Abstract
The decadal changes (from 1992 to 2005) of a community of unattached red algae in the Sea of Japan dominated by Ahnfeltia tobuchiensis were studied for the first time. The most important changes were an increase in species richness and significant changes in the stock of dominant species in the Ahnfeltia bed accompanied by a drastic decrease in the stock and biomass of the cold-temperate-water species Chondrus armatus, which coincided with a significant increase in the stock and biomass of Ahnfeltiopsis flabelliformis, an alga with warm-temperate-water affinity, while the stock and biomass of A. tobuchiensis did not change considerably. These changes led to the alteration of the algal community. The association co-dominated by A. tobuchiensis and C. armatus was noted in 1992, and by 2005, this association was replaced by the A. tobuchiensis and A. flabelliformis co-dominated association. Possible causes for these changes are discussed. It was concluded that warmer waters may be the basic environmental stress factor playing the central role in changing the community structure of the studied Ahnfeltia bed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ahnfeltia tobuchiensis (Kanno et Matsubara) Makienko is one of the most commercially important seaweed producing high-quality agar. This alga forms extensive beds which are used as a sole source of raw material for agar production in the Russian Far East as well as in northern Japan (Cherbadgy and Popova 1998). This phycocolloid is used mainly as a gelling and thickening agent in food industries (Sukhoverkhov et al. 2000). Besides their economic value, Ahnfeltia beds have significant ecological importance. They serve as food and habitat for numerous benthic organisms including commercially valuable species such as scallops, sea cucumbers, and fish (Ivanova et al. 1994). So knowledge of Ahnfeltia community dynamics and the main environmental factors influencing community stability and succession can be important not only for understanding how the community is functioning but also for enhancing our ability to predict changes in the resources of the commercial algae and animals associated with Ahnfeltia beds.
Ahnfeltia beds are truly unique; they are distributed in the cold-temperate coastal zones of Asia Pacific only: in Peter the Great Bay (Sea of Japan), in Busse lagoon (Sakhalin Island), in the Bay of Izmena (Kunashir Island), and on the northern coast of Hokkaido Island (Makienko 1980). Ahnfeltia beds were found on sandy and sandy-muddy bottoms at depths between 5 and 20 m, where it forms a 0.15–1.0 m thick carpet, or stratum, on the seafloor. Ahnfeltia beds represent specific biocoenotic communities which include dozens of macrophyte species and have a complicated structure. These beds are invariably, for a hundred years, present at the same locations where circular currents and bottom morphology prevent the algae from drifting away (Titlyanov et al. 1993). Ahnfeltia communities are formed by red annual and perennial unattached seaweeds, and their epiphytes (Titlyanova 1980). Only perennial long-lived (up to 10 years) species such as A. tobuchiensis and Chondrus armatus dominate in such communities. These algae are sterile and reproduce only vegetatively, mainly by fragmentation. Due to low growth rate of the main species (less than 21–26 % of algal biomass per year), the ratios of dominant algae in a community are not susceptible to either seasonal or interannual changes if environmental conditions are stable. Minor species also have no effect on the seasonal variations of a community’s structure and biomass.
The proportion of A. tobuchiensis in a community differs slightly depending on the locality, but usually 50–100 % of the biomass is made up by this species. In general, the proportion of other species is usually low, less than 5 % (Ivanova et al. 1994; Skriptsova and Nabivailo 2009). However, there are Ahnfeltia beds co-dominated by A. tobuchiensis and other species such as C. armatus, Ptilota filicina, and Sargassum pallidum (Sukhoveeva et al. 1985). For example, the biomass of C. armatus can reach 22.6 % of the community biomass in some parts of the Ahnfeltia bed in the Bay of Izmena and even 50–80 % at some stations (Ivanova et al. 1994). In Baklan Bay (southern part of Peter the Great Bay), a community co-dominated by A. tobuchiensis and P. filicina is formed. The biomass of the latter species at some parts of the bed can reach 80–90 % (Sukhoveeva et al. 1985).
