Abstract
The effect of irradiance and temperature on the photosynthesis of an agarophyte from Thailand, Gelidiella acerosa (Gelidiales, Rhodophyta), was determined using dissolved oxygen sensors and pulse-amplitude modulated fluorometry (PAM). A model of the net photosynthesis–irradiance (P–E) curve at 28 °C revealed that the saturation and compensation irradiances were 83 (95 % Bayesian credible interval (BCI), 52–128) μmol photons m−2 s−1 and 37 (BCI, 29–45) μmol photons m−2 s−1, respectively. Gross photosynthesis and dark respiration were also determined over a range of temperatures (16–40 °C), revealing that the gross photosynthetic rate was highest at 18.4 (BCI, 15.9–20.8) mg O2 gww −1 min−1 at 29.2 (BCI, 26.8–32.1) °C. Dark respiration rate could be fitted to a second-order polynomial regression (P < 0.01) over the range of the experimental temperatures, and the magnitudes of the rates ranged from 0.49 to 0.93 mg O2 gww −1 min−1 °C−1. The highest value of the maximum effective quantum yield (ΦPSII at 0 μmol photons m−2 s−1) occurred at 27.7 (BCI, 26.5–28.9) °C and was 0.57 (BCI, 0.55–0.58), and the values of ΦPSII decreased above and below 27.7 °C. Although this species is considered to be well-adapted to the current seawater temperatures of the region, the results of the experiments also suggest that they are likely close to the limiting temperature conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gelidiella acerosa (Forsskål) Feldmann et Hamel (Gelidiales, Rhodophyta) is an alga that can be found commonly on the surf-exposed and moderately wave-sheltered rocks and reefs in the intertidal and upper sublittoral zones in the tropical and subtropical regions of the world (Santelices and Stewart 1985; Abbott 1999; Lin and Freshwater 2008). This species is also known as one of the more important sources of raw material for agar production (Armisen and Galatas 1987; Ganzon-Fortes 1994), contributing to the regional fisheries in the Indo-Pacific region (e.g., Philippines, China, and India; McHugh 1991; Roleda et al. 1997a, b; Ohno and Largo 1998; Zemke-White and Ohno 1999).
In Thailand this species can be commonly found along the coastlines facing the Andaman Sea and the Gulf of Thailand (Lewmanomont and Ogawa 1995; Coppejans et al. 2011; Tsutsui et al. 2012). Despite the importance as an agarophyte, not to mention its high quality and potential as a fisheries resource, G. acerosa has not been harvested in Thailand, unlike species of Gracilaria and Hydropuntia (Edwards and Tam 1984; Praiboon et al. 2006; Bunsom and Prathep 2012).
The stable production of G. acerosa is largely dependent upon various environmental factors driven by the local climate, and especially by the environmental conditions of its habitat (i.e., temperature and irradiance). Indeed, changes in regional seawater temperature are also of some concern, especially along the coast of the Andaman Sea (Brown et al. 1996). For example, seawater temperatures in Phuket, Andaman Sea, have increased at a rate of 0.161 °C per decade (Brown et al. 1996; Tanzil et al. 2009). Presently, the monthly average seawater temperatures in Phuket range from 28 to 30 °C (Brown et al. 1996; Tanzil et al. 2009), suggesting that any further increases in temperature may be detrimental to many of the corals and macroalgae presently found in the region and leading to change in biodiversity.
Despite the commercial importance of G. acerosa and the presence of previous studies on the ecological and physiological aspects from the Philippines and India (Rao and Mehta 1973; Thomas et al. 1975; Chennubhotla et al. 1986; Ganzon-Fortes 1997a, b, 1999; Roleda et al. 1997a, b; Villanueva et al. 1999), there is a little information regarding its ecophysiology, especially with respect to detailed investigations dealing with the response of photosynthesis to temperature and irradiance.
Our recent studies have reported the temperature and irradiance characteristics of photosynthesis of a number of algae species using pulse amplitude modulated (PAM) fluorometer and dissolved oxygen sensors (Lideman et al. 2013; Vo et al. 2014; Watanabe et al. 2014a, b; Fujimoto et al. 2014a, b). We have great interest regarding the temperature response of the effective quantum yield (ΦPSII) in photosystem II and comparing this response to the temperature response of gross photosynthesis. The information derived from these experiments provides detailed insight on how algae respond to temperature, and provides some knowledge of physiologically important ranges of temperature. Indeed, this yield information on the physiological status of the regulatory processes of photosynthetic energy allocation has been evaluated to be a useful tool for the assessment of photosynthetic activity under various environmental conditions (Schreiber et al. 1986; Edwards and Kim 2010; Schagerl and Möstl 2011).
