Abstract
According to current accounts of social cognition, the emergence of verbal and non-verbal components of social perception might rely on the acquisition of different cognitive abilities. These components might be differently sensitive to the pattern of neuropsychological impairments in congenital neurodevelopmental disorders. Here, we explored the association between social and non-social cognitive domains by administering subtests of the NEPSY-II battery to 92 patients with Intellectual and Developmental Disability (IDD). Regardless the level of intellectual functioning and presence of congenital brain malformations, results revealed that visuospatial skills predicted emotion recognition and verbal component of Theory of Mind, whereas imitation predicted the non-verbal one. Future interventions might focus on spatial and sensorimotor abilities to boost the development of social cognition in IDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Social cognition refers to a complex collection of different psychological processes that allow an individual to infer others’ mental thoughts, feelings, intentions and to interpret others’ behaviors and emotions, thus ultimately supporting social interactions (Beer & Ochsner, 2006). In the last decades, two theories about how we infer others’ mental states have gained major interest: Theory-Theory (TT) and Simulation-Theory (ST).
TT accounts were firstly proposed to explain social cognition deficits shown by persons with Autism Spectrum Disorders (ASD) (Baron-Cohen et al., 1985). These accounts assume that persons with ASD lack of a set of mental concepts and governing principles to represent others’ mental states (Gopnik & Wellman, 1992; Malle, 2005), namely a theory of mind (ToM; (Premack & Woodruff, 1978)). In these accounts a critical role is played by metacognition, that is the ability to reflect upon our mental states and to share them with others as well as to make a correct representational model of the physical and social world (Frith & Frith, 2012). Neuroimaging research has highlighted a cortical network involved in making inferences about one’s own and others’ mental states, which has been called Mentalizing System (MS; (Frith & Frith, 2003; Koster-Hale et al., 2017). The main components of this system are the temporo-parietal junction (TPJ; (Frith & Frith, 2006; Amodio & Frith, 2006), the posterior end of the superior temporal sulcus (pSTS), and the dorsal sub-region of the medial prefrontal cortex (dmPFC; Amodio & Frith 2006). The main function of TPJ would consist in the ability to represent the world from different perspectives (Mai et al., 2016), the pSTS would be involved in the processing of dynamic facial and bodily stimuli (Grossman et al., 2005), while the prefrontal cortex would subtend the ability to plan for the future and to predict what a person is going to think (Overwalle, 2009).
ST accounts propose that we refer to our internal states to understand others, by simulating their cognitive, emotional and behavioral states (Davies et al., 2010; Gordon, 1986). The discovery of mirror neurons, which are activated during both action execution and action observation (Rizzolatti & Craighero, 2004), provided a direct neurobiological basis for these simulation processes. Research in monkeys (for a review, see Casile 2013) and, using different methodologies, in humans (for reviews, see Avenanti et al., 2013; Caspers et al., 2010; Fadiga et al., 2005; Molenberghs et al., 2012; Urgesi et al., 2014) has provided evidence of shared activations for one’s own and others’ actions in a wide Action Observation Network (AON) including premotor, parietal and temporal cortical areas, but also subcortical and cerebellar regions. These mirror-like activations have been integrated in an embodied cognition framework (Barsalou, 2009; Gallese, 2007; Gallese & Goldman, 1998; Gallese & Sinigaglia, 2011; Schmidt et al., 2021). Indeed, by simulating others’ actions into the observer’s motor system, people might rely on their own bodily states to represent and understand others’ mental states and feelings in a pre-reflective, embodied way.
While the theoretical controversial between TT and ST might sound obsolete (Apperly, 2008), there is large agreement that social cognitive processes are underpinned by both the MS and AON neurocognitive systems (Adolphs, 2009; Schurz et al., 2020), which allow for, respectively, a fast, mostly implicit perceptual processing of social stimuli and slower, explicit reflective cognitive operations (Frith & Frith, 2007; Meinhardt-Injac et al., 2018). Although the AON may support the MS in understanding others’ mental states (Isoda, 2016; Overwalle, 2009), the two systems can operate independently by each other (Jacob & Jeannerod, 2005) and follow diverse developmental trajectories (Apperly & Butterfill, 2009). Furthermore, within a neurodevelopmental perspective of social cognition (Happé & Frith, 2014), the emergence of these systems might depend on the acquisition of different, interdependent cognitive and sensorimotor abilities.
With regards to the explicit, higher-level components of social cognition described in TT accounts, most literature investigated associations of social cognition with executive functions and language development (Carlson & White, 2013). There is evidence that the association between executive functions and ToM arises early and becomes stronger in typical development (Bock et al., 2015; Carlson et al., 2015; Carlson & Moses, 2001; Marcovitch et al., 2015; Sodian & Kristen-Antonow, 2015). Studies on atypical development confirmed this association by showing that impairments in executive functions affected social cognition by disrupting metacognitive processes (Leung et al., 2016; Mary et al., 2016). In a similar vein, the development of language and social cognition is tightly intertwined from infancy to childhood (Brooks & Meltzoff, 2015). Indeed, early language abilities seem to predict later acquisition of social skills during infancy (Astington & Jenkins, 1999) and language impairments affect ToM abilities (Farrant et al., 2006; Spanoudis, 2016).
Nevertheless, a crucial prerequisite to gain ToM is the ability to represent others’ visual perspective (Kampis et al., 2017). While understanding whether an individual can see an object (level-1 perspective taking) was reported to emerge early in infancy (Luo & Johnson, 2009; Sodian & Thoermer, 2004), a more refined ability to represent how an individual sees an object (level-2 perspective taking) seems to develop later and would allow one to assume other else’s point of view (Flavell et al., 1981; Moll et al., 2012; Kessler & Rutherford, 2010). Studies on atypical development documented that individuals with ASD may show a specific impairment in the more-refined level-2 perspective taking, and this deficit could be associated with lower ToM abilities (Hamilton et al., 2009). Importantly, the performance of ASD individuals in perspective taking tasks was documented to be strictly associated with the ability to operate mental spatial rotations (Pearson et al., 2016). In a similar vein, research on Williams syndrome, a condition involving important deficits in visual-spatial abilities (Atkinson et al., 1997, 2001), reported deficits in visual perspective taking, which could underlie abnormalities in social behavior often reported in this syndrome (Broadbent et al., 2014; Hirai et al., 2013). All in all, these findings suggest that, at least in conditions of atypical development, impairments in visuospatial abilities might affect the development of perspective taking, with major impact on ToM abilities (Kampis et al., 2017).
As what concerns the implicit processing of social stimuli allowed by the AON in ST accounts, infant research has supported an early relationship between motor abilities and diverse levels of social cognitive processing (Brandone, 2015). For example, Ambrosini and colleagues (2013) documented that 8- and 10-month-old infants correctly interpreted goal-related actions only when they were able to perform such actions. Research in conditions of atypical development has primarily focused on ASD and Developmental Coordination Disorder (DCD; Leonard & Hill 2014). For the former, there is agreement that sensorimotor difficulties contribute to core social behavior deficits (Hannant et al., 2016; Ohara et al., 2019). In a similar vein, poor motor control seems to affect social and emotional abilities in preschool- and school-age children with DCD (Leonard, 2016; Piek et al., 2008). Within this framework, a critical role has been attributed to imitation, as it could underlie the development of high-level socio-cognitive and socio-emotional processes through the experience that the others are ‘like me’ (Meltzoff, 2007; Meltzoff & Decety, 2003). Accordingly, Kenny and colleagues (2016) reported that, in school-age children, imitation was closely related to action understanding. Tough, neither imitation nor motor skills were associated with ToM abilities. This suggests that imitation might have a major role for the development of low-level (non-verbal) motor cognition processes in early infancy, but not for the development of higher-level (verbal) social cognitive processes involved in ToM, at least in conditions of typical development.
