Abstract
The photosynthetic performance of two Indonesian carrageenophytes (Solieriaceae), Eucheuma denticulatum and Kappaphycus sp. (so-called Sumba strain), was investigated under a variety of temperature and light conditions regarding their mariculture performance. A pulse amplitude modulated-chlorophyll fluorometer (Diving-PAM) was used to generate rapid light curves (RLCs) to provide estimates of the relative electron transport rates (rETR) for over 10 temperatures (i.e., from 16 to 34 °C) and at nine levels of photosynthetic active radiation, which ranged from 0 to 1,000 μmol photons m−2 s−1. Underwater irradiance in a cultivation area was also measured at the collection site in South Sulawesi, Indonesia. The initial slope (α), photoinhibition coefficient (β), and the coefficient of maximum photosynthesis assuming no photoinhibition (γ) was calculated by fitting the RLCs to a nonlinear model of the form \( {\text{rETR}} = \gamma \left( {1 - \exp \left( { - \frac{\alpha }{\gamma }{\text{PAR}}} \right)} \right)\left( {\exp \left( { - \frac{\beta }{\gamma }{\text{PAR}}} \right)} \right) \) using a two-level hierarchical Bayesian model. The experiments revealed that E. denticulatum and Kappaphycus sp. required temperatures ranging from 23 to 32 °C and 22 to 33 °C to maintain high rates of photosynthetic activity, respectively. Clearly, both species appear to be well-adapted to the natural light and temperature conditions at the cultivation site, and we expect the results of this study will be useful for the design and sustainable management of similar mariculture activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The red algal genera Eucheuma and Kappaphycus (Solieriaceae) are considered commercially important commodities due to their production of carrageenan (Waaland 1981). They are largely cultivated in tropical areas especially in South East Asia, particularly in Indonesia and the Philippines (Bixler and Porse 2011). Indeed, the mariculture of these genera has supplied a large portion of the world’s carrageenan products (Dawes et al. 1994) and many strains and species have been cultivated. Yet we point out that there are many strains and species, whose physiological response and therefore cultivation strategies remain to be revealed. Many strains under the name of Kappaphycus alvarezii or related species are cultured in Indonesia, such as the Sakol, Tambalang, Cottonii, and Sumba. For example, the Sumba strain (Kappaphycus sp.) is believed to originate from Sumba Island in Indonesia, while the Sakol and Tambalang strains were brought over from the Philippines in the 1990s.
Furthermore, many physiological studies on the photosynthetic response of these carrageenan-producing algae have been carried out using experiments that examine dissolved oxygen production. For example, E. denticulatum and K. alvarezii are the most commonly used species in experiments on carrageenan-producing algae (Dawes et al. 1994; Ohno et al. 1994; Wobeser et al. 2001). These studies on photosynthesis have been mainly conducted by measuring oxygen evolution under relatively low conditions of irradiance (e.g., <650 μmol photons m−2 s−1; Wobeser et al. 2001). Despite such past studies, in Indonesia, the Sumba strain, which is a strain of Kappaphycus, and E. denticulatum are the most prominent species cultivated, and little is known regarding their physiology. Any further enhancements in production rates and efficiency will require further insight regarding the physiology of these organisms. Indeed, the optimal temperature and irradiance requirements of these species cultivated in Indonesia remain to be determined.
Since the late 1980s, pulse amplitude modulated (PAM)-chlorophyll fluorometry has been used for seagrasses as well as many terrestrial plants (Beer et al. 1998; Beer and Björk 2000; Aldea et al. 2006; Kuster et al. 2007; Ralph et al. 1998, 2006) as a quick and efficient way of evaluating the photosynthetic response of whole intact plants. PAM has also been used in aquatic research of macroalgae to elucidate photosynthetic response (Renger and Schreiber 1986; Huppertz et al. 1990; Gevaert et al. 2002; Enriquez and Borowitzka 2011). Clearly, PAM would be an efficient way of clarifying the response of cultured plants of Eucheuma/Kappaphycus (Aguirre-von-Wobeser et al. 2001), and a battery of tests could be designed to clarify the optimal temperature and irradiance conditions that would maximize photosynthesis (i.e., growth) and also be used to diagnose the physiological condition of cultivated macroalgae (i.e., health).
