Abstract
Abnormal perceptual-motor coordination is hypothesized here to be involved in social deficits of autism spectrum disorder (ASD). To test this hypothesis, high functioning children with ASD and typical controls, similar in age as well as verbal and perceptive performance, performed perceptual-motor coordination tasks and several social competence tests. Spontaneous coordination, and intentionally required in-phase and anti-phase were examined. The oscillation kinematics, as well as the accuracy and stability of spontaneous coordination were similar in both groups. In intentional coordination, ASD children produced less accurate, less stable and less complex relative phases than the control group, and in-phase and anti-phase performances that were similar in accuracy, stability, and complexity. An age-dependent relationship between socio-adaptability and coordination skills suggested these skills develop together.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our goal is to test the hypothesis of motor control involvement in the development of perceptual-motor and social deficits in autism. Autism Spectrum Disorder (ASD) is a pervasive neurodevelopmental disorder, affecting one in 68 births (CDC 2016), and diagnosed with regard to deficient social skills and a markedly restricted repertoire of behaviors (DSM-IV-TR, CIM-10, OMS) (APA 2011). The median of worldwide prevalence estimates of autism spectrum disorders was 1/160 (Elsabbagh et al. 2012). Whereas social impairments are the core alerting symptoms (Zwaigenbaum et al. 2005), the major parental concern (Chamak et al. 2011; Guinchat et al. 2012), and the key factor of social exclusion (Le Callenec and Chapel 2016), individuals with ASD have testified to have unusual sensory-motor experiences (Bogdašina 2005). These experiences have been related to an atypical temporal matching of neural events in spatially separate brain regions (Belmonte et al. 2004; Uddin et al. 2013; Maximo et al. 2014). This raises the possibility of a broad impairment affecting the coordination of the whole perceptual-motor system, and manifested by perceptual-motor abnormalities at behavioural level.
Even though motor abnormalities are not used as diagnostic variables (DSM-V), they were already pointed out by Kanner (1968), noticing a limitation of spontaneous activity (p. 219) and/or the failures of body adjustment (p. 225). Later, Leary and Hill (1996) described 36 movement disturbances in ASD persons, such as toe walking, involuntary dance-like movements, the lack of eye contact, etc. Motor scores for children with ASD often fall 1.5 standard deviations (SD) below the mean of typical controls (Fournier et al. 2010) (Whyatt and Craig 2012) and about 50–73% of all children with ASD exhibit delays in the attainment of motor milestones such as head lifting, rolling, sitting, creeping, and walking. (Provost et al. 2007). Moreover, motor abnormalities are among the earliest signs of ASD (Teitelbaum et al. 1998) and faithful predictors of the further persistence of autistic behaviors, captured by Vineland Adaptive Behavior Scale (Sutera et al. 2007). Kinematic analysis in individuals with ASD have revealed abnormal patterns of walking and hopping (Esposito et al. 2011; Moran et al. 2013), of velocity profiles in horizontal sinusoidal arm movements (Cook et al. 2013) together with an abnormal organization and control of motor sequences (Mari et al. 2003; Schmitz et al. 2003).
The present paper focuses on coordination in ASD children, and on their socio-adaptive (SAQ) skills. Basically, coordination refers to the concerted action of perceptual-motor components—as for example limbs, eyes, head, or trunk—and underlies the production of any bodily motion. In ASD infants, coordination deficits have been reported with respect to the kinematic chain linking the limbs, pelvis and shoulder griddle during the execution of primary motor patterns: rolling, crawling, walking etc… (Teitelbaum et al. 1998). In ASD children, 30 to 70 months of age, they were documented between smiles and eye contact (Dawson et al. 1991); in ASD adolescents between speech and gesture motions (de Marchena and Eigsti 2010). These reports, however, have not quantified the body motions in terms of measures capturing the essential properties of coordination—its stability and accuracy—which is indispensable to systematically open an experimental window into the coordination-building process. These measures have been developed in the past 30 years by the dynamical approach to pattern formation in (human) movement (Haken 1983; Thelen and Smith 1996; Turvey 1990), often referred to as coordination dynamics (Kelso 1995; Kelso et al. 2013).
In a nutshell, coordination dynamics (Kelso 1984) aims to get an insight into the process of pattern formation undergoing in complex systems, composed of numerous moving components of any nature—neurons in brain, muscular groups in a body, or humans in a group. Its method consists in looking for universal, lawful principles describing how these components bound together into stable coordination patterns (e.g., waking, running, creeping). Stability expresses how strongly components are likened, and thus resist to perturbations coming from the internal or external environment. In typical subjects, coordination pattern stability has been analysed, among others, between distinct brain areas (Banerjee et al. 2012), between limbs belonging to a unique body (Kelso 1984), between limbs and moving objects (Huys et al. 2004) (Dessing et al. 2007) as well as between distinct individuals engaged in dyadic performances (Schmidt et al. 1990; van Ulzen et al. 2008). Experimental work has shown that without any instruction, learning or effort, healthy subjects spontaneously organize their motor components into two stable coordination patterns only: in-phase and anti-phase (Kelso 1984, 2013). In in-phase coordination, motor components oscillate in synchrony (see Fig. 1a) whereas in anti-phase coordination they oscillate in alternation (see Fig. 1b). In any case, coordination has been shown to display the same lawful properties, indicating that it is a generic process obeying to general rules. Of relevance in the context of the present study, the stabilisation of coordination patterns has been proven to trigger changes in cognition (Kostrubiec et al. 2012): Cognitive processes are embodied, that is, tightly related to motor (coordination) processes (Thelen et al. 2001).