Still, in conditions of atypical development due to genetic syndrome or congenital brain defects, early motor impairments could interfere with the later development of social cognitive abilities (Houwen et al., 2016; Leonard & Hill, 2014; Ritterband-Rosenbaum et al., 2019). Indeed, it has been shown that motor skills are stronger predictors of cognitive and language development in children with intellectual and developmental disabilities (IDD) than in those with typical development (Houwen et al., 2016). Children with IDD show limitations in cognitive and adaptive functioning, including social skills (American Psychiatric Association, 2013) (Baurain & Nader-Grosbois, 2013). Previous studies on development of social skills in children with diagnosis of IDD reported not only delays in both implicit and explicit components of social processing as compared to age- matched controls, but also a different pattern of links between social abilities as compared with IQ-matched controls, pointing to an altered (and not only delayed) development of social abilities in IDD (Baurain & Nader-Grosbois, 2013). However, whether specific motor or cognitive abilities could differently account for diverse components of social cognition in IDD is still to be elucidated.
Moreover, to assess motor, cognitive and social abilities, previous studies mostly adopted tasks of different testing batteries, which were standardized on different samples. This cannot control for the interindividual variations of the developmental curve of each cognitive domain (Russell, E., Russell S & Hill B., 2005), leading to an uncontrollable amount of variability to the findings that poses important limitations to their interpretations. The introduction of the multi-domain neuropsychological battery NEPSY-II (Korkman et al., 2007) allowed us to overcome this issue, since all subtests were standardized on the same sample of children aged 3–16 years. Furthermore, NEPSY-II includes social perception subtests as a separate domain, with specific subtests assessing both the explicit (i.e., verbal ToM subtest) and implicit (i.e., non-verbal ToM subtest; affect recognition) components. This battery has been widely adopted to investigate the neuropsychological profile in typical development and in different clinical populations (epilepsy by Zilli et al., 2015; fetal alcohol spectrum disorder by Rasmussen et al., 2013; anorexia nervosa by Calderoni et al., 2013; preterm born children with spastic diplegia by Di Lieto et al., 2017; ASD by Narzisi et al., 2013, and Barron-Linnankoski et al., 2015).
Considering these premises, we selected NEPSY-II subtests assessing attention, sensorimotor and visuospatial cognitive domains to investigate whether and how basic motor and cognitive abilities could explain performance at social perception subtests in children with IDD. The sample included non-progressive congenital conditions due to either genetic syndromes or unknown etiology and was further divided into four groups according to MRI data reporting, respectively, absence or presence of brain malformations affecting infratentorial, supratentorial structures or both. This way, we verified whether and how congenital malformations of different portions of the brain might affect the developmental links between social cognition and other cognitive abilities in children with IDD. We also considered the full-scale intelligent quotient (FSIQ) in our analyses to control for effects of general cognitive functioning.
We anticipated that, according to TT accounts, performance in the subtests assessing the ability to operate on visual-spatial representations would predict the scores obtained in the verbal part of the ToM subtest. Conversely, according to ST accounts, we expected that performance in the sensorimotor domain should correlate with the ability to mentally recognize others’ intentions and emotions, thus predicting performance at the non-verbal part of the ToM subtest and at the affect recognition subtest. As what concerns the presence and site of brain malformations, we expected that sensorimotor impairments mostly affected the performance in social perception subtests in infratentorial patients, since subcortical areas and particularly the cerebellum have been shown to play a prominent role in both motor control and social cognition (Butti, Corti, et al., 2020; Oldrati et al., 2021; Tavano et al., 2007; Urgesi et al., 2021).
Materials and methods
Participants
Ninety-two children and adolescents (72 males) with a diagnosis of IDD were recruited at the Child Neuropsychiatry and Neurorehabilitation Unit of the Scientific Institute, IRCCS E. Medea. Inclusion criteria were: (i) age between 4 and 16 years, (ii) presence of learning disability and/or neurocognitive disorders due to non-progressive, congenital conditions. Exclusion criteria were: (i) primary acquired brain lesions, (ii) primary diagnosis of ASD, child psychosis or other psychopathologic disorders, (iii) severe sensorial and motor deficits that could interfere with NEPSY-II administration. All patients received imaging and clinical consultations before recruitment, while genetic testing was performed only when a specific diagnosis was suspected in according to the clinical features of each patient. A genetic origin was identified in 15% of the no-malformation patients, 25% of the infratentorial patients, 30% of the supratentorial patients, and 50% of the infra-supratentorial patients. For each participant, the FSIQ derived from the age-corresponding Wechsler Intelligence Scale (Wechsler, 2002, 2003) was obtained through chart review; index scores were not available. The interval between the IQ assessment and the administration of NEPSY-II subtests was no longer than twelve months. Participants were assigned by an expert pediatric neuropsychiatrist (CG or RR) to four different groups (Infratentorial-IF, Supratentorial-ST, Infra-Supratentorial-IST, and No Malformation-NM) according to the malformation presence and location revealed by T1-weighted and T2-weighted images obtained during RM exam. One NM patient received a secondary diagnosis of ASD and another NM patient received a secondary diagnosis of developmental Coordination Disorder. All children and adolescents recruited in the study attended schools; in keeping with Italian school system regulations, children with IDD attend to the same public or private schools with their neuro-typical peers, but the support of a specifically trained teacher is provided and they follow their own individualized educational program within the class. All participants and their parents were informed about aims of the study and parents were asked to sign a written informed consent. The procedures were approved by the local Ethics Committee of the Scientific Institute (IRCCS) E. Medea (Prot. N.34/18 – CE) and were in accordance with the Helsinki Declaration guidelines. A resume of demographic/clinical features of the four groups is reported in Table 1 (see Supplementary Information for a detailed description of clinical features and family information). (Table 1)
Neuropsychological Assessment
All patients recruited in this study at the period of the neuropsychological assessment were hospitalized in our structure for rehabilitative interventions and routine clinical checks. Participants’ competences in four cognitive domains (attention, sensorimotor functioning, visuospatial processing and social perception) were assessed by seven selected subtests of the Italian NEPSY–II version (Korkman et al., 2011). The subtests were selected from the 33 NESY-II subtests to cover these 4 cognitive domains and to be indicated to all children aged 5–16 yo with motor and speech deficits; subtests that require full speech functionality were excluded. A trained psychologist with specific expertise in neuropsychological assessment (EF or NB) administered the NEPSY-II subtests in a silent room. All participants were tested individually in two separate sessions, each lasting approximately 45 min to prevent fatigue. The interval between sessions was less than 10 days.
A short description of the selected NEPSY-II subtests divided for each neuropsychological domain is provided below (for further details please see (Korkman et al., 2011; Urgesi et al., 2011). In the Supplementary Material is reported an example of each subtests’ item.
Attention:
Visual Attention (VA): In this matching task, 3–4 yo children have to identify the figures identical to the given target (i.e., rabbit) on an A3 sheet displaying both distractors and targets in rows. Older children and adolescents (5–16 yo) are required to scan a page containing rows of different faces and to mark targets (i.e., a girl with a sad facial expression, and a boy with a happy facial expression) among similar distractors. Children must solve the task within 180 s. The subtest score is calculated by subtracting the number of false alarms to the number of hits.
Sensorimotor functioning:
Fingertip Tapping (FT): In this task, the child must repeat a series of finger movements demonstrated by the examiner. This subtest has two parts, each one completed with the dominant and non-dominant hand. In the first part, the child taps the tip of the index finger on the tip of the thumb. The examiner records the amount of time employed to produce 20 repetitions of the movement. In the second part, the child repeats a sequence of finger taps on the tip of the thumb progressing from the index to the little finger. The subtest score is the total amount of time spent by the child to produce the two parts with the dominant and non-dominant hand.