This study was undertaken to provide insights regarding the effects of water temperature and irradiance on the photosynthesis of G. acerosa collected from the coastal areas of Krabi, Thailand, facing the Andaman Sea. More specifically, we focused on elucidating the temperature and irradiance optima of photosynthetic from measurements by oxygen sensors and PAM fluorometry and applied Bayesian techniques to provide estimates of these optima. We expect that this knowledge will lead to sustainable utilization and conservation of the G. acerosa as an important agar resource.
Materials and methods
The algae (ca. 60 individuals) were collected from Railay Beach, Krabi, Thailand (8°0′14.57″ N, 98°50′39.43″E) on January 24, 2013, and used for the measurement of oxygen evolution and PAM fluorometry. Collected algae were stored in 1 L plastic bottles with seawater and transported to Japan via an overnight airline trip in a cooler at about 28 °C, which is approximately the same temperature as the sampling date. The samples were maintained for 1 to 3 days before examination at the Faculty of Fisheries, Kagoshima University, in 1 L flasks containing sterile seawater at a salinity of 33 psu. The flasks were placed in an incubator at a water temperature of 24 °C, and under photosynthetic active radiation (PAR) of ca. 100 μmol photons m−2 s−1 (12:12-h light/dark cycle). The PAR was adjusted by changing the distance of cool fluorescent lamps measuring irradiance using a quantum (2π) sensor (LI-190, LI-250A, LI-COR, USA). Voucher specimens were deposited in the Herbarium of Kagoshima University Museum, Kagoshima.
Effect of temperature on photosynthesis
The measurement methods are described in detail in Terada et al. (2013) and Vo et al. (2014). Briefly, the materials were divided into seven temperature treatment groups (16, 20, 24, 28, 32, 36, 40 °C, N = 5 replicates/treatment) and held under 200 μmol photons m−2 s−1, which is higher than saturation irradiance (E k) as revealed by the photosynthetic–irradiance (P–E) curve (as detailed below). Light was provided by a metal-halide lamp (e.g., Nishihara et al. 2004) and temperature was controlled using a glass water bath. PAR in the photosynthetic experiments was measured in the glass water bath by using a submersible spherical (4π) quantum sensor (LI-193, LI-250A, LI-COR, USA).
Dark respiration and net photosynthetic rates were determined by measuring the dissolved oxygen concentration (in milligram per liter) every 5 min for 30 min after a 30-min pre-incubation to acclimate the samples to each experimental condition, and the slope of the linear regression was determined from the data of 30-min measurements to estimate rates. Dissolved oxygen (DO) was measured using a polarographic sensor and a DO meter (Model 58, and 5100, YSI Incorporated, USA).
Explants used in this experiment were approximately 300 mg wet weight (mgww) and were acclimated overnight with sterilized seawater in the incubator at the same temperature and PAR condition as during pre-incubation (Muraoka et al. 1998; Serisawa et al. 2001). To start the experiment we randomly selected at least five explants and placed them in 100-mL BOD bottles containing sterilized natural seawater. The DO sensors were placed in sterilized natural seawater so that no bubbles were trapped and seawater was continuously stirred during the measurement. The exact volume of the BOD bottles was determined after the experiments and was used in the estimates of photosynthesis and dark respiration rates. Seawater medium was renewed after every measurement to avoid any affects due to the depletion of nutrients and dissolved carbon dioxide.
Irradiance effect on the photosynthesis
Photosynthetic rates were determined at 0, 30, 60, 100, 150, 200, 250, and 500 μmol photons m−2 s−1 (N = 5 replicates/level) at 28 °C and the procedure follows those of the temperature experiment.
Temperature and irradiance effect on photosynthetic parameters
Maxi Imaging-PAM (Heinz Walz, Germany) measurements were based on procedures detailed in Lideman et al. (2013) and Vo et al. (2014). Ten replicates of pre-incubated whole algae were randomly selected and placed in a stainless-steel tray (12 × 10 × 3 cm) containing sterilized seawater. Tray temperature was controlled with a block incubator (BI-535A, Astec, Fukuoka, Japan) by placing the tray on the aluminum block of the incubator. Water temperature in the stainless-steel tray was measured with a thermocouple to confirm that the appropriate temperature condition was achieved.
The maximum effective quantum yields (ΦPSII at 0 μmol photons m−2 s−1) were measured from 14 to 40 °C in 2 °C increments. Each increment in temperature occurred over a 30-min period with an additional 30 min allowed for dark and temperature acclimation. One set of experiments typically took more than 6 h to complete.