The population density and proportion of the dominant red algal species are not distributed uniformly but vary spatially. For example, the maximal accumulation of C. armatus in the Ahnfeltia bed located in the Bay of Izmena is concentrated in the south-eastern marginal part of the bed, and the alga occurred only sporadically in the other parts of the bed (Ivanova et al. 1994; Cherbadgy and Popova 1998). The total biomass of the bed and the area occupied by the algal stratum may vary insignificantly from season to season and from year to year (Titlyanov et al. 1993). It was shown that the northern and western boundaries of the Ahnfeltia bed in the Strait of Stark (Peter the Great Bay) can move between 50–100 and 150–250 m from summer to late autumn. The most obvious impact on the bed area is a change in the configuration and total stock of the algae due to strong storms induced by typhoons. Although they can conduce to the periodical turning of the stratum or its parts, the bed structure is generally strongly temporally stable because Ahnfeltia beds are mostly climax communities composed of the species best adapted to average conditions in the area. The community usually returned to its prior state within a year after a disturbance.
The Ahnfeltia beds in Peter the Great Bay were monitored by phycologists in the late twentieth century. The studies have detected general modification and regression of all Ahnfeltia beds in Peter the Great Bay during the last century due to extensive commercial harvesting (Kulepanov et al. 1999). However, since 1992, the harvesting of Ahnfeltia from the native beds in Peter the Great Bay has ceased (Kulepanov et al. 1999). Therefore, any change in the communities which could be found must be attributed to the impact of environmental changes or the natural succession of the communities. Early studies have suggested that abiotic environmental factors, such as temperature, light, and nutritive conditions as well as biotic interactions, are considered as the main factors regulating the dynamics of the major community-forming species in the Ahnfeltia beds (Titlyanov et al. 1993; Nabivailo et al. 2014).
The objectives of this study are (1) to assess the current state of the Ahnfeltia beds in Amursky Bay, (2) to compare it with historical records, (3) to try to relate the evolution of this algal community to environmental changes, and (4) to provide a baseline for further monitoring of this community.
Materials and methods
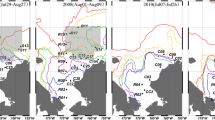
The studied Ahnfeltia beds are located in Amursky Bay near Mt. Stolovaya (between the mouth of the Barabashevka River and Peschanyi Peninsula, from 43° 06′ N, 131° 38′ E to 43° 10′ N, 131° 45′ E) (Fig. 1a). The previous data on the species composition, biomass, and distribution of the dominant species were obtained in the summer of 1992 (own unpublished data). This survey revealed two large accumulations of unattached algae at depths of 4 to 9 m: the southern bed located alongside the Barabashevka’s mouth and the northern bed located opposite Mt. Stolovaya (Fig. 2a, b).
Sampling was done in the Ahnfeltia bed by SCUBA-diving in summer, 1991 at 27 stations and in summer, 1992 and 2005 within the network of 147 sampling stations including those surveyed in 1991 (Fig. 1b). The distance between stations was about two cables (382 m). The positioning of a vessel relative to a station was carried out by use of a GPS navigation device.
The methods of sampling were similar in all undertaken studies. Three quadrats (0.25 m2) were randomly placed over the bottom at each sampling station in 2005, and only one quadrat was collected in both 1991 and 1992. Within each quadrat, all visible plants were collected by hand, placed into a mesh, and transported to the laboratory for further processing. At the laboratory, the samples were sorted by species, which were identified based on Perestenko’s identification keys (1980, 1994). The algae collected in 1992 were identified by T.V. Titlyanova, and the seaweeds collected in 2005, by T.V. Titlyanova and A.V. Skriptsova. The plants were weighed with a digital scale (±0.1 g). The additional collection was done in 2002. Ten quadrats were randomly collected in different parts of the northern bed. These data were not used in the analysis because the coordinates of these sampling locations did not overlap with the coordinates of stations sampled in 1991/92 and 2005. In 2002, the percentage of A. tobuchiensis in the sample ranged from 60 to 80 % (more than 2 kg m−2 on average), the percentage of the second dominant species, Ahnfeltiopsis flabelliformis, was 5–40 % of the total algae biomass (about 1 kg m2 in average) at all locations. C. armatus was not found in the whole set of samples.