Manual Motor Sequences (MMS): In this task, the child imitates and repeats for five times a sequence of unimanual or bimanual gestures demonstrated by the examiner. The subtest score is the sum of the number of times in which the child correctly imitates each sequence.
Imitating Hands Position (IH): In this task, the child copies meaningful or meaningless hand and finger gestures demonstrated by the examiner, with both the dominant and non-dominant hand. The subtest score is the number of positions correctly copied with either hand.
Visuospatial processing:
Block construction (BC): In this task, the child has to use blocks to reproduce models provided by the examiner or to construct 3D representation of bi-dimensional drawings. Each item must be accomplished within 60 s. A correct response is scored 2 when the child employs less than 20 s to build the construction, 1 point when the amount of time spent to build the construction is between 21 and 60 s, and 0 when the construction is incorrect or the child spends more than 60 s to solve task. The subtest score is the sum of all item scores.
Social perception:
Affect Recognition (AR): Depending on their age, participants must: decide whether or not two photographs depict faces with a similar affect; select a pair of pictures with similar affect from a sample of three or four photographs; select one of the four photographs that depicts similar affect as a target photograph at the top of the page. In the last part of the task, the child has to memorize a target emotional face shown for 5 s, and then to select, from a sample of six photographs, a pair of pictures with similar affect as the face previously shown. The subtest score is the sum of correct responses.
Theory of Mind (ToM): This subtest consists of two parts that investigate verbal and non-verbal social abilities, respectively. During the verbal part (ToM A), the child is provided with short stories or illustrations of some social situations and then asked questions that require knowledge of another individual’s point of view to solve the task. In the contextual, non-verbal part (ToM B), the child is shown an illustration depicting a social context in which the protagonist’s face is hidden. The child must select a photograph from four options that depict the appropriate emotion of the protagonist in the illustration. The subtest score for each part is the sum of correctly responded items.
Data Analysis
Raw scores at NEPSY-II subtests were transformed into standard scores (mean = 10, SD = 3) with respect to normative values for the corresponding chronological age of the Italian normative sample (Urgesi et al., 2011), avoiding the approximation of the low and high extremes that is usually employed in normative standardization tables. Since the Finger tapping and Manual motor sequences subtests are administered to children of different ages and both assess the ability to maintain and repeat a motor program, we calculated an aggregate score (FT-MMS) by computing the mean of the standard scores obtained in the two subtests.
We first tested, with factorial Analyses of Variance (ANOVA) models, for age and IQ, or chi square test, for gender, whether the four groups of IDD patients were equally distributed for demographic and clinical variables. Since gender was not uniform in our sample, with more boys (N = 72) than girls (N = 20) across the four groups, preliminarily we ran two-tailed Student’s t- tests (two-tailed) for each NEPSY-II subtest with gender as independent variable, collapsing the four groups of patients. Then, with the aim to check for differences between the clinical groups, the scores obtained at the NEPSY-II subtests (VA, IH, FT-MMS, BC, AR, ToM verbal, ToM, non-verbal) were entered in a Multivariate ANOVA (MANOVA) with Group as between-subject factor. Follow-up univariate factorial ANOVAs were also run for each variable. Standard Multiple Linear Regression Models were then used to identify the relative contribution of specific predictors to the performance obtained at the social perception subtests. In detail, VA, IH, FT-MMS, BC subtest, FSIQ and Group were entered as predictors, while the scores obtained at the AR subtest and at the verbal and non-verbal ToM parts were entered as dependent variables in three separate models. The Group predictor was coded 0 for no malformations, 1 for infratentorial malformations, 2 for supratentorial malformations and 3 for supra- and infra-tentorial malformations. Correlations between continuous predictors were preliminary run in order to verify collinearity assumption.
Statistical analyses were performed with the Statistica software version 8.0 (Statsoft, Tulsa, OK). Significance threshold was set at p = 0.05 for all statistical tests.
Results
Between-groups comparisons
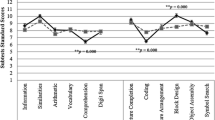
The mean standard scores of each group in these subtests are reported in Table 2. (Table 2)
Preliminary t- tests did not highlight any significant difference between male and female patients (all t > |-0.544 and < 0.998|; all p > 0.321). No difference between the four clinical groups emerged for FISQ (F (3,88) = 0.47, p = 0.705), age (F (3,88) = 1.66, p = 0.181) and gender (X2 (1, N = 91) = 0.108, p = 0.991). The MANOVA on the NEPSY-II subtests showed no significant differences between the group means (Wilks’s λ = 0.814, F (6,18) = 0.980, p = 0.483). Follow-up ANOVAs confirmed that the groups obtained comparable scores in all NEPSY-II subtests (See Table 2). These findings indicated that, independently from the presence and the site of brain malformations, our sample of IDD children showed similar impairments across the subtests.
Multiple linear regression models:
In keeping with data of the US (Korkman et al., 2007) and Italian (Urgesi et al., 2011) normative studies, the variables entered as predictors were only moderately correlated (all r < 0.74), thus ruling out violation of multicollinearity assumption (Table 3).
All regression models were significant (all F > 6.42, all p < 0.001, all Adjusted R2 > 0.26; Table 3). In detail, performance at the AR subtest was significantly predicted by the scores obtained at the BC subtest (β = 0.57, p < 0.001). In a similar vein, BC was the only significant predictor of performance in the ToM verbal part (β = 0.54, p < 0.01). Conversely, manual imitation abilities assessed by the IH subtest predicted performance in the non-verbal, contextual ToM part (β = 0.18, p < 0.01). For all models, Group, FSIQ and other NEPSY-II subtests were not significant (all β < 0.01, all p > 0.07), thus indicating that these associations were reliable independently from the presence and location of brain malformations, general cognitive abilities and the other assessed cognitive functions (Fig. 1; Table 4).
Scatter plots showing the significant results for, respectively, the Affect recognition (a), ToM verbal (b) and ToM non-verbal (c) subtests. Standard scores for all measures are expressed as Mean = 10 and SD = 3, with scores between 7 and 13 indicating performance at the expected level, scores between 4 and 7 or between 13 and 16 indicating performance below or above the expected level, respectively, and scores below 3 or above 16 representing performance well below or well above the expected level, respectively.
Discussion
The present study examined the possible links of social perception skills with attentional, visuospatial and sensorimotor functions in a sample of children with IDD. According to TT accounts (Kampis et al., 2017), we expected that attentional and visuospatial abilities would predict explicit, verbal ToM performance, while, according to ST accounts (Meltzoff, 2007), sensorimotor abilities should predict implicit, non-verbal ToM and AR performance. Partially in line with these expectations, we observed that the ability to operate on visual-spatial representations predicted performance not only at the verbal ToM task, but also at the AR subtest. Conversely, the scores obtained at the manual imitation subtest predicted performance at the non-verbal ToM task, but not at the AR subtest. Notably, these relationships were independent from presence and location of brain malformations and from general cognitive functioning (i.e., FSIQ), thus pointing to a strong link of visuospatial skills and imitation with different social perception abilities in children with IDD regardless of the nature of their atypical development. These findings suggest that TT and ST accounts are not mutually exclusive as they could explain different levels of social cognitive processing, in keeping with an integrative perspective on functioning of the MS and AON neurocognitive networks (Isoda, 2016). Moreover, these results could also be useful to inform rehabilitative interventions addressing social cognition deficits in conditions of atypical development.