Modeling the photosynthetic response to irradiance and temperature
The temperature response of gross respiration and effective quantum yield (ΦPSII) was assumed to follow a non-linear exponential function (Alexandrov and Yamagata 2007; Eq. 1), where y is the response variable, which in this case is the gross photosynthetic rate and K is the temperature in Kelvin. There are four parameters in this model, where y max is the maximal rate of y occurring when the temperature is K opt, H a is the activation energy in kilojoule per mole and H d is the deactivation energy in kilojoule per mole. R in this model is the ideal gas constant, and has a value of 8.314 J kJ mol−1. The gross respiration rates were calculated by adding the dark respiration rates to the net photosynthetic rates, after assuming that the dark respiration rates approximate photorespiration.
The relationship between the dark respiration rate and temperature was initially assumed to follow a simple linear model; however, a second order polynomial was also fitted and statistically examined.
The response of photosynthesis to irradiance was examined by modeling the data using an exponential equation (Jassby and Platt 1976; Webb et al. 1974; Platt et al. 1980; Henley 1993) that included a respiration term, which had the form:
where, P net was the net O2 production rate, P max was the maximum O2 production rate, α was the initial slope of the photosynthesis versus irradiance curve, E was the incident irradiance, and R d was the dark respiration rate. From this model, the saturation irradiance (E k) was calculated as P max/α and the compensation irradiance (E c) was \( {P}_{\max } \ln \left(\frac{P_{\max }}{\left({R}_{\mathrm{d}}-{P}_{\max}\right)}\right)/\alpha \).
Statistical analysis
Statistical analyses of all the models were done using R version 3.0.1 (R Development Core Team 2013) and model fitting was done using rstan version 2.10 (Stan Development Team 2013). The parameters were examined by fitting the relevant models (i.e., Eq. 1 or Eq. 2) using Bayesian inference, since these models are difficult to fit using least squares or maximum likelihood methods. rstan primarily uses a variant of a Hamiltonian Monte Carlo sampler to construct the posterior distributions of the parameters, and four chains of at least 500,000 samples/chain were generated and assessed for convergence. Weakly informative normal priors were placed on all of the parameters of the model, and a half-cauchy prior was placed on the scale parameter of the models (Gelman 2004, 2006). A generalized linear model was used to analyze the dark respiration–temperature relationship, assuming a normal error distribution. A linear and second-order polynomial was fitted to the data and compared using an F test.
Results
Effect of irradiance on the net photosynthesis
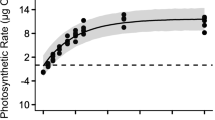
The net photosynthetic rates at 28 °C steadily increased from −10.5 mg O2 gww −1 min−1 (95 % confidence interval (CI) of −13.5 to −7.5 mg O2 gww −1 min−1) at 0 μmol photons m−2 s−1 to a high of 21.2 mg O2 gww −1 min−1 (CI, 18.3–24.2 mg O2 gww −1 min−1) at 500 μmol photons m−2 s−1, respectively (Fig. 1a).
The response of the oxygenic photosynthesis and dark respiration of G. acerosa to temperature and irradiance. a The net photosynthetic rates along an irradiance gradient determined at 28 °C. b The gross photosynthetic rates along a temperature gradient determined at 200 μmol photons m−2 s−1. c The dark respiration rates along a temperature gradient. The dots and vertical lines indicate the mean and 95 % confidence interval of the data (n = 5) and the model lines indicate the expected value. The shaded region in a and b indicates the 95 % Bayesian credible interval of the model, and for c, the shaded region indicates the 95 % confidence interval of the model
Given the model (Eq. 2) and the data, the posterior distribution of the parameters to describe the model was determined to be 27.7 mg O2 gww −1 min−1 (95 % Bayesian credible interval (BCI) of 24.3–31.0 mg O2 gww −1 min−1) for the maximum net photosynthetic rate (P max), 7.8 mg O2 gww −1 min−1 (BCI, 5.0–10.5 mg O2 gww −1 min−1) for the dark respiration rate (R d), and 0.25 mg O2 gww −1 min−1 (μmol photons m−2 s−1)−1 (BCI, 0.17–0.34 mg O2 gww −1 min−1 (μmol photons m−2 s−1)−1) for the initial slope (α) of the model, respectively.
From these parameters, we estimated that the 95 % BCI of the compensation irradiance (E c) was from 29 to 45 μmol photons m−2 s−1, with a mean value of 37 μmol photons m−2 s−1, and that the 95 % BCI of the saturation irradiance (E k) was from 52 to 128 μmol photons m−2 s−1, with a mean value of 83 μmol photons m−2 s−1, respectively.