From the data obtained, the following bed areas were defined: (1) “pure” Ahnfeltia areas are those dominated by only A. tobuchiensis with the biomass of attendant species less than 5 % of the community biomass in the sample; and (2) “mixed” areas, named after the co-occurring dominant species, are comprised of two species, with the biomass of each species varying between 20 and 80 % of the community biomass.
Data analysis
To analyze relative similarity of the algal associations and species lists for different years, the data were visualized in a two-dimensional non-metric multidimensional scale (n-MDS) ordination plot based on Bray-Curtis similarity matrix calculated for standardized biomass data or presence/absence data for species lists. Resulting maximum percentage similarity lines were obtained from a group average dendrogram and overlaid on the n-MDS plot. The significance of the resulting groups was tested using the one-way analysis of similarities (ANOSIM). The null hypothesis states that the mean of differences between groups is zero. All analyses were carried out using the software package PRIMER 6.0. The diagrams of algae distribution were prepared using Surfer 7.0 (GoldenSoftware).
Results
Changes of the floristic composition of the Ahnfeltia bed
Significant changes in the floristic composition of seaweeds were observed in the A. tobuchiensis beds in Amursky Bay over the last 13 years (Table 1). A total of 20 species of macrophytes were found in 1992 (2 species of Chlorophyta, 4 species of Phaeophyceae, and 14 species of Rhodophyta). In 2005, 35 algal species were recorded (2 species of Chlorophyta, 7 species of Phaeophyceae, and 26 species of Rhodophyta). Fifteen species were found in both collections. Additional species (those present in the latest study but not in the earlier) are small epiphytic algae with finely-branched or delicate foliaceous thalli. However, five species were large frondose seaweeds, such as Chorda filum, S. pallidum, Stephanocystis crassipes, and Desmarestia kurilensis. It should be noted that all additional species were detected in the northern bed only. The collections of 1992 and 2005 from the northern bed had a similarity of 65.3 %. Both collections from the southern bed were more similar to each other with a similarity of 88.8 % (Fig. 3). The similarity between total species lists of 1992 and 2005 was 64 %.
Changes in the stock and biomass of the dominant species
The total stock of the macrophytobenthos in both Ahnfeltia beds decreased from 17,100 t in 1992 to 14,650 t in 2005, although the patterns of changes in various parts of the bed were different. The stock decreased in the southern bed (from 12,600 to 9850 t), while in the northern one, the seaweed resources did not change significantly (4500 t in 1992 and 4800 t in 2005). The stratum thickness was higher in 2005 as compare with 1992 (Fig. 2a, b).
Three algal species had the highest biomass in the Ahnfeltia beds (A. tobuchiensis, A. flabelliformis, and C. armatus). These species constitute more than 90 % of the total biomass of macrophytobenthos. Significant changes in the biomass of this species have been found in the study area over the last 13 years. The total A. tobuchiensis biomass slightly decreased from 1992 to 2005. This reduction occurs mainly due to stock decline in the northern bed, while the A. tobuchiensis stock in the southern bed was unaltered. The stock of A. flabelliformis increased by five times during the study period. The stock of C. armatus decreased by 20 times (Table 2).
A. tobuchiensis was a dominant species in both beds in the 1990s and in 2005. Its biomass per square meter was almost constant over the last 13 years (Fig. 2a, b). Major variations in the areas occupied by species and their biomasses were found for A. flabelliformis and C. armatus. The biomass of A. flabelliformis, occurring in the northern bed only, increased by 2–4 times (from 0.1–0.8 kg m−2 in 1992 to 0.2–3.5 kg m−2 in 2005). The area of the algae accumulations also significantly expanded (Fig. 2e, f). The proportion of this species in the community increased from 4 to 15 % of the community biomass in 1992 to 10–60 % in 2005. The biomass of C. armatus decreased from 0.5–3.6 to 0.05–0.2 kg m−2 during the same period, coinciding with reduction in the area of the alga accumulation (Fig. 2g, h). The algal proportion in the community decreased from 20 to 50 % of the community biomass in 1992 to 0.1–4 % in 2005.