While previous studies pointed to executive functions and language acquisition as fundamental blocks of social cognition (Amadó et al., 2016; Hippolyte et al., 2010; Leung et al., 2016), within a neurodevelopmental perspective an association between visual-spatial skills and social cognition may be even more important, as this relationship might emerge earlier and, thus, affect widely the development of social perception abilities (Kampis et al., 2017). Accordingly, our findings provide evidence that impairments of the ability to form and manipulate visual-spatial representations might affect social perception at multiple levels.
With regards to verbal, reflective operations underlying higher-order ToM abilities, deficits in visuospatial skills in children with IDD might interfere with the acquisition of perspective taking, leading to a deficient ability to infer others’ mental states. Indeed, the verbal ToM subtest required participants to form social mental representations of others and to assume a different perspective to understand social relationships among the different characters. Similarly, in the Block Construction subtest, children had to build a 3-D construction that matched a 3-D model or a 2-D model in a picture. During the execution of this task, children saw only one face of the construction and were not allowed to rotate the 3-D model or the instruction card, thus preventing the view of the construction from another perspective. This way, in order to solve the task, children had to imagine how the model looked from the other perspective and infer the position of the cubes. This visual perspective taking requires an embodied mental transformation to assume others’ point of view (Kessler & Thomson, 2010) and is considered to be critical for social cognition (Saxe, 2006).
These results are in line with previous studies on perspective taking in atypical development (Hamilton et al., 2009; Hirai et al., 2013; Pearson et al., 2016) and allow educated speculations about specific brain areas involved by both visual-spatial tasks and social cognition. In detail, assumption of others’ perspectives, as required by both the BC and verbal ToM subtests, might rely on TPJ (Schurz et al., 2013; Aichhorn et al., 2006). Neuroimaging studies documented that the specialization of TPJ occurs early in infancy and subtends mental operations on visual-spatial representations, ultimately leading to track others’ beliefs (Hyde et al., 2018). Xiao and colleagues (2019), in their resting-state MRI study, showed that maturation of right TPJ and development of neural connections with other social network areas correlate with the acquisition of different ToM abilities. In a similar vein, Santiesteban and colleagues (2012) reported that interferences with right TPJ activity through transcranial direct current stimulation did not directly disrupt mental states attribution, but rather the self-other representation entailed by perspective taking.
Interestingly, impairments of the ability to represent visual-spatial relations were also associated with worse facial affect recognition, which represents a faster, perceptual component of social cognition (Apperly & Butterfill, 2009; Meinhardt-Injac et al., 2018). Previous studies documented that emotion processing follows two distinct developmental trajectories in childhood, one associated to verbal skills (expressive emotion knowledge) and the other linked to the development of visuospatial ability (Vicari et al., 2000). The claim that emotion-matching accuracy is predicted by shape-matching accuracy in children with typical development was also suggested by Herba and colleagues (2006). As concerns atypical development, an increasing number of studies has suggested that deficits in affect recognition in congenital conditions, such as Down and Williams syndromes, might reflect an impairment in processing and integrating visual-spatial features of face stimuli rather than specific alterations of social processing (Gagliardi et al., 2003; Dimitriou et al., 2015). Our results support this hypothesis, since deficits of visuospatial skills due to IDD might have posed constraints to the development of emotion recognition abilities (Martínez-Castilla et al., 2015).
While recognizing facial emotions seemed to rely on visuospatial skills, impairments in recognizing emotions elicited by a specific social context were predicted by poor performance in the hand posture imitation task. This result highlights the importance of simulation mechanisms, strictly related to imitation (Meltzoff, 2007), at least for inferring other individual’s emotion on the basis of contextual information (Bastiaansen et al., 2009; Gallese, 2007). Previous studies pointed to a crucial role of imitation and simulation processes in implicit, non-verbal tracking of others’ intentions as involved by action understanding (Jeannerod, 2001; Kenny et al., 2016). Even though the NEPSY-II non-verbal ToM subtest does not present dynamic stimuli such as action videos, simulation processes are required to predict others’ mental states according to context (Brown & Brüne, 2012). Conversely, partially in contrast to previous research on atypical development (Cummins et al., 2005; Leonard & Hill, 2014), we did not find an association between social cognition and motor skills entailing motor planning and coordination as assessed by the FT-MMS subtests. In this sense, imitation is a complex motor skill that requires to transform visually specified goals (i.e., experimenter’s hand orientation and hand shape) into motor acts, distinguishing one’s own motor repertoire from that of the other (de Guzman et al., 2016). Beyond the classical mirror neuron system, imitation and simulation processes recruit the wide AON (Urgesi et al., 2014), in order to trace intentions from subtle kinematics’ differences (Koul et al., 2018) and from context (Amoruso & Urgesi, 2016). Specifically, when contextual information is crucial to disambiguate other individual’s intention (Wurm et al., 2017), frontal associative areas, such as the dorsolateral prefrontal cortex (Amoruso et al., 2018; Kalbe et al., 2010), and the cerebellum (Abdelgabar et al., 2019; Butti, Corti, et al., 2020; Urgesi et al., 2021) would provide a contextual predictive output as a result of simulation processes (Maranesi et al., 2014; Urgen & Miller, 2015). Notably, this predictive simulation operates across domains (Siman-Tov et al., 2019) but might play a more critical role in social cognition (Koster-Hale & Saxe, 2013; Oldrati et al., 2021). In keeping with this literature, we could speculate that impairments in imitation due to IDD might affect simulation processes, ultimately leading to low performance in the contextual, non-verbal ToM task.
It is to note that our results were reliable across all groups, regardless the presence and location of brain malformations. On the one hand, this could be due to the limits of our classification, which did not allow disentangling the role of specific areas. On the other hand, the lack of differences between groups might suggest that the complex nature of congenital disorders presented by our samples could have widely affected functional and structural brain connections (Gagliardi et al., 2018), even beyond the areas involved by the malformation through developmental diaschisis (Stoodley & Limperopoulos, 2016). Furthermore, rather than relying on the activity of specific areas, development of social cognition processes and their links with other cognitive functions is likely to be supported by complex cortical-subcortical networks (Alcalá-López et al., 2018).
The findings reported here sustain the importance of visuospatial skills and imitation for the development of different social perception abilities and could inform rehabilitative interventions for IDD. According to the neurodevelopmental perspective sustained by this study, promoting early interventions (Lipkin et al., 2020; Purpura et al., 2017) and intensive rehabilitation training (Gagliardi et al., 2015) in IDD would have beneficial effects not only on the specific targeted functions but also on social cognitive skills. Within an embodied framework, early body-centered interventions could promote habilitating sensorimotor experiences and imitation, which in turn could foster cognitive development (Ritterband-Rosenbaum et al., 2019) and, as suggested by our findings, also contextual, non-verbal ToM abilities. As regards visuospatial skills, our results indicate that boosting the abilities to form mental representations of the external physical world could also improve social perspective taking, with likely effects on both low-level and high-level social cognitive processes. In this light, immersive Virtual Reality (VR) might be particularly useful to implement neurorehabilitation programs that simultaneously provide embodied sensorimotor experiences and boost visuospatial skills (Maggio et al., 2019; Tieri et al., 2018). Accordingly, VR interventions have been proposed to address social cognition deficits in congenital neurodevelopmental disorders trough explicit training of social skills (Kandalaft et al., 2013) or by enhancing predictive simulation processes (Butti, Biffi et al., 2020: Urgesi et al., 2021). In a similar vein, VR showed its efficacy in boosting the ability to build visual-spatial representations in children with cerebral palsy (Biffi et al., 2020), and it could be extended to congenital conditions in order to improve ToM and emotion recognition abilities.