Effect of temperature on the gross photosynthesis and dark respiration
The measured gross photosynthetic rates was highest at 28 °C, with a maximum value of 17.4 mg O2 gww −1 min−1 (CI, 12.4–22.4) and lowest at 40 °C with a minimum value of 3.3 mg O2 gww −1 min−1 (CI, 1.5–5.1 mg O2 gww −1 min−1) (Fig. 1b).
The model (Eq. 1) fit to the data indicated that the optimal temperature (T GPopt ) where the maximal gross photosynthetic rates (GP max = 18.4 mg O2mgww chl-a −1 min−1 (BCI, 15.9–20.8 mg O2mgww chl-a −1 min−1)) would occur was 29.2 °C (BCI, 26.8–32.1 °C). The activation energy (H GPa ) was determined to be 62.1 kJ mol−1 (BCI, 28.3–111 kJ mol−1) and the deactivation energy (H GPd ) was 296 kJ mol−1 (BCI, 197–533 kJ mol−1).
The measured dark respiration rates increased in magnitude from 0.9 ± 0.9 mg O2 gww −1 min−1 °C−1 (mean ± SE) at 16 °C to 6.7 ± 1.4 mg O2 gww −1 min−1 °C−1 at 32 °C (Fig. 1c). Two generalized linear models of the dark respiration rate were fitted to the data assuming that linear model was the null model and the second order model was the alternative. An F test revealed that the second order model best fit the data, with F (1,38) = 9.27 (P < 0.01). Therefore, we assumed that the dark respiration rates and temperature relationship could be modeled in the form, y = b 0 + b 1 x + b 2 x 2, were y is the respiration rate and x is the temperature. The coefficients b0, b1, and b2 were all significant (P < 0.01), and were estimated to be 13.8, −1.24, and 0.019, respectively. Hence, the expected values of the respiration rates were −0.49 mg O2 gww −1 min−1 at 16 °C and −0.93 mg O2 gww −1 min−1 at 40 °C.
Effect of temperature on and maximum quantum yield (ΦPSII)
The temperature response of the effective quantum yield (ΦPSII) at 0 μmol photons m−2 s−1 was similar to that of the photosynthesis–temperature relationship (Fig. 2). Indeed, ΦPSII was low at low temperatures, and the measured value (mean ± SE) was 0.22 ± 0.01 at 14 °C. However, the ΦPSII climbed to a peak of 0.59 ± 0.01 at 26 °C, and then decreased to another low of 0.20 ± 0.04 by 40 °C.
The temperature response of the maximum effective quantum yield (ΦPSII at 0 μmol photons m−2 s−1) in G. acerosa. The dots and vertical bars indicate the mean value of the data (n = 10) and the 95 % confidence interval of the mean. The model lines indicate the expected value. The shaded regions indicate the 95 % Bayesian confidence interval of the model
Given the model (Eq. 1) and the data, the maximum ΦPSII (Φ PS I I max) occurring at the optimal temperature (T ΦPSIIopt = 27.7 (BCI, 26.5–28.9) °C) was 0.57 (BCI, 0.55–0.58). The activation and deactivation energy was determined to be 10.4 (6.4–15.6) kJ mol−1 and 247 (194–308) kJ mol−1, respectively.
Discussion
In Thailand, G. acerosa can be found on the substrata throughout the year and is reported to be particularly abundant during the rainy season (e.g., October; Prathep 2005). The laboratory study of G. acerosa, clearly revealed that temperature influences their photosynthetic activity in addition to the rates of dark respiration. Indeed, the temperature response of oxygen evolution (gross photosynthetic rate) showed a characteristic dome-like shape, and the highest gross photosynthetic rate occurred at 29.2 °C (Fig. 1b). Likewise, an empirical second-order linear model suggested that respiration rates increased nonlinearly with temperature and are restricted at higher temperatures (Fig. 1c). Although this model is difficult to interpret, perhaps the restriction of the respiration rate above 36 °C might be related to denatured enzymes and damage of protein structures that was caused by the thermal stress. More experiments will be needed to confirm this hypothesis.
Nevertheless, the apparent optimal temperatures determined for photosynthetic activity was within the ranges reported for their natural habitat in the Philippines (27 to 31 °C; Roleda et al. 1997b), Taiwan (18 to 25 °C; Lin and Freshwater 2008), and the Ryukyu Islands, Japan (18 to 28 °C; Chihara and Kamura 1963; Vo et al. 2014). It is relevant to note that in a prior study of the macroalgal community found in the lower intertidal zone and near the collection site, the seawater temperatures were recorded to range from 27.8 to 34.5 °C (Prathep et al. 2007).