The MDS ordination plot graphically represents the variation in the Ahnfeltia bed over time. Five clusters based on the dominant species biomass were separated (Fig. 4a). The overlay of the MSD plot with the cluster dendrogram similarity lines indicated the respective maximum boundary values for discrimination of clusters. The maximum relative similarity of all clusters was only 15.8 % (Bray-Curtis similarity). The clusters formed by the C. armatus or A. flabelliformis dominated stations were less similar to all the other (17.3 %). The collections of 1991–1992 and 2005 from the northern bed were separated at a similarity level of 58.4 %. The collections from the southern bed were more similar to each other with an 80 % similarity. The obtained clusters are strongly determined by the biomasses of A. tobuchiensis, A. flabelliformis, and C. armatus (Fig. 4b–d, respectively). There were no significant differences between the samples of 1991 and 1992 (Global R: 0.093, significance level: 1.6 %, no. of permutations: 999, no. of permuted R statistics greater than or equal to Global R: 15, for the results of the pair-wise test see Table 3). No correlation between depth and species distribution in the northern bed was found.
Two-dimensional non-metric multidimensional scaling ordination (n-MDS) of similarities of community (a) and biomass of Ahnfeltia tobuchiensis (b), Ahnfeltiopsis flabelliformis (c), and Chondrus armatus (d) for different time periods. N5 northern bed 2005, N92 northern bed 1992, N91 northern bed 1991, S5 southern bed 2005, S92 southern bed 1992, S91 southern bed 1991. In b–d, the algae biomass (kg m−2) is given
Thus, a total of three areas were described in the studied Ahnfeltia beds in the 1990s and in 2005. These were (1) “pure” Ahnfeltia areas in the southern bed in both sampling periods and in the south-eastern part of the northern bed in 1992, (2) “mixed” A. tobuchiensis and A. flabelliformis area in the northern bed in 2005, and (3) “mixed” A. tobuchiensis and C. armatus area mainly in the southern and western parts of the northern bed in the 1990s (Fig. 5).
Discussion
The results of the present research revealed a community alteration in the Ahnfeltia bed in Amursky Bay since 1992. The most important changes are (1) the increase in species richness and (2) the significant changes in the stock of the dominant species in the bed, i.e., the drastic decrease in the biomass of C. armatus and the simultaneous significant increase in the biomass of A. flabelliformis, while the biomass of A. tobuchiensis did not change considerably from 1992 to 2005. These changes led to the alteration of the community structure in the northern bed. Here, the association co-dominated by A. tobuchiensis and C. armatus was recorded in 1992, and by 2005, this association was succeeded by the A. tobuchiensis and A. flabelliformis co-dominated association.
Although in the present study the number of species differed significantly between the collections made in 1992 and 2005, the floristic similarity value of both collections was high (Bray-Curtis similarity 64 %). New for the community records are generally rare in the beds and may be absent from the transects. It should be noted that all the species collected in 2005 as well as in 1992 are common in Peter the Great Bay (Perestenko 1980), and most of them were previously recorded in the other Ahnfeltia beds of the bay (Titlyanova 1980). Therefore, the differences found in the species compositions of the tested Ahnfeltia beds between 1992 and 2005 are strongly believed to be rather caused by higher sampling efforts in 2005, during which three parallel samplings were carried out at each station than the influx of biota from the surrounding coast.
The most obvious change was found in the community structure of the northern bed, where C. armatus was replaced by A. flabelliformis. It must be taken into consideration that under a long-term study of marine communities, the measured differences between two distant samplings may be a short-term process, such as seasonal or interannual fluctuations in the community, instead of long-term trends. The seasonal changes of dominant species biomass in the studied Ahnfeltia beds can be repudiated because the samplings were done at the same time (in July). Moreover, it was shown that the community is just slightly variable between seasons and years due to slow growth and failing of seaweeds senescence after reproduction. A reinvestigation of the same station during 1991 and 1992 did not show any changes in the proportions of the dominant species from 1991 to 1992. In stark contrast, the decadal (13-year) pattern of change recorded at the 37 stations located in the northern bed and resurveyed in 1991, 1992, and 2005 is similar to the widespread changes in the proportion of the dominant species. This confirms the data obtained from the whole station set. Moreover, an additional survey done in 2002 revealed that C. armatus almost disappeared in the northern bed and the coenotic role of A. flabelliformis increased, i.e., its percentage rose from 4 to 15 % of the total community biomass in 1992 to 5–40 % in 2002 (data of 2002 are not shown). So this result suggests that the observed Ahnfeltia bed changes over 13 years may not represent short-term fluctuations in the biomass of species. The gradual substitution of C. armatus by A. flabelliformis in the northern bed has been observed since 2002 at least.