In this study, we focused on elucidating the temperature and irradiance conditions needed to promote photosynthesis in cultured E. denticulatum and Kappaphycus sp. (Sumba strain) from Indonesia using PAM fluorometry. We use this technology to perform the first trial study of Indonesian Eucheuma/Kappaphycus photobiology, with the hope that this knowledge will lead to an advancement in cultivation output and efficiency of these species.
Materials and methods
Cultured specimens of Eucheuma denticulatum and Kappaphycus sp. (Sumba strain) (Fig. 1) were collected in a farming area in Funga, South Sulawesi, Indonesia (5°34′56.62″N, 119°27′42.56″E) on August 15, 2010.
For each species, 10 cultured plants that were attached to a rope at the depth of 1–2 m were collected by the first author and stored in 1,000-mL plastic bags in a cool box at approximately 24 °C. They were directly transported within 2 days to a laboratory on the T/S “Kagoshima Maru”, a research vessel of the Faculty of Fisheries, Kagoshima University, anchored in the Pelabuhan Benoa, Bali Indonesia. During the experiments, specimens were maintained in a tank (1.0 × 1.0 × 0.5 m) containing natural seawater (salinity at around 33 PSU, pH 8.0) that was placed in the laboratory. Water temperature was maintained at 24 °C by water-temperature controller. Photosynthetic active radiation (PAR) was provided by room fluorescent lamps at around 90 μmol photons m−2 s−1 with 12:12 light/dark cycle. After transport, the samples were acclimatized for around 4 h before experiment. Voucher herbarium specimens of the two taxa were deposited in the herbarium of Marine Botany, Kagoshima University Museum (KAG).
Underwater temperature and photosynthetic active radiation
Underwater irradiance and water temperature was measured in a coral lagoon, which was located inside of the mariculture farm during noon on August 15, 2010 (fine weather with no clouds). A PAR light meter with underwater spherical quantum sensor (LI-193, LI-250, LI-COR, USA) and thermometer (YSI model 85, USA) were used for measuring PAR and surface seawater temperature, respectively. Specifically, PAR was measured 300 m from the shoreline at a depth of 4 m, 700 m from the shoreline at a depth of 5 m, and 1,000 m from the shoreline at a depth of 5 m. PAR measurements were the used to determine the extinction coefficient (K) that of the Beer–Lambert equation:
where, E (Z) is PAR at the some depth (Z) in meters, E (0) is surface PAR coefficient, and K is the extinction coefficient. Terminology and abbreviation in this study are based on Cosgrove and Borowitzka (2011).
Rapid light curves
Rapid light curves (RLCs) were generated by running the standard algorithm of the PAM fluorometer (Diving-PAM, Heinz Walz GmbH, Germany) using an incremental sequence of actinic illumination periods, with PAR intensities increasing in nine steps from 0 to 1,000 μmol photons m−2 s−1. Relative electron transport rate (rETR) was calculated using the equation:
where, Y is the effective quantum yield of PSII (\( {\Phi_{\text{PSII}}} = \left( {F - {F_{\text{m}}}\prime } \right)/{F_{\text{m}}}\prime \), where F is the initial fluorescence, and F m’ is the maximum fluorescence), the factor 0.5 assumes that half of the photons are absorbed by PSII (Schreiber et al. 1995), and AF is the fraction of incident light assumed to be absorbed by the sample (i.e., 0.84).
Temperature and light effect on photosynthetic parameters
From each collected specimen, 2-cm long portions of the thalli were placed in a 50 × 40 × 50 cm plastic tank with seawater, with four replicates for each species. Temperature was adjusted and maintained by temperature-controlled water bath. Water temperature in the tank was measured with a thermocouple in order to confirm that the water reached the desired temperature setting. The relative electron transport rates were determined by generating RLCs with nine PAR levels, for every 2 °C increment of temperature ranging from 16 to 34 °C. When we changed the temperature condition, we spent for at least 1 h to change 2 °C. We also acclimatized the algae for more than 1 h before each temperature experiment.