It is also notable that the maximum effective quantum yield (ΦPSII at 0 μmol photons m−2 s−1) was temperature dependent, given that it gradually increased with increasing temperature until 27 °C, and then quickly decreased above 30 °C (Fig. 2). More importantly, it closely corresponded with the temperature response of oxygen evolution (gross photosynthesis) and the optimum temperature for maximum effective quantum yield and oxygen evolution were almost identical.
Previous studies also indicated that the maximum effective quantum yield was temperature dependent and its optimum temperature was almost related to the temperature in the natural habitat during the period when each species or sexual phase occurred, as reported for Pyropia tenera (Kjellman) Kikuchi et al. (Watanabe et al. 2014a) and Agardhiella subulata (C. Agardh) Kraft et Wynne (Vo et al. 2014). For this study, the maximum effective quantum yield also corresponded with those of the natural habitat in the Andaman Sea coast of Thailand (Prathep et al. 2007).
Despite the temperature response in the ΦPSII in the present study, the maximum quantum yield (Fv/Fm) is typically known as being temperature independent. Nevertheless, the effective quantum yield (ΦPSII) is temperature dependent in physiologically appropriate temperature ranges and will vary with species (Dongsansuk et al. 2013; Salvucci and Crafts-Brandner 2004). As we mentioned in Watanabe et al. (2014a), the reduction in gross photosynthesis at high temperatures are partly attributable to increased respiration rates, as demonstrated by the dark respiration experiments and in part to reduced CO2 uptake as rubisco activity declines (Salvucci and Crafts-Brandner 2004). However, the mechanisms associated with the decline in ΦPSII are uncertain. Typically in higher plants, the decrease of the ΦPSII at high temperatures can be associated PSII reaction centers inactivation (Roháček 2002).
However, unlike higher plants and other orders of macroalgae, red algae are known to have light harvesting antennae that include phycobilisomes, which capture a wide band (580 to 680 nm) of light energy (Yokono et al. 2011; Larkum 2003; Larkum and Vesk 2003). Approximately half of the energy captured by phycobilisomes is then transferred to photosystem II (PSII), and when all the photochemical traps of PSII are closed, up to 100 % of this energy can be transferred to PSI (Kowalczyk et al. 2013). Therefore, for high-temperature PAM experiments, the decreases in ΦPSII might be due to changes in the state of PSII or to the proportion of energy transferred to PSI and PSII.
Despite the concerns of tolerable temperature in the tropical waters, G. acerosa was considered to be adapted to the current range of seawater temperatures in this region. This insight might be related to the habitat characteristics of this species that is growing on the lower intertidal zone, which is exposed by the lowest spring tide. In the tropical region, seawater temperature in the shallow intertidal zone is somewhat unstable (Prathep et al. 2007; Tanzil et al. 2009). Direct sunlight in shallow waters undergoing tidal fluctuation have different thermal properties associated with decreased water mass during shallow periods and increased water mass during relatively deep periods. Indeed, characteristics of thermal and desiccation tolerance have been reported for some intertidal species (Dring and Brown 1982; Lüning 1984; Smith and Berry 1986; Bell 1993; Kübler and Davison 1993; Davison and Perason 1996) including Gelidium pusillum (Stackhouse) Le Jolis from Thailand (Prathep et al. 2009). It is also likely that G. acerosa can tolerate short-term desiccation, because it is sometimes exposed from the seawater by the lowest spring tide. However, the eventual bleaching of this species after long periods of emersion were also reported by Ganzon-Fortes (1997a, b), suggesting that further studies of the desiccation tolerance must be required in future.
The experiments revealed that photosynthetic rates of this species were optimal within the range of 26 to 32 °C, but are close to their marginal temperatures in the natural environment. If summer seawater temperatures continue to rise in these regions, as has been shown in previous studies (Tanzil et al. 2009), the continuous and compounded effects of high temperature, direct sunlight, and emersion may lead to the decreased abundance of this species (Roleda et al. 1997b).
Regarding the PAR response of G. acerosa, the experiments revealed that the net photosynthetic rate at 28 °C was strongly dependent on irradiance when PAR < 83 μmol photons m−2 s−1, which is the E k determined from the model (Fig. 1a). Therefore, we can hypothesize that the vertical distributional limit of these species is connected to the magnitude of E k. In contrast, we did not detect any evidence of photoinhibition, which was similar to other intertidal species (e.g., Gracilariopsis chorda (Holmes) Ohmi and P. tenera) determined from our previous studies (Terada et al. 2013; Watanabe et al. 2014a). Consider that G. acerosa can be found in the lower intertidal and upper sublittoral zones (i.e., up to 2 m in deep), and therefore, it is likely that this species is periodically exposed to direct sunlight during the daytime low tide. Therefore, the evidence for the lack of photoinhibition remains inconclusive, given that the experimental PAR did not exceed 500 μmol photons m−2 s−1.