The revealed alterations in the Ahnfeltia bed community structure may be the result of environmental changes in Peter the Great Bay, namely water quality change, water warming, physical disturbance by strong storms, or dramatic reduction in community disturbance due to ceased harvesting of seaweeds from the bed. However, they may not be responsible for the observed community alterations to the same degree.
For example, strong storms can occasionally disrupt Ahnfeltia beds (Titlyanov et al. 1993). During strong waves, powerful near-bottom currents are formed that conduce to loosening and moving of an algal stratum, its peripheral parts breaking into fragments that can be stranded (Titlyanov et al. 1993). Big accumulations of wrack algae after strong storms confirm the disruption of Ahnfeltia strata (Kulepanov et al. 1999). Moreover, storms can induce periodical turning of an algal stratum or its parts (Titlyanova 1980). Consequently, the algae that occur chiefly on the surface of a stratum are removed to the lower layer. Here, the algae are practically subjected to total darkness, hence only the species resistant to extremely low light intensity can occupy this niche. As it was earlier shown, these species are A. tobuchiensis and A. flabelliformis, while C. armatus inhabiting the bed’s surface is less tolerant to low light (Varfolomeeva et al. 1994). Therefore, if C. armatus happens to get under the bed, it dies because of light deficiency. Individual fragments of the alga are source for C. armatus stock restoration. So the stratum turning can lead to the temporary modification of the community structure. Usually, in favorable environmental conditions, the community returns to its prior state within the year after disturbance. So, we strongly suppose that turning of the studied stratum by strong waves cannot be responsible in itself for decadal community changes.
The cessation of the Ahnfeltia bed exploitation could cause the alteration of the community structure. Dragging, similarly to storm disturbance, results in the break and overturn of parts of the bed (Titlyanov et al. 1993). Certainly, the stratum becomes stable when harvesting stops, and it might cause gradual structural changes in the community. In case of stratum stabilization, photophilous species, such as C. armatus, stay on the bed surface under the most favorable light conditions that allow this species to grow fast and enlarge its biomass if other environmental conditions do not limit the algal growth. Conversely, our data showed significant reduction in the biomass of this species in the study area. Similar catastrophic reduction (by 20 times) in C. armatus stock occurred in Ismena Bay from 1991 to 2008 (own unpublished data). The elimination of C. armatus from the studied bed could be explained by its competitive supplantation by the dominant species A. tobuchiensis. However, our experiments did not show inhibitive effect of A. tobuchiensis on the photosynthetic rates and growth of C. armatus at least in a short time period (about 3 weeks) (Nabivailo et al. 2014). A significant increase in the stock and biomass of A. flabelliformis in the studied bed cannot also be explained by harvesting termination. This species is physiologically similar to A. tobuchiensis: both species have similar requirements in light and nutrients and show similar growth rates in the same growth conditions (Nabivailo et al. 2014). One of the possible explanations of the increase in A. flabelliformis stock is the influx of spores or seedlings from the surrounding coasts which resupply the population of the alga in the bed. However, it was shown that A. flabelliformis found within the Ahnfeltia bed is genetically distant from the one growing in the nearest water area (Shibneva and Zaslavskaya 2012) and the latter cannot be a source for the algal population increment in the studied community.
So we strongly suggest that both storm disturbance and harvesting cessation cannot be responsible for the observed community alteration in themselves; however, they might be a trigger of the succession maintained by environmental changes. In general, secondary succession does not return the ecosystem to its historic state if environmental conditions change. A new-forming community will be best adapted to average conditions in the area and will reach a new stable equilibrium. It is considered that in general, long-term alterations in stable macroalgal communities are caused by unidirectional varying long-affected factors. Nutrients, water temperature, and light levels are the major factors regulating the dynamics of macroalgal communities at local scale.
