttmAC1, 2 and two TptmAC1 and TptmAC2 from T. pseudonana (Fig. 2b, open circle). PtsAC C2 domain possessed Ala instead of Thr (Fig. 2b) but it was shown by Cann et al. (2003) that the stimulating effect by HCO3 − on Anabaena CyaB1 was retained when Ala was substituted for Thr721 (Fig. 2b, Anabaena CyaB1 T721A). Asp substituted for Thr721 in Anabaena CyaB1 caused a loss of stimulation of activity by HCO3 − (Cann et al. 2003) (Fig. 2b, Anabaena CyaB1 T721D), and ACs, which possess Asp at the corresponding site, are known to be CO2/HCO3 − insensitive (Cann et al. 2003) (Fig. 2b). These data strongly suggest the possibility of occurrence of CO2/HCO3 − sensitive soluble and transmembrane ACs in these two diatoms.
Sequence analyses of diatom ACs. a The phylogenetic tree was constructed using multiple alignments of P. tricornutum ACs and ACs in several origins as follows: Anabaena spirulensis CyaA (Protein database, PDB ID, P43524); A. spirulensis CyaB1 (Genbank ID, BAA13998); A. spirulensis CyaB2 (Genbank ID, BAA13999); A. spirulensis CyaC (Genbank ID, BAA14000); Anopheles gambiae AC (Genbank ID, XP_001237598); Chloroflexus aurantiacus Chlo1066 (Genbank ID, ZP_00018085); C. aurantiacus Chlo1187; (Genbank ID, ZP_00018205); C. aurantiacus Chlo1431 (Genbank ID, ZP_00018442); Cryptococcus neoformans Cac1 (Genbank ID, AAG60619); Dictyostelium discoideum SgcA (Genbank ID, AAK92097); Spirulna platensis CyaC (Genbank ID, BAA22997); Stigmatella aurantiaca CyaA (Genbank ID, CAA11549); S. aurantiaca CyaB (PDB ID, P40183); Synechosystis sp. PCC6803 Cya1 (Genbank ID, NP_441200); Synechocystis sp. PCC6803 CyaA2 (Genbank ID, BAA16969); Mycobacterium leprae AC (Genbank ID, CAA19149); Mesorhizobium loti Cya3 (Genbank ID, BAB50205); Rattus norvegicus sAC (Genbank ID, AAD04035); Mus musculus sAC (Genbank ID, NP_766617); Plasmodium falciparum AC β (Genbank ID, NP_704518); Oryctolagus cuniculus sAC (Genbank ID, AAO38673); Mycobacterium Rv1264 (Genbank ID, NP_215780); Mycobacterium Rv1319c (Genbank ID, NP_215835); Homo sapiens tmAC4 (Genbank ID, AAM94373); Bos taurus tmAC1 (Genbank ID, AAA79957); Rattus norvegicus tmAC3, M55075; Mus musculus tmAC9, CAA03415; P. tricornutum sAC (Genbank ID, XP_002177591); P. tricornutum tmAC1 (Genbank ID, XP_002185871); P. tricornutum tmAC2 (Genbank ID, XP_002186366); T. pseudonana tmAC1 (Genbank ID, XP_002289271); T. pseudonana, tmAC2 (Genbank ID, XP_002293237). * indicates the CO2/HCO3 − sensitive ACs and black boxes shows the ACs from marine diatoms. b Amino acid sequence alignment of the catalytic domains of ACs from various species. Amino acid residues important for enzyme activity are indicated by bold characters and symbols; residues for, asterisk, substrate definition; filled inverted triangle, divalent metal ion coordination; open circle, substrate definition and full catalysis of ACs, filled circle, transition state stabilization. Numbers of amino acid between two domains are indicated in parenthesis. Presence or absence of Ci dependency is indicated at the right of the alignment
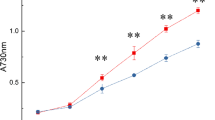
We tested the effects of DIC on AC activity in P. tricornutum lysate. Cells grown under air conditions were disrupted by sonication and the crude lysate separated into supernatant and pellet by centrifugation. The resulting crude lysate, supernatant, and pellet fractions were subjected to AC activity measurement using ELISA method in the reaction medium strongly buffered by 50 mM Tris–HCl (pH 7.0) in the presence or absence of 40 mM NaHCO3. As a result, the activities of ACs in all fractions increased to 2–7 fold in the presence of NaHCO3 compared to that in the absence of NaHCO3 (Fig. 3a), strongly suggesting the occurrence of CO2/HCO3 − sensitive soluble and transmembrane ACs in P. tricornutum. However, critical Ci-dependent AC molecules have not so far been identified and these ACs activities are yet to be related to CCM regulation in diatoms. Three candidates of Ci dependent ACs in P. tricornutum seemed to be expressed constitutively in the cells independent of CO2 concentrations in the inflow air (Fig. 3b).