Lastly, this study supports the use of a multidimensional neuropsychological battery such as NEPSY-II, which can help clinicians to localize child’s weaknesses and strengths and tailor the rehabilitation programs to the specific neuropsychological profile of the child, also taking into account the developmental interdependencies of different cognitive and social domains. Moreover, the adoption of different social perception tasks allows capturing diverse dimensions of social cognition and ToM rather than assessing them as single constructs (Warnell & Redcay, 2019), providing a more complex glimpse of social functioning in children with IDD (Fiasse & Nader-Grosbois, 2012).
Caution in the interpretation of our findings is needed in the light of limitations. First, the relatively small sample size and variety of patients without or with different (clinically visible) brain malformations may limit the generalization of our results to the general population of individuals with IDD. Moreover, we obtained a prevalence of male children in our sample, albeit preliminary analyses did not highlight significant effects of gender. A prevalence of male in child samples with IDD, however, has been reported by previous research (Lai et al., 2012). Furthermore, we did not administer subtests of the linguistic domain and could not estimate contribution of language development to social processing in IDD. Still, a strict link between verbal skills and social cognition has been documented in IDD children (Thirion-Marissiaux & Nader-Grosbois, 2008). Nevertheless, our models explained a wide part of the variance even for the verbal ToM task (Adj R2 > 0.50), thus ensuring the strength of our findings beyond the likely effects of linguistic abilities on social perception. Finally, we could not have quantitative measures of alterations in different brain areas from our IDD patients, thus preventing us from investigating the neural bases of the links of social processes with other cognitive domains. Future studies with larger sample and more precise brain structure and functioning measures are required to better investigate the commonalities between the brain structures accounting for cognitive and social functioning impairments in children with IDD.
References
Abdelgabar, A. R., Suttrup, J., Broersen, R., Bhandari, R., Picard, S., Keysers, C. … Gazzola, V. (2019). Action perception recruits the cerebellum and is impaired in patients with spinocerebellar ataxia. Brain. 1;142(12):3791–3805. doi: https://doi.org/10.1093/brain/awz337
Adolphs, R. (2009). The social brain: neural basis of social knowledge. Annu Rev Psychol, 60, 693–716. doi:https://doi.org/10.1146/annurev.psych.60.110707.163514
Aichhorn, M., Perner, J., Kronbichler, M., Staffen, W., & Ladurner, G. (2006). Do visual perspective tasks need theory of mind? Neuroimage, 15;30(3):1059-68. doi: https://doi.org/10.1016/j.neuroimage.2005.10.026
Alcalá-López, D., Smallwood, J., Jefferies, E., Van Overwalle, F., Vogeley, K., Mars, R. B. … Bzdok, D. (2018). Computing the social brain connectome across systems and states. Cerebral cortex, 28(7), 2207–2232
Amadó, A., Serrat, E., & Vallès-Majoral, E. (2016). The role of executive functions in social cognition among children with down syndrome: relationship patterns. Frontiers in psychology, 7, 1363
Ambrosini, E., Reddy, V., de Looper, A., Costantini, M., Lopez, B., & Sinigaglia, C. (2013). Looking Ahead: Anticipatory Gaze and Motor Ability in Infancy. Plos One, 8(7), 1–9. https://doi.org/10.1371/journal.pone.0067916
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author
Amodio, D. M., & Frith, C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7, 268–277
Amoruso, L., & Urgesi, C. (2016). Contextual modulation of motor resonance during the observation of everyday actions. Neuroimage, 134, 74–84
Amoruso, L., Finisguerra, A., & Urgesi, C. (2018). Contextualizing action observation in the predictive brain: Causal contributions of prefrontal and middle temporal areas. Neuroimage, 177, 68–78
Apperly, I. A. (2008). Beyond Simulation-Theory and Theory-Theory: why social cognitive neuroscience should use its own concepts to study “theory of mind”. Cognition, 107(1), 266–283
Apperly, I. A., & Butterfill, S. A. (2009). Do humans have two systems to track beliefs and belief-like states? Psychological Review, 116, 953–970.https://doi.org/10.1037/a0016923
Astington, J. W., & Jenkins, J. M. (1999). A longitudinal study of the relation between language and theory-of-mind development. Developmental Psychology, 35, 1311–1320
Atkinson, J., Anker, S., Braddick, O., Nokes, L., Mason, A., & Braddick, F. (2001). Visual and visuo-spatial development in young Williams Syndrome children. Developmental Medicine and Child Neurology, 43, 330–333
Atkinson, J., King, J., Braddick, O., Nokes, L., Anker, S., & Braddick, F. (1997). A specific deficit of dorsal stream function in Williams’ syndrome. Neuroreport, 8(8), 1919–1922. https://doi.org/10.1097/00001756-199705260-00025
Avenanti, A., Candidi, M., & Urgesi, C. (2013). Vicarious motor activation during action perception: beyond correlational evidence. Frontiers in human neuroscience, 7, 185
Baron-Cohen, S., Leslie, A. M., & Frith, U. (1985). Does the autistic child have a ‘Theory of mind’? Cognition, 21, 37–46
Barron-Linnankoski, S., Reinvall, O., Lahervuori, A., Voutilainen, A., Lahti-Nuuttila, P., & Korkman, M. (2015). Neurocognitive performance of children with higher functioning autism spectrum disorders on the NEPSY-II. Child Neuropsychology, 21(1), 55–77
Barsalou, L. W. (2009). Situating concepts
Bastiaansen, J. A. C. J., Thioux, M., & Keysers, C. (2009). Evidence for mirror systems in emotions. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 364(1528), 2391–2404. https://doi.org/10.1098/rstb.2009.0058
Baurain, C., & Nader-Grosbois, N. (2013). Theory of mind, socio-emotional problem-solving, socio-emotional regulation in children with intellectual disability and in typically developing children. Journal Of Autism And Developmental Disorders, 43, 1080–1097. doi: https://doi.org/10.1007/s10803-012-1651-4
Beer, J. S., & Ochsner, K. N. (2006). Social cognition: a mult level analysis. Brain Research, 1079, 98–105
Biffi, E., Gagliardi, C., Maghini, C., Genova, C., Panzeri, D., Redaelli, D. F., & Turconi, A. C. (2020). Learning my way: a pilot study of navigation skills in Cerebral Palsy in Immersive Virtual Reality. Frontiers in Psychology, 11
Bock, A. M., Gallaway, K. C., & Hynd, A. M. (2015). Specifying links between executive functioning and theory of mind during middle childhood: cognitive flexibility predicts social understanding. J. Cogn. Dev. 16, 509–521. doi: https://doi.org/10.1080/15248372.2014.888350
Brandone, A. C. (2015). Theory of mind and behavior. Emerging Trends in the Social and Behavioral Sciences: An Interdisciplinary, Searchable, and Linkable Resource, 1–16
Broadbent, H. J., Farran, E. K., & Tolmie, A. (2014). Egocentric and allocentric navigation strategies in Williams syndrome and typical development. Developmental Science, 17(6), 920–934
Brooks, R., & Meltzoff, A. N. (2015). Connecting the dots from infancy to childhood: A longitudinal study connecting gaze following, language, and explicit theory of mind. Journal of Experimental Child Psychology, 139, 67–78
Brown, E. C., & Brüne, M. (2012). The role of prediction in social neuroscience. Frontiers in human neuroscience, 6, 147
Butti, N., Biffi, E., Genova, C., Romaniello, R., Redaelli, D. F., Reni, G. … Urgesi, C. (2020). Virtual Reality Social Prediction Improvement and Rehabilitation Intensive Training (VR-SPIRIT) for paediatric patients with congenital cerebellar diseases: Study protocol of a randomised controlled trial. Trials, 21(1), 1–12. https://doi.org/10.1186/s13063-019-4001-4
Butti, N., Corti, C., Finisguerra, A., Bardoni, A., Borgatti, R., Poggi, G., & Urgesi, C. (2020). Cerebellar Damage Affects Contextual Priors for Action Prediction in Patients with Childhood Brain Tumor. Cerebellum, 19(6):799–811. doi: https://doi.org/10.1007/s12311-020-01168-w. PMID: 32699945
Calderoni, S., Muratori, F., Leggero, C., Narzisi, A., Apicella, F., Balottin, U. … Urgesi, C. (2013). Neuropsychological functioning in children and adolescents with restrictive-type anorexia nervosa: An in-depth investigation with NEPSY–II. Journal of Clinical and Experimental Neuropsychology, 35(2), 167–179
Carlson, S. M., Claxton, L. J., & Moses, L. J. (2015). The relation between executive function and theory of mind is more than skin deep. Journal of Cognition and Development, 16(1), 186–197
Carlson, S. M., & Moses, L. J. (2001). Individual differences in inhibitory control and children’s theory of mind. Child Development, 72, 1032–1053
Carlson, S. M., & White, R. E. (2013). Executive function, pretend play, and imagination
Casile, A. (2013). Mirror neurons (and beyond) in the macaque brain: an overview of 20 years of research. Neuroscience Letters, 540, 3–14. doi: https://doi.org/10.1016/j.neulet.2012.11.003
Caspers, S., Zilles, K., Laird, A. R., & Eickhoff, S. B. (2010). ALE meta-analysis of action observation and imitation in the human brain. Neuroimage, 50, 1148–1167. doi: https://doi.org/10.1016/j.neuroimage.2009.12.112
Cummins, A., Piek, J. P., & Dyck, M. J. (2005). Motor coordination, empathy, and social behaviour in school-aged children. Developmental Medicine & Child Neurology, 47(7), 437–442
Davies, M., Stone, T., & Ohear, A. (2010). Folk Psychology and Mental Simulation. Current Issues in Philosophy of Mind.