Deterioration of the water quality due to eutrophication or increase of pollution is considered as one of the basic reasons of the decadal and interdecadal changes of seaweed communities (Eriksson et al. 2002). However, it should be noted that, though the coastal ecosystem of Amursky Bay is subject to considerable anthropogenic stress since mid-twentieth century, no marked increase in nutrient concentrations or other polluting agents due to coastal run-off has been observed in the bay in the last two decades, and some reports even revealed its stabilization or decrease (Ogorodnikova 2001; Belan et al. 2007). The decrease in anthropogenic pressure on Amursky Bay during the last decades led to some improvement in the state of the ecosystem and its biodiversity (Belan et al. 2007). Moreover, it did not show any significant changes in nutrient concentrations in the studied area from 1986 to 2005 (Moshchenko et al. 2011). So, the changes detected in the Ahnfeltia bed do not seem to be explained by either a nutrient supply increase or rise of pollution in this area.
The next possible cause of the changes found in the Ahnfeltia bed may be water warming during the past two decades. In Amursky Bay, the annual sea surface temperature (SST) has increased by 1.7 °C during the last 50 years (Gayko 2005; Moshchenko et al. 2011). The heating caused dramatic changes in seasonal extreme temperatures. Gayko showed that before 1988, the temperatures varied little around the long-term average annual level, but since 1989, positive annual SST anomalies increased to 1–1.4 °C over the normal SST.
These changes could more likely cause the decadal change in the biomass of community-forming species in the studied Ahnfeltia beds. We found significant decrease in C. armatus biomass and increase in A. flabelliformis biomass with relatively small change in the biomass of A. tobuchiensis from 1990s to 2005. These alterations can be explained by the prediction of Harley et al. (2006): “species living near their range boundaries are exhibited to thermal stress, and their distribution and abundance may shift when environmental conditions are more favorable.” As a consequence, the abundance of southern species should increase and the abundance of northern species should decrease in response to water warming, especially at the edge of their geographic range (Sagarin et al. 1999; Harley et al. 2006). Analysis of latitudinal distribution of species dominating in the studied Ahnfeltia beds revealed that C. armatus is a boreal species with cold-temperate-water affinity. It is an endemic of the Sea of Japan. Here, it occurs along the continental coast and at the west coast of Sakhalin Island, as well as at Honshu Island and at the Southern Kuril Islands. Peter the Great Bay is close to the southern margin of C. armatus range. On the contrary, A. flabelliformis is a warm-temperate species with tropical and subtropical to temperate distribution. In the northern hemisphere, it occurs mainly in Vietnam, Philippines, Taiwan, Hawaii, China, and along the continental and insular coasts of the Sea of Japan (Guiry and Guiry 2013). Peter the Great Bay is the northern margin of its distribution. A. tobuchiensis occurs only in Peter the Great Bay, Aniva Bay (south of Sakhalin Island), at Honshu and Kunashir islands, and is therefore a species with temperate-water affinity. Therefore, our findings strongly support the hypothesis that the increase in water temperature observed during past decades can cause changes in the Ahnfeltia bed community.
It should be noted that changes in the abundance of algae with warm-water and cold-water affinity are well-documented in the temperate zone of the oceans. These include an increase in the abundance of “southern species” in subtidal algal communities in Norway (Husa et al. 2008), a drastic decrease in the abundance of relatively cold-water species such as Saccharina japonica, Kjellmaniella crassifolia, and Costaria costata, and an increase in the biomass of warmer-water species Undaria pinnatifida, Undaria peterseniana, and Ecklonia stolonifera along with an SST rise of 1 °C near northern Honshu Island, Japan (Kirihara et al. 2006). Similarly, a decline of the Ecklonia cava population associated with an increase in seawater temperature was observed in southern Japan (Serisawa et al. 2004; Tanaka et al. 2012). Similarly, temperate species such as Sargassum micracanthum and Sargassum okamurae that dominated during the 1970s in Isoyake areas (Japan) have been decreasing, and the subtropical species Sargassum duplicatum has become a major component of the flora since the 1990s (Nagai et al. 2011).