We modeled the rETR versus PAR to calculate the maximum rETR rate in the absence of photoinhibition (γ), the initial slope (α) of the photosynthesis–irradiance curve (P-I curve) and the photoinhibition coefficient (β) by fitting the RLCs to a nonlinear model modified after Platt et al. (1980):
Based on these parameters, we then estimated the values of E k, which defines PAR when rETR begins to saturate (Eq. 4) and E opt, which defines PAR when the rETR is at a maximum.
Furthermore, by computing the derivative of Eq. 4 with respect to PAR, and solving the equation when \( \frac{{drETR}}{{dPAR}} = 0 \), the value of PAR at the rETR maxima can be estimated from the first real root:
By substituting PARopt into Eq. 4, we arrive at the value of rETR at the maximum (rETRmax) of the P-I curve. Saturating PAR (E k) was calculated using the equation:
Statistical analysis
Statistical analyses of photosynthetic parameters of γ, α, and β were done using R (R Development Core Team 2011) and model fitting was done using OpenBUGS (Thomas et al. 2006). The parameters were examined by fitting the RLCs to the nonlinear model (Eq. 3) using hierarchical Bayesian methods. OpenBUGS primarily uses a Gibbs sampler to construct the posterior distributions of the parameters, and four chains of at least 20,000 samples were generated and assessed for convergence. Uniform priors were placed on all of the hyper parameters of the model.
Model formulation and selection of the relationship between the estimated parameters and experimental water temperature were also examined based on the methods used by Bhujel (2008). Based on the model results, a range of optimum temperatures for the photosynthetic activity of these species could be defined as at least 95 % of the estimated maximum or minimum parameter values. A general linear model was used to examine temperature and species effect and the interaction of them on the photosynthetic parameters. Levene’s homogeneity test was used to test equality of error of variances. The curve estimation and general linear model were analyzed by using SPSS v.17 (SPSS Inc.).
Results
Irradiance and temperature in habitat of specimen
At the collection site, the cultured E. denticulatum and Kappaphycus sp. were found to be attached to a rope that was set to a depth of 1–2 m. The irradiances were 1,448 to 393 μmol photon m−2 s−1 between depths of 0.5 and 3 m for the location 300 m from the shoreline. At 700 m from shore, PAR ranged from 1,482 to 109 μmol photon m−2 s−1 between depth of 0.5 and 5 m, and at 1,000 m from shore, PAR ranged from 1,213 to 138 μmol photon m−2 s−1 between a depth of 0.5 and 5 m (Fig. 2). The average sea surface temperature was 29 °C. According to the Beer–Lambert equation, 300 m from shoreline, the irradiance can be described as E (Z) = 1,848.4 e−0.54·Z (R² = 0.9713); 700 m from the shoreline, it was E (Z) = 1,964.8e−0.60·Z (R² = 0.9925); and 1,000 m from the shoreline, it was E (Z) = 1,666.3 e−0.53·Z (R² = 0.9752). Note that the extinction coefficients (K) determined for waters at 300, 400, and 700 m from the shore line were 0.54, 0.60, and 0.53, respectively, and the coefficient of surface PAR were 1,848, 1,964, and 1,666 μmol photons m−2 s−1, respectively. Based on these estimated parameters and assuming a surface PAR of 2,000 μmol photons m−2 s−1, the estimated PAR at the depth of cultured plant (1–2 m deep) can be estimated to range from 600 and 1,100 μmol photons m−2 s−1.
Rapid light curves
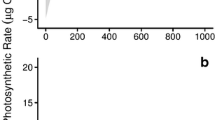
Generally, the rapid light curves of these species increased until reaching some asymptote, and photoinhibition was not readily apparent until PAR reached 1,000 μmol m−2 s−1 (Fig. 3). At any given temperature and PAR, the rETR of Kappaphycus sp. tended to be higher than that of E. denticulatum. By fitting Eq. 4 to the results using hierarchical Bayesian methods, we were able to elucidate the parameters of the model (γ, α, and β) across all water temperatures, as well as derive estimates of rETRmax, E k, and E opt.