De Guzman, M., Bird, G., Banissy, M. J., & Catmur, C. (2016). Self–other control processes in social cognition: from imitation to empathy. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1686), 20150079
Di Lieto, M. C., Brovedani, P., Pecini, C., Chilosi, A. M., Belmonti, V., Fabbro, F. … Sicola, E. (2017). Spastic diplegia in preterm-born children: Executive function impairment and neuroanatomical correlates. Research in developmental disabilities, 61, 116
Dimitriou, D., Leonard, H. C., Karmiloff-Smith, A., Johnson, M. H., & Thomas, M. S. (2015). Atypical development of configural face recognition in children with autism, D own syndrome and Williams syndrome. Journal of Intellectual Disability Research, 59(5), 422–438
Fadiga, L., Craighero, L., & Olivier, E. (2005). Human motor cortex excitability during the perception of others’ action. Current opinion in neurobiology, 15(2), 213–218
Farrant, B. M., Fletcher, J., & Maybery, M. T. (2006). Specific language impairment, theory of mind: and visual perspective taking: evidence for simulation theory and the developmental role of language. Child Development, 77, 1842–1853
Fiasse, C., & Nader-Grosbois, N. (2012). Perceived social acceptance, theory of mind and social adjustment in children with intellectual disabilities. Research in developmental disabilities, 33(6), 1871–1880
Flavell, J. H., Everett, B. A., Croft, K., & Flavell, E. R. (1981). Young children’s knowledge about visual perception: Further evidence for the Level 1–Level 2 distinction. Developmental psychology, 17(1), 99
Frith, C. D., & Frith, U. (2012). Mechanisms of social cognition. Annual Review of Psychology, 63, 287–313. https://doi.org/10.1146/annurev-psych-120710-100449
Frith, C. D., & Frith, U. (2007). Social Cognition in Humans. Current Biology, 17(16), 724–732. https://doi.org/10.1016/j.cub.2007.05.068
Frith, C. D., & Frith, U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–534
Frith, U., & Frith, C. D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 358(1431), 459–473
Gagliardi, C., Arrigoni, F., Nordio, A., De Luca, A., Peruzzo, D., Decio, A. … Borgatti, R. (2018). A Different Brain: Anomalies of Functional and Structural Connections in Williams Syndrome. Frontiers in neurology, 9, 721. https://doi.org/10.3389/fneur.2018.00721
Gagliardi, C., Brenna, V., Romaniello, R., Arrigoni, F., Tavano, A., Romani, M. … Borgatti, R. (2015). Cognitive rehabilitation in a child with Joubert Syndrome: Developmental trends and adaptive changes in a single case report. Research in developmental disabilities, 47, 375–384
Gagliardi, C., Frigerio, E., Burt, D. M., Cazzaniga, I., Perrett, D. I., & Borgatti, R. (2003). Facial expression recognition in Williams syndrome. Neuropsychologia; 41(6):733-8. doi: https://doi.org/10.1016/s0028-3932(02)00178-1. PMID: 12591030
Gallese, V. (2007). Before and below ‘theory of mind’: embodied simulation and the neural correlates of social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1480), 659–669
Gallese, V., & Goldman, A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences, 2, 493501
Gallese, V., & Sinigaglia, C. (2011). What is so special about embodied simulation? Trends in cognitive sciences, 15(11), 512–519
Gopnik, A., & Wellman, H. M. (1992). Why the child’s theory of mind really is a theory. Mind & Language, 7(1–2), 145–171. https://doi.org/10.1111/j.1468-0017.1992. tb00202.x
Gordon, R. (1986). Folk psychology as simulation. Mind & Language, 1, 158–171
Grossman, E. D., Battelli, L., & Pascual-Leone, A. (2005). Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Research, 45(22), 2847e2853. http://doi.org/10. 1016/j.visres.2005.05.027
Hamilton, A. F. D. C., Brindley, R., & Frith, U. (2009). Visual perspective taking impairment in children with autistic spectrum disorder. Cognition, 113(1), 37–44
Hannant, P., Cassidy, S., Tavassoli, T., & Mann, F. (2016). Sensorimotor difficulties are associated with the severity of autism spectrum conditions.Frontiers in Integrative Neuroscience,10
Happé, F., & Frith, U. (2014). Annual research review: Towards a developmental neuroscience of atypical social cognition. Journal of Child Psychology and Psychiatry, 55(6), 553–577
Herba, C. M., Landau, S., Russell, T., Ecker, C., & Phillips, M. L. (2006). The development of emotion-processing in children: Effects of age, emotion, and intensity. Journal of Child Psychology and Psychiatry, 47(11), 1098–1106
Hippolyte, L., Iglesias, K., Van der Linden, M., & Barisnikov, K. (2010). Social reasoning skills in adults with Down syndrome: The role of language, executive functions and socio-emotional behaviour. Journal of Intellectual Disability Research, 54(8), 714–726
Hirai, M., Muramatsu, Y., Mizuno, S., Kurahashi, N., Kurahashi, H., & Nakamura, M. (2013). Developmental changes in mental rotation ability and visual perspective-taking in children and adults with Williams syndrome. Frontiers in Human Neuroscience, 7856. https://doi.org/10.3389/fnhum.2013.00856
Houwen, S., Visser, L., van der Putten, A., & Vlaskamp, C. (2016). The interrelationships between motor, cognitive, and language development in children with and without intellectual and developmental disabilities. Research in Developmental Disabilities, 53, 19–31
Hyde, D. C., Simon, C. E., Ting, F., & Nikolaeva, J. I. (2018). Functional organization of the temporal–parietal junction for theory of mind in preverbal infants: a near-infrared spectroscopy study. Journal of Neuroscience, 38(18), 4264–4274
Isoda, M. (2016). Understanding intentional actions from observers’ viewpoints: a social neuroscience perspective. Neuroscience research, 112, 1–9
Jeannerod, M. (2001). Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage, 14(1), S103–S109
Kalbe, E., Schlegel, M., Sack, A. T., Nowak, D. A., Dafotakis, M., Bangard, C. … Kessler, J. (2010). Dissociating cognitive from affective theory of mind: a TMS study. Cortex; A Journal Devoted To The Study Of The Nervous System And Behavior, 46(6), 769–780
Kampis, D., Fogd, D., & Kovács, Á. M. (2017). Nonverbal components of Theory of Mind in typical and atypical development. Infant Behavior and Development, 48, 54–62. https://doi.org/10.1016/j.infbeh.2016.11.001
Kandalaft, M. R., Didehbani, N., Krawczyk, D. C., Allen, T. T., & Chapman, S. B. (2013). Virtual reality social cognition training for young adults with high-functioning autism. Journal of autism and developmental disorders, 43(1), 34–44