Measurement of AC activities in lysate of P. tricornutum and occurrences of three AC transcripts in P. tricornutum. a Activities of ACs in crude lysate, supernatant and pellet in the presence and absence of NaHCO3. Each sample was mixed with reaction mixture containing 50 mM Tirs-HCl (pH7.0), 5 mM MnCl and 0.2 mM IBMX. The reaction was started by adding to 5 mM ATP and with or without 40 mM NaHCO3 at 30°C for 30 min. Reaction was stopped by boiling the reaction mixture for 5 min, centrifuged and the amount of cAMP in supernatant was measured by ELISA method. White bars and grey bars indicate the activity in the absence and the presence of NaHCO3, respectively. Values are mean ± SD of three separate experiments. b Analysis of transcript levels of PtsAC, PttmAC1, and PttmAC2 by semi-quantitative RT-PCR. Total RNA samples were isolated from P. tricornutum cells grown under 5% CO2 (H) or air (A) conditions. One μg of total RNA was reverse-transcribed using Oligo(dT)20 primer and reverse transcriptase to form single strand cDNA. Semi-quantitative RT-PCR was carried out with a set of specific primer of each gene. The glyceraldehydes-3-phosphate dehydrogenase gene, gapC2 was used as an internal control
Our previous studies using P. tricornutum gave the first evidence for algal CCM regulation via CO2 signal transduction mediated by cAMP (Harada et al. 2006). Considering the relatively common occurrence of the CO2 sensing mechanism in heterotrophs by direct control of sAC activity as described above and the preliminary data shown in Figs 2 and 3, it is possible that an analogous system is present for the regulation of the CCM and other CO2 responsive processes in marine diatoms. However, it is also true that CCM expression and transcriptional control of the Pptca1 are highly dependent on light as well as CO2 (Harada et al. 2005). This strongly suggests the existence of a system in diatoms which enables the crosstalk between the CO2-responsive cAMP signaling pathway, and that which respond to light, although there is no molecular evidence for such an interaction in diatoms at present.
Concluding remarks and perspectives
The entry of CO2 to the cells of photoautotrophs is the first critical step to maintain their productivity. The molecular mechanisms behind this process must be of very diverse forms among cyanobacteria, eukaryotic algae, higher plants, and vary depending upon their habitat. Marine diatom is one of the most intriguing organisms to study this aspect. In this review, we pointed out that inorganic carbon uptake occurs in all marine diatoms so far tested despite the contradictory on the mechanisms of subsequent carbon metabolism, C3 or C4. Analogously to the land plant evolution, diatom cells also have experienced the evolutionary diversification in cell size, which is apparently accompanied by multiplication of plastid. Carbon acquisition metabolism might also be evolved concomitantly with such morphological evolution which would be a future topic of interest in this field. Besides substantial diversity of mechanisms in eukaryotic CCMs, there seems to be a common biochemical component of the pyrenoid, that is, LCIB/C homologues of Chlamydomonas seems generally occur as a common frame structure of the pyrenoid of eukaryotic algae. Probably, very diverse biochemical component cooperate to LCIB/C orthologous system to constitute the algal CCMs, which is an apparently highly convergent system has incorporated many components from different origins. One of the most marked diversification factors of algal CCM would be a membrane structure surrounding the plastid. Diatom system concentrates α-CAs in four-layered membrane matrix of the chloroplast and the function of this layered membrane in inorganic carbon traffic across cytosol and stroma would be a critical object to understand the CCM in diatoms.
Molecular mechanisms of CO2 response in marine photoautotrphs are also the critical factor for the issue of global primary production, as it regulates the CO2 acquisition under changing ambient pCO2. From past literatures, eukaryotic algae seemed to incorporate more direct system to recognize CO2 concentration compared to cyanobacteria. It was indeed clarified in a marine diatom that cAMP plays a critical role in CO2 signaling pathway as a second messenger. We showed in this review the possibility that CO2 sensing system analogous to those widely conserved in ACs in animals may also occur in diatoms. Such sophisticated sensing-signaling pathway would contribute to increase cross talk point with other environmental signals via protein kinase/phosphatase activity and/or metabolic feedback.