Effect of temperature on the photosynthetic parameters
The mean values of the maximum rETR in the absence of photoinhibition (γ), ranged from 13.1 to 25.0 μmol e− m−2 s−1 for E. denticulatum and 14.3–34.6 μmol e− m−2 s−1 for Kappaphycus sp. over the range of temperatures examined, and they monotonically increased with increasing temperature (Fig. 4a). The parameter γ for both E. denticulatum and Kappaphycus sp. increased linearly with temperature (F (1,39) = 112.992, P < 0.001 and F (1,39) = 207.365, P < 0.001, respectively). The models of γ vs. temperature (t) could be described by equation: γ = 0.127 + 0.753 t (R 2 = 0.748) for E. denticulatum and γ = −5.243 + 0.73 t (R 2 = 0.845) for Kappaphycus sp. Temperature and species had a significant effect on γ (F (9,80) = 54.905, P < 0.001 and F (9,80) = 148.159, P < 0.001, respectively), and there was also an interaction between temperature and species (F (1,80) = 4.914, P < 0.001), which suggests that γ for Kappaphycus sp. responded to temperature differently when compared to E. denticulatum.
The photosynthetic parameters at some temperature levels of cultured E. denticulatum (triangle) and Kappaphycus sp. (black circle) and the fitted models for E. denticulatum (gray lines) and Kappaphycus sp. (solid lines). a The maximum relative electron transport rates (rETR) in absence of photoinhibition, γ. b The initial slope of the rapid light curves, α. c The photoinhibition coefficient, β. d The maximum rETR that was observed when photosynthetic active radiation (PAR) was optimum, rETRmax. e The PAR at which rETR rates begin to saturate, E k. f The PAR at which maximum rETR was observed, E opt. Bars indicate one standard deviation. n = 4 samples for each temperature and PAR
The mean values of the initial slope (α) of E. denticulatum and Kappaphycus sp. ranged from 0.076 to 0.148 μmol e− (μmol photons)−1 and 0.123 to 0.181 μmol e− (μmol photons)−1, respectively, and were dome-shaped with respect to temperature (Fig. 4b). The model between temperature and α of E. denticulatum and Kappaphycus sp. can be described by a quadratic function (F (2,39) = 51.151, P < 0.001 and F (2,39) = 31.098, P < 0.001, respectively) and they could be described by the equation: α = −0.2726 + 0.0313 t − 0.0006 t 2 (R 2 = 0.734) for E. denticulatum and α = −0.1651 + 0.0256 t − 0.0005 t 2 (R 2 = 0.627) for Kappaphycus sp. Based on these models, the highest α of E. denticulatum and Kappaphycus sp. were estimated at 0.135 and 0.163 μmol e− (μmol photons)−1 and occurred at temperatures of 26.1 and 25.6 °C, respectively. Hence, for α, 95 % of the maximum would be 0.128 μmol e− m−2 s−1 for E. denticulatum and 0.154 μmol e− m−2 s−1 for Kappaphycus sp., which leads to temperature range of 22.6–29.6 °C and 21.5–29.7 °C, respectively. The α values were significantly affected by temperatures (F (9,80) = 16.860, P < 0.001), while the interactions between temperature and species were insignificant (F (1,80) = 4.914, P = 0.976). However, a species effect was detected (F (9,80) = 149.863, P < 0.001), indicating that the α values of Kappaphycus sp. were significantly higher than E. denticulatum. There was an estimated 0.03 μmol e− (μmol photons)−1 difference in the parameter estimates of the maximum α rate between these species.