Kenny, L., Hill, E., & Hamilton, A. F. D. C. (2016). The relationship between social and motor cognition in primary school age-children. Frontiers in psychology, 7, 228
Kessler, K., & Rutherford, H. (2010). The two forms of visuo-spatial perspective taking are differently embodied and subserve different spatial prepositions. Frontiers in Psychology, 1, 213
Kessler, K., & Thomson, L. A. (2010). The embodied nature of spatial perspective taking: Embodied transformation versus sensorimotor interference. Cognition, 114(1), 72–88. https://doi.org/10.1016/j.cognition.2009.08.015
Korkman, M., Kirk, U., Kemp, S. L., Urgesi, C., & Fabbro, F. (2011). [Eds.]). Firenze, Italia:Giunti O.S. Organizzazioni Speciali
Korkman, M., Kirk, U., & Kemp, S. (2007). NEPSY-II: A develop- mental neuropsychological assessment. San Antonio, TX: The Psychological Corporation
Koster-Hale, J., Richardson, H., Velez, N., Asaba, M., Young, L., & Saxe, R. (2017). Mentalizing regions represent distributed, continuous, and abstract dimensions of others’ beliefs. Neuroimage, 161, 9–18
Koster-Hale, J., & Saxe, R. (2013). Theory of mind: a neural prediction problem. Neuron, 79(5), 836–848
Koul, A., Cavallo, A., Cauda, F., Costa, T., Diano, M., Pontil, M., & Becchio, C. (2018). Action observation areas represent intentions from subtle kinematic features. Cerebral Cortex, 28(7), 2647–2654
Jacob, P., & Jeannerod, M. (2005). The motor theory of social cognition: a critique. Trends Cogn Sci. 9(1):21 – 5. doi: 10.1016/j.tics.2004.11.003. PMID: 15639437
Lai, D. C., Tseng, Y. C., Hou, Y. M., & Guo, H. R. (2012). Gender and geographic differences in the prevalence of intellectual disability in children: Analysis of data from the national disability registry of Taiwan. Research in developmental disabilities, 33(6), 2301–2307
Leonard, H. C. (2016). The impact of poor motor skills on perceptual, social and cognitive development: The case of developmental coordination disorder. Frontiers in Psychology, 7, 1–4. https://doi.org/10.3389/fpsyg.2016.00311
Leonard, H. C., & Hill, E. L. (2014). The impact of motor development on typical and atypical social cognition and language: A systematic review. Child and Adolescent Mental Health, 19(3), 163–170. DOI: 1–33.https://doi.org/10.1111/camh.12055
Leung, R. C., Vogan, V. M., Powell, T. L., Anagnostou, E., & Taylor, M. J. (2016). The role of executive functions in social impairment in Autism Spectrum Disorder. Child Neuropsychology, 22(3), 336–344
Lipkin, P. H., Macias, M. M., & COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS. (2020). Promoting Optimal Development: Identifying Infants and Young Children With Developmental Disorders Through Developmental Surveillance and Screening. Pediatrics, 145(1), e20193449. https://doi.org/10.1542/peds.2019-3449
Luo, Y., & Johnson, S. C. (2009). Recognizing the role of perception in action at 6 months. Developmental science, 12(1), 142–149
Mai, X., Zhang, W., Hu, X., Zhen, Z., Xu, Z., Zhang, J., & Liu, C. (2016). Using tDCS to Explore the Role of the Right Temporo-Parietal Junction in Theory of Mind and Cognitive Empathy. Frontiers in psychology, 7, 380. https://doi.org/10.3389/fpsyg.2016.00380
Maggio, M. G., Maresca, G., De Luca, R., Stagnitti, M. C., Porcari, B., Ferrera, M. C. … Calabrò, R. S. (2019). The Growing Use of Virtual Reality in Cognitive Rehabilitation: Fact, Fake or Vision? A Scoping Review. Journal of the National Medical Association, 111(4), 457–463. https://doi.org/10.1016/j.jnma.2019.01.003
Malle, B. F. (2005). Folk theory of mind: Conceptual foundations of human social cognition. In R. R. Hassin, J. S. Uleman, & J. A. Bargh (Eds.), The new unconscious (pp. 225–255). New York, NY: Oxford University Press.
Maranesi, M., Livi, A., Fogassi, L., Rizzolatti, G., & Bonini, L. (2014). Mirror neuron activation prior to action observation in a predictable context. Journal of Neuroscience, 34(45), 14827–14832
Marcovitch, S., O’Brien, M., Calkins, S. D., Leerkes, E. M., Weaver, J. M., & Levine, D. W. (2015). A longitudinal assessment of the relation between executive function and theory of mind at 3, 4, and 5 years. Cognitive development, 33, 40–55
Martínez-Castilla, P., Burt, M., Borgatti, R., & Gagliardi, C. (2015). Facial emotion recognition in Williams syndrome and Down syndrome: A matching and developmental study. Child Neuropsychology, 21(5), 668–692
Mary, A., Slama, H., Mousty, P., Massat, I., Capiau, T., Drabs, V., & Peigneux, P. (2016). Executive and attentional contributions to Theory of Mind deficit in attention deficit/hyperactivity disorder (ADHD). Child Neuropsychology, 22(3), 345–365
Meinhardt-Injac, B., Daum, M. M., Meinhardt, G., & Persike, M. (2018). The two-systems account of theory of mind: Testing the links to social-perceptual and cognitive abilities. Frontiers in human neuroscience, 12, 25
Meltzoff, A. N. (2007). ‘Like me’: a foundation for social cognition. Developmental science, 10(1), 126–134
Meltzoff, A. N., & Decety, J. (2003). What imitation tells us about social cognition: a rapprochement between developmental psychology and cognitive neuroscience. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 358(1431), 491–500
Molenberghs, P., Sale, M. V., & Mattingley, J. B. (2012). Is there a critical lesion site for unilateral spatial neglect? A meta-analysis using activation likelihood estimation. Front. Hum. Neurosci. 6, 78. doi: 10.3389/ fnhum.2012.00078
Moll, H., Meltzoff, A. N., Merzsch, K., & Tomasello, M. (2012). Taking versus confronting visual perspectives in preschool children.Developmental Psychology: 49.,646–654
Narzisi, A., Muratori, F., Calderoni, S., Fabbro, F., & Urgesi, C. (2013). Neuropsychological profile in high functioning autism spectrum disorders. Journal of autism and developmental disorders, 43(8), 1895–1909
Ohara, R., Kanejima, Y., Kitamura, M., & Izawa, P., K (2019). Association between Social Skills and Motor Skills in Individuals with Autism Spectrum Disorder: A Systematic Review. European Journal of Investigation in Health Psychology and Education, 10(1), 276–296. https://doi.org/10.3390/ejihpe10010022
Oldrati, V., Ferrari, E., Butti, N., Cattaneo, Z., Borgatti, R., Urgesi, C., & Finisguerra, A. (2021). How social is the cerebellum? Exploring the effects of cerebellar transcranial direct current stimulation on the prediction of social and physical events. Brain Struct Funct, 226(3), 671–684. https://doi.org/10.1007/s00429-020-02198-0
Pearson, A., Marsh, L., Ropar, D., & Hamilton, A. (2016). Cognitive Mechanisms underlying visual perspective taking in typical and ASC children. Autism Research, 9(1), 121–130