These molecular mechanisms of CO2 acquisition and CO2 response will deepen our knowledge on estimation of future transition of natural environment under changing climate, and also become critical information when we apply diatom and other algal cells to technology such as oil production.
References
Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD (1998) The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot 76:1052–1071
Beardall J, Mukerji D, Glover HE, Morris I (1976) The path of carbon in photosynthesis by marine phytoplankton. J Phycol 12:409–417
Bowler C et al (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244
Bozzo GG, Colman B (2000) The induction of inorganic carbon transport and external carbonic anhydrase in Chlamydomonas reinhardtii is regulated by external CO2 concentration. Plant Cell Environ 23:1137–1144
Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR (1999) Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA 96:79–84
Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D (2001) CO2 and HCO3 − uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr 46:1378–1391
Cann MJ, Hammer A, Zhou J, Kanacher T (2003) A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J Biol Chem 278:35033–35038
Carré IA, Edmunds LN Jr (1993) Oscillator control of cell division in Euglena: cyclic AMP oscillations mediate the phasing of the cell division cycle by the circadian clock. J Cell Sci 104:1163–1173
Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J (2000) Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289:625–628
Chen X, Qiu CE, Shao JZ (2006) Evidence for K+-dependent HCO3 − utilization in the marine diatom Phaeodactylum tricornutum. Plant Physiol 141:731–736
Coleman JR, Colman B (1981) Photosynthetic carbon assimilation in the blue-green alga Coccochloris peniosystis. Plant Cell Environ 4:285–290
Colman B, Rotatore C (1995) Photosynthetic inorganic carbon uptake and accumulation in two marine diatoms. Plant Cell Environ 18:919–924
Colman B, Rotatore C (1988) Uptake and accumulation of inorganic carbon by a freshwater diatom. J Exp Bot 39:1025–1032
Colman B, Huertus IE, Bhatti S, Dason JS (2002) The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct Plant Biol 29:261–270
Dionisio-Sese ML, Fukuzawa H, Miyachi S (1990) Light-Induced carbonic anhydrase expression in Chlamydomonas reinhardtii. Plant Physiol 94:1103–1110
Dou Z, Heinhorst S, Williams EB, Murin CD, Shively JM, Cannon GC (2008) CO2 fixation kinetics of Halothiobacillus neapolitanus mutant carboxysomes lacking carbonic anhydrase suggest the shell acts as a diffusional barrier for CO2. J Biol Chem 283:10377–10384
Falkowski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281:200–206
Falkowski P, Scholes RJ, Boyle E, Canadell J, Canfield D, Elser J, Gruber N, Hibbard K, Högbeg P, Linder S et al (2000) The global carbon cycle: a test of our knowledge of Earth as a system. Science 290:291–296
Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR (2004) The evolution of modern eukaryotic phytoplankton. Science 305:354–360
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Fukuzawa H, Miura K, Ishizaki K, Kucho KI, Saito T, Kohinata T, Ohyama K (2001) Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci USA 98:5347–5352
Funke RP, Kovar JL, Weeks DP (1997) Intracellular carbonic anhydrase is essential to photosynthesis in Chlamydomonas reinhardtii at atmospheric levels of CO2. Demonstration via genomic complementation of the high-CO2-requiring mutant ca-1. Plant Physiol 114:237–244
Gibbs SP (1981) The chloroplast endoplasmic reticulum: structure, function and evolutionary significance. Int Rev Cytol 72:49–99
Hammer A, Hodgson DR, Cann MJ (2006) Regulation of prokaryotic adenylyl cyclases by CO2. Biochem J 396:215–218
Harada H, Matsuda Y (2005) Identification and characterization of a new carbonic anhydrase in the marine diatom Phaeodactylum tricornutum. Can J Bot 83:909–916
Harada H, Nakatsuma D, Ishida M, Matsuda Y (2005) Regulation of the expression of intracellular β-carbonic anhydrase in response to CO2 and light in the marine diatom Phaeodactylum tricornutum. Plant Physiol 139:1041–1050