The mean values of the photoinhibition coefficient (β) of these species ranged from 0.001 to 0.010 μmol e− (μmol photons)−1 for E. denticulatum and from 0.002 to 0.09 μmol e− (μmol photons)−1 for Kappaphycus sp., and were U-shaped in nature (Fig. 4c). This parameter can also be modeled as a quadratic relationship with temperature for both E. denticulatum (F (2,39) = 18.921, P < 0.001) and Kappaphycus sp. (F (2,39) = 19.164, P < 0.001) and they could be described by equation: β = 0.0589 − 0.0046 t + 0.0000918 t 2 (R 2 = 0.506) for E. denticulatum and β = 0.0232 − 0.0019 t + 0.0000452 t 2 (R 2 = 0.509) for Kappaphycus sp. A β minimum could be estimated at temperatures near 25.0 and 21.0 °C for E. denticulatum and Kappaphycus sp., respectively. In case of β, 95 % of the minimum value would be 0.00139 μmol e− m−2 s−1 for E. denticulatum and 0.00342 μmol e− m−2 s−1 for Kappaphycus sp., which leads to a temperature range of 24.1–26.0 °C and 19.2–22.8 °C, respectively. Although temperatures had significant effect on β (F (9,80) = 7.065, P < 0.001), species differences did not appear to be a significantly important factor (F (1,80) = 1.304, P = 0.258), and the interactions among species and temperature was not strong (F (9,80) = 2.174, P = 0.037).
The mean values of rETRmax of E. denticulatum and Kappaphycus sp. ranged from 9.02 to 22.26 μmol e− m−2 s−1 and 12.44 to 30.84 μmol e− m−2 s−1, respectively (Fig. 4d). A model describing the relationship between temperature and rETRmax of E. denticulatum and Kappaphycus sp. was quadratic in form (F (2,39) = 100.046, P < 0.001 and F (2,39) = 141.720, P < 0.001, respectively). The relationship between temperature and rETRmax could be described by equation: rETRmax = −22.991 + 2.576 t − 0.039 t 2 (R 2 = 0.844) for E. denticulatum and rETRmax = −40.590 + 4.275 t − 0.067 t 2 (R 2 = 0.885) for Kappaphycus sp. Based on these models, the maximum rETRmax of E. denticulatum and Kappaphycus sp. was 19.5 and 27.6 μmol e− m−2 s−1 and occurred at temperatures of 31.1 and 31.5 °C, respectively. Moreover, for α, 95 % of the maximum would be 18.6 μmol e− m−2 s−1 for E. denticulatum and 26.2 μmol e− m−2 s−1 for Kappaphycus sp., which leads to a temperature range of 28.0–34.0 and 27.4–34.0 °C, respectively. The rETRmax values were significantly affected by temperatures (F (9,80) = 111.081, P < 0.001), and there were interactions between temperature and species (F (1,80) = 7.831, P < 0.001). A species effect was detected (F (9,80) = 418.296, P < 0.001), which suggests that rETRmax of Kappaphycus sp. was significantly higher than E. denticulatum.
Regarding the mean values for E k, which indicates the value of PAR when rETR begins to saturate, they increased with increasing temperature (Fig. 4e). Temperature were significant factors in the differences determined for E k (F (9,80) = 19.951, P < 0.001), and there were interactions among temperature and species (F (9,80) = 2.207, P < 0.05); however, there were no significant species effect on E k (F (1,80) = 3.462, P = 0.068). A model describing the relationship between temperature and E k of E. denticulatum and Kappaphycus sp. were linear (F (2,39) = 29.482, P < 0.001 and F (2,39) = 116.522, P < 0.001, respectively). Furthermore, the relationship between temperature and E k of E. denticulatum and Kappaphycus sp. could be described by the equation: E k = 49.3 + 3.199 t (R 2 = 0.437) and E k = 24.364 + 4.461 t (R 2 = 0.754), respectively. Their E k values are shown in Table 1.