So we assume that water warming may be the most possible environmental stress factor playing a central role in changing the community structure of the studied Ahnfeltia beds. However, biotic interactions cannot be excluded from consideration. As it was shown before, environmental changes may strongly alter interspecific interactions in algal communities. For example, an increase in light intensity and nutrient accessibility significantly enhanced the competitive ability of A. tobuchiensis, C. armatus, and A. flabelliformis and decreased the negative influence of A. tobuchiensis on attendant species (Nabivailo et al. 2014). So it can be suggested that water warming of only a few degrees could have an effect on community competition, altering the competitive ability of relative warm-temperate-water and cold-temperate-water competitors, and therefore affecting community composition. Our preliminary data also support this assumption. It was shown that C. armatus is more sensitive to elevated temperature than A. tobuchiensis and A. flabelliformis. Both the photosynthetic rate and growth rate of C. armatus were depressed under 15 °C, whereas in A. flabelliformis, these parameters increased with temperature increase from 5 to 15 °C. At low temperature (5–10 °C), the growth rates of C. armatus were twice as high compared to A. flabelliformis, whereas at 15 °C, the growth rate of A. flabelliformis was 1.8 times higher (own unpublished data). This data suggest that the competitive ability and productivity of A. flabelliformis increase and of C. armatus decrease with water warming. The temperature above 12 °C is an average summer-autumn temperature in Peter the Great Bay. Rising water temperature in warm period of a year allows A. flabelliformis to grow actively for a longer period and suppresses the growth of C. armatus. This may apparently allow A. flabelliformis to gradually replace C. armatus in the bed, and the latter wins in the competition for light and nutrients. However, this hypothesis requires experimental confirmation.
Summing up, probably the main factor that caused the alteration of the Ahnfeltia bed community located in Amursky Bay is water warming. The hypothetical scenario explaining the evolution of the Ahnfeltia bed communities from 1992 to 2005 is given at Fig. 6.
References
Belan TA, Moschenko AV, Lishavskaya TS (2007) Long-term variations in the level of pollution of sea environment and benthos in Peter the Great Bay. In: Tarasov VG (ed) Dynamics of marine ecosystems and modern problems of conservation of biological resources of the Russian seas. Dalnauka, Vladivostok, pp 50–75
Cherbadgy II, Popova LI (1998) Distribution, biomass and primary production of an Ahnfeltia tobuchiensis (Ahnfeltiales, Rhodophyta) population in the Bay of Izmena, Kunashir Island. Phycol Res 46:1–10
Eriksson BK, Johansson G, Snoeijs P (2002) Long-term changes in the macroalgal vegetation of the inner Gullmar fjord, Swedish Skagerrak coast. J Phycol 38:284–296
Gayko LA (2005) Peculiarities of hydrometeorological regime of the coastral zone in the Peter-the-Great Bay (Sea of Japan). Dal’nauka, Vladivostok (in Russian)
Guiry M D, Guiry GM (2013) AlgaeBase version 4.2. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org (accessed 28 Apr 2013)
Harley CD, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJ, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impact of climate change in coastal marine systems. Ecol Lett 9:228–241
Husa V, Sjøtun K, Brattenborg N, Lein TE (2008) Changes of macroalgal biodiversity in sublittoral sites in southwest Norway: impact of an introduced species or higher temperature? Mar Biol Res 4:414–428
Ivanova MB, Novozhilov AV, Tsurpalo AP (1994) Conditions of habitation and some peculiarities of floral-faunistic composition of commercially used natural fields of Ahnfeltia tobuchiensis in Stark Stark Strait (Peter the Great Bay, Sea of Japan) and in Izmeny Bay (Kunashir Isle, Kuril Islands). Izv TINRO 113:83–99 (in Russian)
Kirihara S, Nakamura T, Kon N, Fujita D, Notoya M (2006) Recent fluctuations in distribution and biomass of cold and warm temperature species of Laminarialean algae at Cape Ohma, nothren Honshu, Japan. J Appl Phycol 18:521–527