We stress that the results of this study are based on short-term laboratory measurements of photosynthesis and respiration using both dissolved oxygen and fluorescence measurements, and that the temperature characteristics of these species remains to be fully elucidated. For example, temperature acclimation has not been examined for photosynthesis and respiration, which was observed in higher plants (Atkin and Tjoelker 2003). The results are extrapolated with caution, and we acknowledge that studies of longer timescale be conducted to verify our hypotheses, especially regarding climate change induced increases in temperature.
In conclusion, we recommend the continued monitoring of seawater temperature to better forecast the future state of G. acerosa growing in the tolerable environments and to include dissolved oxygen derived estimates of photosynthetic activity, given that fundamentally different processes of light energy harvesting and transfer of the photosynthetic apparatus in red algae.
References
Abbott IA (1999) Marine red algae of the Hawaiian Islands. Bishop Museum Press, Honolulu, 477 pp
Alexandrov GA, Yamagata Y (2007) A peaked function for modeling temperature dependence of plant productivity. Ecol Model 200:189–192
Armisen R, Galatas F (1987) Production, properties and uses of agar. In McHugh DJ (ed) Production and Utilization of Products from Commercial Seaweeds. FAO Fish. Tech. Pap. 288: 1-57
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Bell EC (1993) Photosynthetic response to temperature and desiccation of the intertidal alga Mastocarpus papillatus. Mar Biol 117:337–346
Brown BE, Dunne RP, Chansang H (1996) Coral bleaching relative to elevated seawater temperature in the Andaman Sea (Indian Ocean) over the last 50 years. Coral Reefs 15:151–152
Bunsom C, Prathep A (2012) Effect of salinity, light intensity and sediment on growth, pigments, agar production and reproduction in Gracilaria tenuistipitata from Songkhla Lagoon in Thailand. Phycol Res 60:169–178
Chennubhotla VSK, Kalimuthu S, Najmuddin M, Panigrahy R, Selvaraj M (1986) Changes in growth and phycocolloid content of Gelidiella acerosa and Gracilaria edulis. Seaweed Res Util 9:45–48
Chihara M, Kamura S (1963) On the germination of tetraspores of Gelidiella acerosa. Phycologia 3:69–74
Coppejans E, Prathep A, Leliaert F, Lewmanomont K, De Clerck O (2011) Seaweeds of Mu Ko Tha Lae Tai (SE Thailand). Methodologies and field guide to the dominant species. Biodiversity Research and Training Program (BRT), Bangkok, 274 pp
Davison IR, Perason GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32:197–211
R Development Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Dongsansuk A, Lutz C, Neuner G (2013) Effects of temperature and irradiance on quantum yield of PSII photochemistry and xanthophyll cycle in a tropical and temperate species. Photosynthetica 51:13–21
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Edwards MS, Kim KY (2010) Diurnal variation in relative photosynthetic performance in giant kelp Macrocystis pyrifera (Phaeophyceae, Laminariales) at different depths as estimated using PAM fluorometry. Aquat Bot 92:119–128
Edwards P, Tam DM (1984) The potential for Gracilaria farming in Thailand. Hydrobiologia 116/117:246–248
Fujimoto M, Nishihara GN, Terada R (2014a) The effect of irradiance and temperature on the photosynthesis of two agarophytes Gelidium elegans and Pterocladiella tenuis (Gelidiales) from Kagoshima, Japan. Fish Sci 80:695–703
Fujimoto M, Nitta K, Nishihara GN, Terada R (2014b) Phenology, irradiance and temperature characteristics of a freshwater red alga, Nemalionopsis tortuosa (Thoreales), from Kagoshima, southern Japan. Phycol Res 62:77–85
Ganzon-Fortes ET (1994) Gelidiella. In: Akatsuka I (ed) Biology of economic algae. SPB Academic Publishing, Hague, pp 149–184
Ganzon-Fortes ET (1997a) Diurnal and diel patterns in the photosynthetic performance of the agarophyte Gelidiella acerosa. Bot Mar 40:93–100
Ganzon-Fortes ET (1997b) Influence of tidal location on morphology, photosynthesis and pigments of the agarophyte, Gelidiella acerosa, from Northern Philippines. J Appl Phycol 9:525–532
Ganzon-Fortes ET (1999) Photosynthetic and respiratory responses of the agarophyte Gelidiella acerosa collected from tidepool, intertidal and subtidal habitats. Hydrobiologia 398/399:321–328