Piek, J. P., Dawson, L., Smith, L. M., & Gasson, N. (2008). The role of early fine and gross motor development on later motor and cognitive ability. Human Movement Science, 27, 668–681. https://doi.org/10.1016/j.humov.2007.11.002
Premack, D., & Woodruff, G. (1978). Does the chimpanzee have a theory of mind? Behavioral And Brain Sciences, 1, 515–526
Purpura, G., Cioni, G., & Tinelli, F. (2017). Multisensory-based rehabilitation approach: translational insights from animal models to early intervention. Frontiers in neuroscience, 11, 430
Rasmussen, C., Tamana, S., Baugh, L., Andrew, G., Tough, S., & Zwaigenbaum, L. (2013). Neuropsychological impairments on the NEPSY-II among children with FASD. Child Neuropsychology, 19(4), 337–349
Ritterband-Rosenbaum, A., Justiniano, M. D., Nielsen, J. B., & Christensen, M. S. (2019). Are sensorimotor experiences the key for successful early intervention in infants with congenital brain lesion? Infant Behavior and Development, 54, 133–139
Rizzolatti, G., & Craighero, L. (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192
Russell, E. W., Russell, S. L., & Hill, B. D. (2005). The fundamental psychometric status of neuropsychological batteries. Archives of Clinical Neuropsychology, 20(6), 785–794
Santiesteban, I., Banissy, M. J., Catmur, C., & Bird, G. (2012). Enhancing social ability by stimulating right temporoparietal junction. Current Biology, 22, 2274–2277
Saxe, R. (2006). Uniquely human social cognition. Current Opinion In Neurobiology, 16, 235–239
Schmidt, S. N. L., Hass, J., Kirsch, P., & Mier, D. (2021). The human mirror neuron system-A common neural basis for social cognition? Psychophysiology. 58(5):e13781. doi: https://doi.org/10.1111/psyp.13781. Epub 2021 Feb 11. PMID: 33576063
Schurz, M., Maliske, L., & Kanske, P. (2020). Cross-network interactions in social cognition: A review of findings on task related brain activation and connectivity. Cortex,130, 142–157
Schurz, M., Aichhorn, M., Martin, A., & Perner, J. (2013). Common brain areas engaged in false belief reasoning and visual perspective taking: a meta-analysis of functional brain imaging studies. Frontiers in human neuroscience, 7, 712
Siman-Tov, T., Granot, R. Y., Shany, O., et al. (2019). Is there a prediction network? Meta-analytic evidence for a cortical-subcortical network likely subserving prediction. Neuroscience And Biobehavioral Reviews, 105, 262–275. https://doi.org/10.1016/j.neubiorev.2019.08.012
Sodian, B., & Kristen-Antonow, S. (2015). Declarative joint attention as a foundation of theory of mind. Developmental psychology, 51(9), 1190
Sodian, B., & Thoermer, C. (2004). Infants’ understanding of looking, pointing, and reaching as cues to goal-directed action. Journal of Cognition and Development, 5, 289–316
Spanoudis, G. (2016). Theory of mind and specific language impairment in school-age children. Journal of Communication Disorders, 61, 83–96
Stoodley, C. J., & Limperopoulos, C. (2016). Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med Oct, 21(5), 356–364. doi: https://doi.org/10.1016/j.siny.2016.04.010
Tavano, A., Grasso, R., Gagliardi, C., Triulzi, F., Bresolin, N., Fabbro, F., & Borgatti, R. (2007). Disorders of cognitive and affective development in cerebellar malformations. Brain, 130(10), 2646–2660. https://doi.org/10.1093/brain/awm201
Tieri, G., Morone, G., Paolucci, S., & Iosa, M. (2018). Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies. Expert review of medical devices, 15(2), 107–117
Thirion-Marissiaux, A. F., & Nader-Grosbois, N. (2008). Theory of mind “beliefs”, developmental characteristics and social understanding in children and adolescents with intellectual disabilities. Research in developmental disabilities, 29(6), 547–566
Urgen, B. A., & Miller, L. E. (2015). Towards an empirically grounded predictive coding account of action understanding. Journal of Neuroscience, 35(12), 4789–4791
Urgesi, C., Campanella, F., & Fabbro, F. (2011). NEPSY-II, Contributo alla Taratura Italiana. Firenze: Giunti OS
Urgesi, C., Candidi, M., & Avenanti, A. (2014). Neuroanatomical substrates of action perception and understanding: An anatomic likelihood estimation meta-analysis of lesion-symptom mapping studies in brain injured patients. Frontiers in Human Neuroscience, 8(MAY), 1–17. https://doi.org/10.3389/fnhum.2014.00344
Urgesi, C., Butti, N., Finisguerra, A., Biffi, E., Valente, E. M., Romaniello, R., & Borgatti, R. (2021). Social prediction in pediatric patients with congenital, non-progressive malformations of the cerebellum: From deficits in predicting movements to rehabilitation in virtual reality. Cortex; A Journal Devoted To The Study Of The Nervous System And Behavior, 144, 82–98. https://doi.org/10.1016/j.cortex.2021.08.008
Van Overwalle, F. (2009). Social cognition and the brain: a meta-analysis. Human brain mapping, 30(3), 829–858
Vicari, S., Snitzer Reilly, J., Pasqualetti, P., Vizzotto, A., & Caltagirone, C. (2000). Recognition of facial expressions of emotions in school-age children: The intersection of perceptual and semantic categories. Acta Paediatrica, 89(7), 836–845. doi:https://doi.org/10.1111/j.1651-2227.2000.tb00392.x
Xiao, Y., Geng, F., Riggins, T., Chen, G., & Redcay, E. (2019). Neural correlates of developing theory of mind competence in early childhood. Neuroimage, 184, 707–716
Warnell, K. R., & Redcay, E. (2019). Minimal coherence among varied theory of mind measures in childhood and adulthood. Cognition, 191, 103997
Wechsler, D. (2003). Wechsler Intelligence Scale for Children, 4th Edn San Antonia, TX: PsychCorp
Wechsler, D. (2002). WPPSI-III-Wechsler preschool and primary scale of intelligence. San Antonio, TX: Psychological Corporation
Wurm, M. F., Artemenko, C., Giuliani, D., & Schubotz, R. I. (2017). Action at its place: Contextual settings enhance action recognition in 4- to 8-year-old children. Developmental Psychology, 53(4), 662–670. https://doi.org/10.1037/dev0000273
Zilli, T., Zanini, S., Conte, S., Borgatti, R., & Urgesi, C. (2015). Neuropsychological assessment of children with epilepsy and average intelligence using NEPSY II. Journal of clinical and experimental neuropsychology, 37(10), 1036–1051
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferrari, E., Butti, N., Gagliardi, C. et al. Cognitive predictors of Social processing in congenital atypical development. J Autism Dev Disord 53, 3343–3355 (2023). https://doi.org/10.1007/s10803-022-05630-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-022-05630-y