Kulepanov VN, Dzizyurov VD, Zhiltsova LV (1999) Contemporary stage of fields of Ahnfeltia tobuchiensis (Kanno et Matsubara) Mak. in the Bay of Peter the Great (Japan Sea). Rastitel’nye Resursy 1:116–122 (in Russian)
Makienko VF (1980) On the history of Ahnfeltia plicata (Huds) Fries study. Ahnfeltia species near the Far eastern coasts of the USSR. In: Biology of Ahnfeltia. DVNTS AN SSSR, Vladivostok, p 5--14
Moshchenko A, Belan T, Korostelev Y (2011) Long-term changes of marine environment and benthic communities in the north part of Amursky Bay (The Sea of Japan) Abstrs. of the PICES–2011 Annual Meeting. Khabarovsk, Russia, p 93
Nabivailo YV, Skriptsova AV, Titlyanov EA (2014) The interspecific relationships of seaweeds and their role in the formation of communities of Ahnfeltia tobuchiensis (Kanno et Matsubara, 1932) Makienko 1970 (Rhodophyta). Rus J Mar Biol 40:344--353
Nagai S, Yoshida G, Tarutani K (2011) Change in species composition and distribution of algae in the coastal waters of western Japan. In: Casalegno S (ed) Global Warming Impacts - Case Studies on the Economy, Human Health, and on Urban and Natural Environments. InTech, Rijeka, pp 209–236
Ogorodnikova AA (2001) The ecological-economic evaluation of coastal contamination sources’ effect on environment and bioresources in Peter the Great Bay. TINRO-centre, Vladivostok (in Russian)
Perestenko LP (1980) Algae of Peter the Great Bay. Nauka, Leningrad (In Russian)
Perestenko LP (1994) Red algae of the Far-Eastern Seas of Russia. Olga Publishing House, St Peterburg (In Russian)
Sagarin RD, Barry JP, Gilman SE, Baxter CH (1999) Climate related change in an intertidal community over short and long time scales. Ecol Monogr 69:465–490
Serisawa Y, Imoti Z, Ishikawa T, Ohno M (2004) Decline of the Ecklonia cava population associated with increased seawater temperatures in Tosa Bay, southern Japan. Fish Sci 70:189–191
Shibneva SY, Zaslavskaya NI (2012) Attached and unattached forms of two species of red seaweeds in Peter the Great Bay: morphological plasticity and genetic determination. In: Proceedings of XI regional conference of students and post-graduated students of Russian Far-East: Actual problems of ecology, marine biology and biotechnology. May 3–4, 2012. Vladivostok, p. 313–315 (in Russian)
Skriptsova AV, Nabivailo YV (2009) Spatial distribution of algae in Ahnfeltia tobuchiensis beds in Amursky Bay (Sea of Japan). Rus J Mar Biol 35:1–7
Sukhoveeva MV, Paimeeva LG, Zhmakin AF, Umudova LL (1985) Raspredelenie i zapasi promislovih vodorosley v Japonskom more. Otchet TINRO. №19489, Vladivostok (in Russian)
Sukhoverkhov SV, Kadnikova IA, Podkorytova AV (2000) Production of agar and agarose from the red alga Ahnfeltia tobuchiensis. Appl Biochem Microbiol 36:201–203
Tanaka K, Taino S, Haraguchi H, Prendergast G, Hiraoka M (2012) Warming off southwestern Japan linked to distributional shifts of subtidal canopy-forming seaweeds. Ecol Evol 2:2854–2865
Titlyanov EA, Novozhilov AV, Cherbadgy II (1993) Ahnfeltia tobuchiensis: Biology, ecology, productivity. Moskow, p 224
Titlyanova TV (1980) Species composition and distribution of seaweeds in the Ahnfeltia tobuchiensis stratum in Starka Strait. Biologia Ahnfeltii, Vladivostok, pp 15–20 (in Russian)
Varfolomeeva SV, Titlyanov EA, Cherbagy II (1994) Physiological features of competing algae in Ahnfeltia tobuchiensis community. Biol Morya 20:34–41 (in Russian)
Acknowledgments
This work was financially supported by the Far-East Branch of Russian Academy of Science (project № 12-I-OBN-08). We wish to extend our deep gratitude to Prof. E.A. Titlyanov for critical comments to paper and help in discussion of the results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skriptsova, A.V., Sabitova, L.I. & Cherbadgy, I.I. The decadal changes in the Ahnfeltia bed in the Peter the Great Bay (Sea of Japan): possible causes. J Appl Phycol 28, 417–427 (2016). https://doi.org/10.1007/s10811-015-0524-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0524-6