Gelman A (2004) Parameterization and Bayesian modeling. J Am Stat Assoc 99:537–545
Gelman A (2006) Prior distributions for variance parameters in hierarchical models. Bayesian Anal 1:515–533
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photo inhibition and diel changes. J Phycol 29:729–39
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Kowalczyk N, Rappaport F, Boyen C, Wollman FA, Collen J, Joliot P (2013) Photosynthesis in Chondrus crispus: the contribution of energy spill-over in the regulation of excitonic flux. Biochim Biophys Acta Bioenerg 1827:834–842
Kübler JE, Davison IR (1993) High-temperature tolerance of photosynthesis in the red alga Chondrus crispus. Mar Biol 117:325–335
Larkum AWD (2003) Light-harvesting systems in algae. In: Larkum AWD, Douglas SE, Raven JA (eds) Photosynthesis in algae. Kluwer, Dordrecht, pp 277–304
Larkum AWD, Vesk M (2003) Algal plastids: their fine structure and properties. In: Larkum AWD, Douglas SE, Raven JA (eds) Photosynthesis in algae. Kluwer, Dordrecht, pp 11–28
Lewmanomont K, Ogawa H (1995) Common seaweeds and seagrasses of Thailand. Faculty of Fisheries, Kasetsart University, Bangkok, 163 p
Lideman, Nishihara GN, Noro T, Terada R (2013) Effect of temperature and light on the photosynthesis as measured by chlorophyll fluorescence of cultured Eucheuma denticulatum and Kappaphycus sp. (Sumba strain) from Indonesia. J Appl Phycol 25:399–406
Lin SM, Freshwater DW (2008) The red algal genus Gelidiella (Gelidiales, Rhodophyta) from Taiwan, including Gelidiella fanii sp. nov. Phycologia 47:168–176
Lüning K (1984) Temperature tolerance and biogeography of seaweeds: the marine algal flora of Helgoland (North Sea) as an example. Helgol Meeresunters 38:305–317
McHugh DJ (1991) Worldwide distribution of commercial resources of seaweeds inc1uding Gelidium. Hydrobiologia 221:12–29
Muraoka D, Yamamoto H, Yasui H, Terada R (1998) Formation of wound tissue of Gracilaria chorda Holmes (Gracilariaceae) in culture. Bull Fac Fish Hokkaido Univ 49:31–39
Nishihara GN, Terada R, Noro T (2004) Photosynthesis and growth rates of Laurencia brongniartii J. Agardh (Rhodophyta, Ceramiales) in preparation for cultivation. J Appl Phycol 16:303–308
Ohno M, Largo DB (1998) The seaweed resources of Japan. In: Critchley AT, Ohno M (eds) Seaweed Resources of the World. Japan International Cooperation Agency (JICA), Yokosuka, pp 1–14
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Praiboon J, Chirapart A, Akakabe Y, Bhumibhamond B, Kajiwara T (2006) Physical and chemical characterization of agar polysaccharides extracted from the Thai and Japanese species of Gracilaria. Sci Asia 32:11–17
Prathep A (2005) Spatial and temporal variations in diversity and percentage cover of macroalgae at Sirinart Marine National Park, Phuket Province, Thailand. Sci Asia 31:225–233
Prathep A, Wichachucherd B, Thongroy P (2007) Spatial and temporal variation in density and thallus morphology of Turbinaria ornata in Thailand. Aquat Bot 86:132–138
Prathep A, Lewmanomont K, Buapet P (2009) Effects of wave exposure on population and reproductive phenology of an algal turf, Gelidium pusillum (Gelidales, Rhodophyta), Songkhla, Thailand. Aquat Bot 90:179–183
Rao PS, Mehta VB (1973) Physiological ecology of Gelidiella acerosa (Forsskal) Feldmann et Hamel. J Phycol 9:333–335
Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40:13–29
Roleda MY, Montaño NE, Ganzon-Fortes ET, Villanueva RD (1997a) Acetic acid pretreatment in agar extraction of Philippine Gelidiella acerosa (Forsskaal) Feldmann et Hamel (Rhodophyta, Gelidiales). Bot Mar 40:63–70
Roleda MY, Ganzon-Fortes ET, Montaño NE, de los Reyes FN (1997b) Temporal variation in the biomass, quantity and quality of agar from Gelidiella acerosa (Forsskål) Feldmann et Hamel (Rhodophyta: Gelidiales) from Cape Bolinao, NW Philippines. Bot Mar 40:487–496
Salvucci M, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol 134:1460–1470
Santelices B, Stewart JG (1985) Pacific species of Gelidium Lamouroux and other Gelidiales (Rhodophyta), with keys and descriptions to the common or economically important species. In: Abbott IA, Norris JN (eds) Taxonomy of economic seaweds with reference to some Pacific and Caribbean species. California Sea Grant College Program, La Jolla, pp 17–31