The PAR, where rETRmax was observed (at E opt), increased with increasing temperature and reached a peak at higher temperatures (Fig. 4f), with a significant temperature effect (F (9,80) = 25.049, P < 0.001) and a significant species effect (F (1,80) = 20.920, P < 0.001). There were also significant interactions describing the relationship between species and temperature (F (1,24) = 2.980, P < 0.01), where E opt for Kappaphycus sp. was generally higher and more sensitive to temperature compared to E. denticulatum. A model describing the relationship between temperature and E opt of E. denticulatum and Kappaphycus sp. was quadratic in form (F (2,39) = 25.169, P < 0.001 and F (2,39) = 48.174, P < 0.001, respectively) and could be described by equation: E opt = −468.441 + 70.780 t − 1.152 t 2 (R 2 = 0.576) and E opt = −786.596 + 100.889 t − 1.751 t 2 (R 2 = 0.723), respectively (Table 1).
Discussion
Temperature and irradiance response of photosynthetic activity
The drop in irradiance with increasing depth was clearly evident in the measurements taken during this study; based on these observations, we estimate that the local farmers were cultivating E. denticulatum and Kappaphycus sp. at PAR values from 602 to 1,177 μmol photons m−2 s−1 (at noon), given that they set their cultivation rope between 1 and 2 m. The extinction coefficients (K) varied from 0.53 to 0.60 in this study, which is higher than typically observed in the coral reef which ranged from 0.11 to 0.22 PAR m−1 (Glynn et al. 1983; Bejarano 2006), because in coral reefs, advection caused by wave action can help reduce turbidity caused by plankton and silt (Anthony et al. 2004). Given that the farming area were located 1,000 m from the reef edge, it is likely that such processes were not occurring in these locations, closer to shore.
Light availability is expected to influence the sensitivity of response to changing light climate, and hence affect values of α and β, which are the coefficients of photosynthesis and photoinhibition, respectively. However, these coefficients also can be temperature dependent, which can strongly influence the photosynthetic ability of these species. This study clearly shows that these two coefficients reached a maxima (e.g., α) or a minima (e.g., β) within similar ranges of temperature that correspond well to environmental temperature that ranged from 25 to 30 °C (Tomascik et al. 1997). Indeed, under the temperature and irradiance conditions determined in the ponds, it is likely that photoinhibition remains low, whereas photosynthesis can quickly respond to changes in irradiance, which is essential given that extinction coefficient was large (>0.50).
From the perspective of the aquaculture farmer, it is important to anticipate productivity with respect to changing environmental conditions. Indeed, to maximize production, it is desirable to know the temperatures that will ensure the maximization of production. Although PAM measures photosynthetic activity directly, and is not a direct indicator of productivity per se, it is an excellent surrogate, given the ease and speed of measurements. Hence, the parameter, rETRmax, provides some insight on the productivity of these seaweeds, with respect to temperature (Fig. 4d). Indeed, at temperatures from 28 to 34 °C, rETRmax was maximal for both species. More importantly, these temperatures correspond well with the water temperatures determined for the mariculture farm, as well as those recorded in Indonesian waters (Soegiarto et al. 1976; Nonji 1993; Tomascik et al. 1997; Amin et al. 2008). Moreover, these temperature ranges for maximal rETRmax include estimates from a study based on dissolved oxygen evolution, where Wobeser et al. (2001) estimated that maximum photosynthesis (P max) and respiration of K. alvarezii (red and green morphotypes from the Philippines) reach a maximum value at 30 °C. Other studies on photosynthesis and growth of carrageenan-producing subtropical red algae have similar ranges, where K. alvarezii expressed highest growth rates at temperatures of 25–28 °C (Ohno et al. 1994) and the growth of Meristotheca papulosa was optimal between 20 and 24 °C (Lideman et al. 2011).
It is also reasonable to suggest that the environmental conditions and species cultivated are well-matched in this region. However, it is important to note that the ranges in temperature provided are for each individual parameter, and should not be interpreted as the “best” range of temperatures for the cultivation of these species. It would be better if we could combine the information regarding these three important parameters so that we can derive a more robust range of optimal temperatures for the cultivation of these species. Hence, let the optimal temperature range be the range of temperatures that are the union of the temperature ranges determined for each of the parameters, particularly the values of α, β and rETRmax, which can be argued as the parameters that most strongly dictate the rates of photosynthetic activity. This reveals that the optimal temperature range for E. denticulatum was 23–32 °C and for Kappaphycus sp. the range was 22–33 °C.