Harada H, Nakajima K, Sakaue K, Matsuda Y (2006) CO2 sensing at ocean surface mediated by cAMP in a marine diatom. Plant Physiol 142:1318–1328
Holdsworth ES, Colbeck J (1976) The pattern of carbon fixation in the marine unicellular alga Phaeodactylum tricornutum. Mar Biol 38:189–199
Iglesias-Rodriguez MD, Merrett MJ (1997) Dissolved inorganic carbon utilization and the development of extracellular carbonic anhydrase by the marine diatom Phaeodactylum tricornutum. New Phytol 135:163–168
Iseki M, Matsunaga S, Murakami A, Ohno K, Shiga K, Yoshida K, Sugai M, Takahashi T, Hori T, Watanabe M (2002) A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 415:1047–1051
Johnston AM, Raven JA (1996) Inorganic carbon accumulation by the marine diatom Phaeodactylum tricornutum. Eur J Phycol 31:285–290
Kanacher T, Schultz A, Linder JU, Schultz JE (2002) A GAF-domain-regulated adenylyl cyclase from Anabaena is a self-activating cAMP switch. EMBO J 21:3672–3680
Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YI, Husic HD, Moroney JV, Samuelsson G (1998) A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J 17:1208–1216
Kitao Y, Harada H, Matsuda Y (2008) Localization and targeting mechanisms of two chloroplastic beta-carbonic anhydrases in the marine diatom Phaeodactylum tricornutum. Physiol Plant 133:68–77
Kitao Y, Matsuda Y (2009) Formation of macromolecular complexes of carbonic anhydrases in the chloroplast of a marine diatom by the action of the C-terminal helix. Biochem J 419:681–688
Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schröppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, Levin LR, Buck J, Mühlschlegel FA (2005) Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15:2021–2026
Kohinata T, Nishino H, Fukuzawa H (2008) Significance of zinc in a regulatory protein, CCM1, which regulates the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol 49:273–283
Korb RE, Saville PJ, Johnston AM, Raven JA (1997) Sources of inorganic carbon for photosynthesis by three species of marine diatom. J Phycol 33:433–440
Kroth PG et al (2008) A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS One 3:e1426
Kuchitsu K, Tsuzuki M, Miyachi S (1991) Polypeptide composition and enzyme activities of the pyrenoid and its regulation by CO2 concentration in unicellular green algae. Can J Bot 69:1062–1069
Kucho K, Ohyama K, Fukuzawa H (1999) CO2-responsive transcriptional regulation of CAH1 encoding carbonic anhydrase is mediated by enhancer and silencer regions in Chlamydomonas reinhardtii. Plant Physiol 121:1329–1337
Kucho K, Yoshioka S, Taniguchi F, Ohyama K, Fukuzawa H (2003) Cis-acting elements and DNA-binding proteins involved in CO2-responsive transcriptional activation of Cah1 encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plnat Physiol 133:783–793
Lane TW, Morel FMM (2000) A biological function for cadmium in marine diatoms. Proc Natl Acad Sci USA 97:4627–4631
Lane TW, Saito MA, George GN, Pickering IJ, Prince RC, Morel FMM (2005) A cadmium enzyme from marine diatom. Nature 435:42
Litchman E, Klausmeier CA, Yoshiyama K (2009) Contrasting size evolution in marine and freshwater diatoms. Proc Natl Acad Sci USA 106:2665–2670
Ludwig M, Gibbs SP (1985) DNA is present in the nucleomorph of cryptomonads: further evidence that the chloroplast evolved from a eukaryotic endosymbiont. Protoplasma 127:9–20
Marcus Y, Harel E, Kaplan A (1983) Adaptation of the cyanobacterium Anabaena variabilis to low CO2 concentration in their environment. Plant Physiol 71:208–210
Masuda S, Ono TA (2005) Adenylyl cyclase activity of Cya1 from the cyanobacterium Synechocystis sp. srain PCC6803 inhibited by bicarbonate. J Bacteriol 187:5032–5035
Matsuda Y, Colman B (1995a) Induction of CO2 and bicarbonate transport in green alga Chlorella ellipsoidea. I Time course of induction of two systems. Plant Physiol 108:247–252
Matsuda Y, Colman B (1995b) Induction of CO2 and bicarbonate transport in green alga Chlorella ellipsoidea. II Evidence for induction in response to external CO2 concentration. Plant Physiol 108:253–260