Schagerl M, Möstl M (2011) Drought stress, rain and recovery of the intertidal seaweed Fucus spiralis. Mar Biol 158:2471–2479
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Serisawa Y, Yokohama Y, Aruga Y, Tanaka J (2001) Photosynthesis and respiration in bladelet of Ecklonia cava Kjellman (Laminariales, Phaeophyta) in two localities with different temperature conditions. Phycol Res 49:1–11
Smith CM, Berry JA (1986) Recovery of photosynthesis after exposure of intertidal algae to osmotic and temperature stresses: comparative studies of species with differing distributional limits. Oecologia 70:6–12
Stan Development Team (2013) Stan: A C++ Library for Probability and Sampling, Version 1.3. URL: http://mc-stan.org
Tanzil JTI, Brown BE, Tudhope AW, Dunne RP (2009) Decline in skeletal growth of the coral Porites lutea from the Andaman Sea, South Thailand between 1984 and 2005. Coral Reefs 28:519–528
Terada R, Inoue S, Nishihara GN (2013) The effect of light and temperature on the growth and photosynthesis of Gracilariopsis chorda (Gracilariales, Rhodophtya) from geographically separated locations of Japan. J Appl Phycol 25:1863–1872
Thomas PC, Rao KR, Subbaramaiah K (1975) Changes in the natural growth of Gelidiella acerosa (Forskal) Feldmann et Hamel in an exploited population. Bot Mar 18:241–243
Tsutsui I, Hamano K, Aue-umneoy D, Songphatkaew J, Srisapoome P, Ruangsomboon S, Klomkling S, Ganmanee M, Taveekijararn P, Maeno Y (2012) Common underwater plants in coastal areas of Thailand. International Agriculture Series No. 21. Japan International Research Center for Agricultural Studies, Tsukuba, 171 pp
Villanueva RD, Montaño NE, Romero JB, Aliganga AKA, Enriquez EP (1999) Seasonal variations in the yield, gelling properties, and chemical composition of agars from Gracilaria eucheumoides and Gelidiella acerosa (Rhodophyta) from the Philippines. Bot Mar 42:175–182
Vo TD, Nishihara GN, Shimada S, Watanabe Y, Fujimoto M, Kawaguchi S, Terada R (2014) Taxonomic identity and the effect of temperature and light on the photosynthesis of an indoor tank-cultured red alga, Agardhiella subulata, from Japan. Fish Sci. 80:281–292
Watanabe Y, Nishihara GN, Tokunaga S, Terada R (2014a) The effect of irradiance and temperature on the photosynthesis of a cultivated red alga, Pyropia tenera (=Porphyra tenera), at the southern limit of distribution in Japan. Phycol Res. 62:187–196
Watanabe Y, Nishihara GN, Tokunaga S, Terada R (2014b) The effect of irradiance and temperature responses and the phenology of a native alga, Undaria pinnatifida (Laminariales), at the southern limit of its natural distribution in Japan. J Appl Phycol. doi:10.1007/s10811-014-0264-z
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia 17:281–291
Yokono M, Murakami A, Akimoto S (2011) Excitation energy transfer between photosystem II and photosystem I in red algae: larger amounts of phycobilisome enhance spillover. Biochim Biophys Acta Bioenerg 1807:847–853
Zemke-White WL, Ohno M (1999) World seaweed utilisation: an end-of-century summary. J Appl Phycol 11:369–376
Acknowledgments
We express our gratitude to Khanjanapaj Lewmanomont, Eric Coppejans and Ken-ichi Hayashizaki, for their valuable suggestions at the field survey in Krabi, Thailand. We also thank Jaruwan Mayakun, Anuchit Darakrai, Supattra Pongparadon, Ekkalak Ratanachot, and Takashi Tsunoda for their kind assistances to complete the field survey. This research was sponsored in part by a JSPS-Asian CORE Program in the Atmosphere and Ocean Research Institute, the University of Tokyo (AP and RT), and by a Grant-in-Aid for Scientific Research (nos. 22510033, 25340012, and 25450260) from the Japanese Ministry of Education, Culture, Sport and Technology (RT and GNN); partially funded by Plant Genetic Conservation Project Under the Royal Initiation of Her Royal Highness Princess Maha Chakri Sirindhorn (AP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujimoto, M., Nishihara, G.N., Prathep, A. et al. The effect of irradiance and temperature on the photosynthesis of an agarophyte, Gelidiella acerosa (Gelidiales, Rhodophyta), from Krabi, Thailand. J Appl Phycol 27, 1235–1242 (2015). https://doi.org/10.1007/s10811-014-0409-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0409-0