Species-specific differences in model parameters
The model equation fit the observed values of rETR well for either species, and the relationship between each parameter and temperature were similar. Given the data, it appears that Kappaphycus sp. has a higher capacity for photosynthetic activity and can responds more quickly to a dynamic light environment, given the higher values of rETRmax and alpha, respectively. However, similarities in model parameters was apparent for E k (Fig. 4e) and E opt (Fig. 4f), where the values of these parameters were almost identical (P > 0.05). This strongly suggests that both species are adapted to similar temperature and light conditions and that only the capacity of photosynthesis differs. Detailed cultivation experiments will be needed if we are to elucidate if there are also differences in productivity between these two species. We hypothesize that (1) both species will grow well under similar conditions and (2) Kappaphycus sp. will out-compete E. denticulatum in growth rates under optimal temperature and light conditions.
In conclusion, the mariculture of E. denticulatum and Kappaphycus sp. are an important industry to many Southeast Asian countries, including China and parts of Africa. Although it is clear from many studies, including ours, that for the Southeast Asian region, the species and environment appear to be well-matched; detailed understanding of how the photobiology of these and other species respond to environmental change is an important precursor to the increased efficiency of cultivation and the optimization of production. Our study provides one clue on to how the photosynthetic activities of two species of carrageenan-producing red algae respond to irradiance and temperature, and we provide an estimate of the range in temperatures where cultivation should be expected to be successful. However, the detailed response of productivity with respect to temperature and light remain to be determined. Finally, PAM-based assessments of photosynthesis and the appropriate interpretation of rETR models can be expected to contribute to the advancement of seaweed mariculture.
References
Aguirre-von-Wobeser E, Figueroa FL, Cabello-Pasini A (2001) Photosynthesis and growth of red and green morphotypes of Kappaphycus alvarezii (Rhodophyta) from the Philippines. Mar Biol 138:679–686
Aldea M, Frank TD, DeLucia EH (2006) A method for quantitative analysis of spatially variable physiological processes across leaf surface. Photosynth Res 90:161–172
Amin M, Rumayar TP, Femmi NF, Keemur D, Suwitra IK (2008) The assessment of seaweed (Eucheuma cottonii) growing practice of different systems and planting seasons in Bangkep Regency Central Sulawesi. Indonesian J Agricul Sci 1:132–139
Anthony KRN, Ridd PV, Orpin AR, Larcombe P, Lough J (2004) Temporal variation of light availability in coastal benthic habitats. Effects of clouds, turbidity, and tides. Limnol Oceanogr 49:2201–2211
Beer S, Björk M (2000) Measuring rates of photosynthesis of two tropical seagrasses by pulse amplitude modulated (PAM) fluorometry. Aquatic Bot 66:69–76
Beer S, Vilenkin B, Weill A, Veste M, Susell L, Eshel A (1998) Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry. Mar Ecol Prog Ser 174:293–300
Bejarano RI (2006) Relationships between reef fish communities, water and habitat quality on coral reefs. M.S. thesis, University of Puerto Rico, Mayagüez
Bhujel RC (2008) Statistics for aquaculture. Wiley-Blackwell, Iowa
Bixler HJ, Porse H (2011) A decade of change in seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and developments. Developments in Applied Phycology 4. Springer, Dordrecht, pp 1–17
Dawes CJ, Lluisma AO, Trono GC (1994) Laboratory and field growth studies of commercial strains of Eucheuma denticulatum and Kappaphycus alvarezii in the Philiphines. J Appl Phycol 6:21–24
Enriquez S, Borowitzka MA (2011) The use of the fluorescence signal in studies of seagrass and macroalgae. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and developments. Developments in Applied Phycology 4. Springer, Dordrecht, pp 187–208