Matsuda Y, Hara T, Colman B (2001) Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom Phaeodactylum tricornutum. Plant Cell Environ 24:611–620
Mayo WP, Williams TG, Birch DG, Turpin DH (1986) Photosynthetic adaptation by Synechococcus leopoliensis in response to exogenous dissolved inorganic carbon. Plant Physiol 80:1038–1040
McFadden GI, Gilson P (1995) Something borrowed, something green: lateral transfer of chloroplasts by secondary endosymbiosis. Trends Ecol Evol 10:12–17
McGinn PJ, Morel FMM (2008) Expression and inhibition of the carboxylating and decarboxylating enzymes in the photosynthetic C4 pathway of marine diatoms. Plant Physiol 146:300–309
Miller AG, Espie GS, Canvin DT (1990) Physiological aspects of CO2 and HCO3 − transport by cyanobacteria: a review. Can J Bot 68:1291–1302
Mitchell C, Beardall J (1996) Inorganic carbon uptake by an Antarctic sea-ice diatom, Nitzschia frigida. Polar Biol 16:95–99
Mitra M, Lato SM, Ynalvez RA, Xiao Y, Moroney JV (2004) Identification of a new chloroplast carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 135:173–182
Miura K, Kohinata T, Yoshioka S, Ohyama K, Fukuzawa H (2002) Regulation of a carbon concentrating mechanism through CCM1 in Chlamydomonas reinhardtii. Funct Plant Biol 29:211–219
Miura K, Yamano T, Yoshioka S, Kohinata T, Inoue Y, Taniguchi F, Asamizu E, Nakamura Y, Tabata S, Yamato KT, Ohyama K, Fukuzawa H (2004) Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol 135:1595–1607
Montsant A, Jabbari K, Maheswari U, Bowler C (2005) Comparative genomics of the pennate diatom Phaeodactylum tricornutum. Plant Physiol 137:500–513
Moustafa A, Beszteri B, Maier UG, Bowler C, Valentin K, Bhattacharya D (2009) Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 324:1724–1726
Nishimura T, Takahashi Y, Yamaguchi O, Suzuki H, Maeda S, Omata T (2008) Mechanism of low CO2-induced activation of the cmp bicarbonate transporter operon by a LysR family protein in the cyanobacterium Synechococcus elongatus strain PCC 7942. Mol Microbiol 68:98–109
Omata T, Gohta S, Takahashi Y, Harano Y, Maeda S (2001) Involvement of a CbbR homolog in low CO2-induced activation of the bicarbonate transporter operon in cyanobacteria. J Bacteriol 183:1891–1898
Patel BN, Merrett MJ (1986) Inorganic-carbon uptake by the marine diatom Phaeodactylum tricornutum. Planta 169:222–227
Price GD, Badger MR, Woodger FJ, Long BM (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59:1441–1461
Pronina NA, Semenenko VE (1984) Localization of membrane-bound and soluble forms of carbonic anhydrase in the Chlorella cell. Fiziol Rast (Moscow) 31:241–251
Quarmby LM (1994) Signal transduction in the sexual life of Chlamydomonas. Plant Mol Biol 26:1271–1287
Raven JA (1994) Carbon fixation and carbon availability in marine phytoplankton. Photosynth Res 39:259–273
Raven JA (1997) CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ 20:147–154
Rawat M, Moroney JV (1995) The regulation of carbonic anhydrase and ribulose-1, 5-bisphosphate carboxylase/oxygenase activase by light and CO2 in Chlamydomonas reinhardtii. Plant Physiol 109:937–944
Reinfelder JR, Kraepiel AML, Morel FMM (2000) Unicellular C4 photosynthesis in a marine diatom. Nature 407:996–999
Reinfelder JR, Milligan AJ, Morel FMM (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol 135:2106–2111
Roberts SB, Lane TW, Morel FMM (1997) Carbonic anhydrase in the marine diatom Thalassiosira weissflogii (Bacillariophyceae). J Phycol 33:845–850
Roberts K, Granum E, Leegood RC, Raven JA (2007) C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 145:230–235
Rost B, Riebesell U, Burkhardt S, Sültemeyer D (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48:55–67
Rotatore C, Colman B (1992) Active uptake of CO2 by the diatom Navicula pelliculosa. J Exp Bot 249:571–576
Rotatore C, Colman B, Kuzuma M (1995) The active uptake of carbon dioxide by the marine diatoms Phaeodactylum triconrutum and Cyclotella sp. Plant cell Environ 18:913–918
Satoh D, Hiraoka Y, Colman B, Matsuda Y (2001) Physiological and molecular biological characterization of intracellular carbonic anhydrase from the marine diatom Phaeodactylum tricornutum. Plant Pysiol 126:1459–1470