Gevaert F, Creach A, Davoult D, Holl AC, Seuront L, Lemoine Y (2002) Photo-inhibition and seasonal photosynthetic performance of the seaweed Laminaria saccharina during a simulated tidal cycle: chlorophyll fluorescence measurements and pigment analysis. Plant Cell Environ 25:859–872
Glynn PW, Welington GM, Wells JW (1983) Corals and coral reefs of the Galápagos Islands. University of California Press, London
Huppertz K, Hanelt D, Nultsch W (1990) Photoinhibition of photosynthesis in the marine brown algae Fucus serratus as studied in field experiments. Mar Ecol Prog Ser 66:175–182
Kuster A, Pohl K, Altenburger R (2007) A fluorescence-based bioassay for aquatic macrophytes and its suitability for effect analysis of non-photosystem II inhibitors. Env Sci Pollut Res 14:377–383
Lideman, Nishihara GN, Noro T, Terada R (2011) In vitro growth and photosynthesis of three edible seaweeds, Betaphycus gelatinus, Eucheuma serra and Meristotheca papulosa (Solieriaceae, Rhodophyta). Aquaculture Sci 59:563–571
Nonji A (1993) Indonesian Ocean. Djambatan, Jakarta
Ohno M, Largo DB, Ikumoto T (1994) Growth rate, carrageenan yield and gel properties of cultured kappa-carrageenan producing red alga Kappaphycus alvarezii (Doty) Doty in the subtropical waters of Shikoku. Japan J Appl Phycol 6:1–5
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Ralph PJ, Gademann R, Dennison WC (1998) In situ seagrass photosynthesis measured using a submersible, pulse-amplitude modulated fluorometer. Mar Biol 32:367–373
Ralph PJ, Schreiber U, Gademann R, Kuhl M, Larkum AWD (2006) Coral photobiology studied with a new imaging pulse amplitude modulated fluorometer. J Phycol 41:335–342
Renger G, Schreiber U (1986) Practical applications of fluorometric methods to algae and higher plant research. In: Govindjee, Amesz J, Fork DD (eds) Light emission by plants and bacteria. Academic Press, Orlando
Schreiber U, Hormann H, Neubauer C, Klughammer C (1995) Assessment of photosystem II, photochemical quantum yield by chlorophyll fluorescence quenching analysis. Aust J Plant Physiol 22:209–220
Soegiarto A, Birowo S, Sukarno (1976) Map of Indonesian Ocean. Indonesian Science Institute
Thomas A, O’Hara B, Ligges U, Sturtz S (2006) Making BUGS Open. R News 6:12–17
Tomascik T, Mah AJ, Nonji A, Moosa MK (1997) The ecology of Indonesian seas. Part Two. Periplus Editions (HK) Ltd, Singapore
Waaland JR (1981) Commercial utilization. In: Loban CS, Wynne MJ (eds) The biology of seaweeds. University of California Press, Berkley, pp 726–741
Wobeser EA, Figueroa FL, Cabello-Pasini A (2001) Photosynthesis and growth of red and green morphotypes of Kappaphycus alvarezii (Rhodophyta) from the Philippines. Mar Biol 138:679–686
Acknowledgments
This research was sponsored in part by Grant-in-Aid for Scientific Research (#22510033) from the Japanese Ministry of Education, Culture, Sport, and Technology (RT), and the Nagasaki University Strategy for Fostering Young Scientist with funding provided by the Special Coordination Funds for Promoting Science and Technology of Ministry of Education, Culture, Sport, Science and Technology (GNN). We would like to expresses our thanks to Director of Central for Brackish-water Aquaculture Development Center, for their kind contributions to the collections of samples and measurement of underwater irradiance in their cooperating farming area, South Sulawesi, Indonesia. We also thank Dr. Michael A. Borowitzka, and two reviewers for their valuable suggestion to improve the manuscript. This research was also the part of dissertation submitted by the first author in partial fulfillment of the Ph.D. degree.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lideman, Nishihara, G.N., Noro, T. et al. Effect of temperature and light on the photosynthesis as measured by chlorophyll fluorescence of cultured Eucheuma denticulatum and Kappaphycus sp. (Sumba strain) from Indonesia. J Appl Phycol 25, 399–406 (2013). https://doi.org/10.1007/s10811-012-9874-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9874-5