Sawaya MR, Cannon GC, Heinhorst S, Tanaka S, Williams EB, Yeates TO, Kerfeld CA (2006) The structure of beta-carbonic anhydrase from the carboxysomal shell reveals a distinct subclass with one active site for the price of two. J Biol Chem 281:7546–7547
So AK, Espie GS, Williams EB, Shively JM, Heinhorst S, Cannon GC (2004) A novel evolutionary lineage of carbonic anhydrase (ε class) is a component of the carboxysome shell. J Bacteriol 186:623–630
Sültemeyer DF (1998) Carbonic anhydrase in eukaryotic algae: characterization, regulation, and possible function during photosynthesis. Can J Bot 76:962–972
Sültemeyer DF, Fock HP, Canvin DT (1991) Active uptake of inorganic carbon by Chlamydomonas reinhardtii: evidence for simultaneous transport of HCO3 − and CO2 and characterization of active CO2 transport. Can J Bot 69:995–1002
Tachibana M, Allen AE, Kikutani S, Endo Y, Bowler C, Matsuda Y (2011) Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynth Res
Tanaka Y, Nakatsuma D, Harada H, Ishida M, Matsuda Y (2005) Localization of soluble β-carbonic anhydrase in the marine diatom Phaeodactylum tricornutum. Sorting to the chloroplast and cluster formation on the girdle lamellae. Plant Physiol 138:207–217
Tchernov D, Hassidim M, Luz B, Sukenik A, Reinhold L, Kaplan A (1997) Sustained net CO2 evolution during photosynthesis by marine microorganism. Curr Biol 7:723–728
Terauchi K, Ohmori M (2004) Blue light stimulates cyanobacterial motility via a cAMP signal transduction system. Mol Microbiol 52:303–309
Tréguer P, Nelson DM, Van Bennekom AJ, Demaster DJ, Leynaert A, Quéguiner B (1995) The silica balance in the world ocean: a reestimate. Science 268:375–379
Trimborn S, Lundholm N, Thomas S, Richter KU, Krock B, Hansen PJ, Rost B (2008) Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: the effect of pH-induced changes in seawater carbonate chemistry. Physiol Plant 133:92–105
Tripp BC, Smith K, Ferry JG (2001) Carbonic anhydrase: new insights for an ancient enzyme. J Biol Chem 276:48615–48618
Van K, Spalding MH (1999) Periplasmic carbonic anhydrase structural gene (Cah1) mutant in Chlamydomonas reinhardtii. Plant Physiol 120:757–764
Vance P, Spalding MH (2005) Growth, photosynthesis, and gene expression in Chlamydomonas over a range of CO2 concentrations and CO2/O2 ratios: CO2 regulates multiple acclimation states. Can J Bot 83:796–809
Wang HL, Postier BL, Burnap RL (2004) Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J Biol Chem 279:5739–5751
Wang Y, Sun Z, Horken KM, Im CS, Xiang Y, Grossman AR, Weeks DP (2005) Analyses of CIA5, the master regulator of the carbon-concentrating mechanism in Chlamydomonas reinhardtii, and its control of gene expression. Can J Bot 83:765–779
Woodger FJ, Bryant DA MR, Price GD (2007) Transcriptional regulation of the CO2-concentrating mechanism in a euryhaline, coastal marine cyanobacterium, Synechococcus sp. Strain PCC 7002: role of NdhR/CcmR. J Bacteriol 189:3335–3347
Xiang Y, Zhang J, Weeks DP (2001) The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 98:5341–5346
Xu Y, Feng L, Jeffrey PD, Shi Y, Morel FMM (2008) Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 452:56–61
Yamano T, Miura K, Fukuzawa H (2008) Expression analysis of genes associated with the induction of the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol 147:340–354
Yamano T, Tsujikawa T, Hatano K, Ozawa S, Takahashi Y, Fukuzawa H (2010) Light and low-CO2 dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol 51:1453–1468
Acknowledgments
We thank Ms. Nobuko Higashiuchi for her technical assistance and Ms. Miyabi Inoue for her skilful secretarial aid. This research was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Kwansei-Gakuin University, Research Center for Environmental Bioscience), and by Steel Industry Foundation for the Advancement of Environmental Protection Technology (to Y. M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuda, Y., Nakajima, K. & Tachibana, M. Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynth Res 109, 191–203 (2011). https://doi.org/10.1007/s11120-011-9623-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-011-9623-7