In line with the hypothesis of embodied social cognition (Gallagher 2008), motor coordination between distinct individuals has come to be viewed as a potential means by which the humans become socially bonded with one another (Launay et al. 2016). In social groups, healthy individuals have a tendency to coordinate their motion: people spontaneously walk, rock, sing, or clap hands in unison (McNeill 1995; Launay et al. 2016; Richardson et al. 2007), and this effect bolsters their feelings of social cohesion (Fischer et al. 2013; Lakens 2010; Schmidt and Fitzpatrick 2016). Participants who have matched each other’s movements tend to express higher levels of subjective liking (Hove and Risen 2009), of empathy (De Coster et al. 2013), of mutual trust (Fischer et al. 2013), of altruism (Valdesolo and DeSteno 2011), and of cooperativity (Wiltermuth and Heath 2009). Such pro-social effects of interpersonal coordination are already present in children (Kirschner and Tomasello 2010) as young as 14-month of age: Cirelli et al. (2014) have shown that if the assistant holding the child bounced synchronously with the experimenter, the child was then more likely to help that experimenter than when the bouncing was asynchronous.
Since inter-personal perceptual-motor coordination is tied to social behavior and its experience in typical individuals, there may well be a relationship between the social and perceptual-motor coordination deficits in ASD. In order to investigate this issue, we therefore use the tools of coordination dynamics to capture eventual coordination abnormalities in ASD children and to assess their relationship with social deficits.
In healthy individuals, coordination has been first investigated in the bimanual motion paradigm (Kelso 1984) in which an individual is merely asked to oscillate both his/her index fingers, for instance, in the sagittal plane (i.e., up and down). When the positions of both limbs, which can be conceived of as oscillators when performing oscillatory motions, are recorded, they result in two overlapping or in two alternating times series, for the in-phase and anti-phase mode, respectively (Fig. 1). Such times series are coined as motors signals. The phase captures the part of an oscillation cycle completed by an oscillator at a given instant; Assessed in degrees, it grows from between 0° to 360° (Pikovsky et al. 2001). In a system of two oscillators, the coordination between them is reliably captured by relative phase (RP), which refers to the difference between their respective phases, giving 0° for in-phase and 180° for anti-phase (Kelso 1984). The (coordination) pattern stability is most often assessed via the RP’s variance: the more the RP varies, the less the pattern is stable, and inversely (Schöner et al. 1986). If a specific coordination is required, pattern accuracy may be computed by the discrepancy between the required and produced RP (Kostrubiec et al. 2013).
With the above-mentioned measures at hand, experimenters have established the main features of coordination. In healthy subjects, anti-phase has been experimentally shown to be less stable and accurate (Kelso et al. 1986), more attention-wise (Temprado et al. 2001), and recruiting a larger neural network as compared to in-phase (Jantzen and Kelso 2007). The same coordinative properties were observed in dyadic coordination where the motion of a dominant limb—say the dominant arm—in two distinct individuals watching each other was investigated (Amazeen et al. 1995; Schmidt et al. 1998, 1990; Richardson et al. 2007) (Amazeen et al. 1995).
Beyond the RP variance, assessing the amount of RP variability, the structure of fluctuations in motor signals provide further information about the motor system’s performance (Slifkin and Newell 1999). We here quantify the complexity of RP fluctuations using approximate entropy (Pincus 1991; Pincus and Goldberger 1994; Slifkin and Newell 1999). A complex, unpredictably fluctuating time series has a high approximate entropy (Apen), and inversely. Pathology has been associated with lowered complexity, evidenced for instance in voluntary movement in Parkinsonians (Powell et al. 2014) or in EEG signals in schizophrenic patients (Akar et al. 2016). Simulations with mathematical models suggest that weakly complex times series are generated by weakly connected systems (Pincus 1994).
If ASD children display a coordination impairment, their coordination patterns should be less stable, less accurate, less predictable, as compared to controls in any condition. To date, coordination in ASD children has been assessed in a rocking chair paradigm, providing the children the opportunity to spontaneously coordinate with a parent. The child-parent dyads exhibited less in-phase patterns than typical control dyads (Marsh et al. 2013). Similarly, on a bimanual drumming task, ASD children were shown to be less able to maintain the intentionally required 0° and 180° patterns than typical controls (Isenhower et al. 2012; Fitzpatrick et al. 2013). Similar results were observed in ASD adolescents in inter-personal pendulum swinging (Fitzpatrick et al. 2016).
To provide further data to the hypothesis of deviant coordination in ASD, the present study investigates sensorimotor coordination in ASD children, more specifically, the perceptual-motor linkage between ASD children and a non-human oscillating stimulus: a red dot. In the present study, ASD and typically developing (TD) children were instructed to oscillate their finger to move a green dot left–right that was displayed on a screen where the red dot was oscillating autonomously. Three coordination conditions, spontaneous, and intentionally required 0° and 180° were investigated, and the RP between the green and the red dots was measured. We expected that in all the three conditions ASD children would produce less accurate, less stable, and less predictable coordination as compared to TD children. Finally, in line with the hypothesis of embodied social cognition, we anticipate the existence of a relationship between the coordination and the socio-adaptive skills, assessed by a set of psychological tests.
Method
Participants
Twenty children with high functioning ASD (without mental retardation), and 21 children with a history of TD participated in the study. High functioning ASD children were chosen to ensure that possible differences in coordination and social skills can be attributed to autism rather than to mental disorder. Children with ASD were recruited through advertisements at local therapist offices and the TD children from local families and schools. The autistic children met the DSM-IV criteria for ASD (APA 2011) and the clinicians administered Autism Diagnostic Interview, Revised (ADI-R; Lord et al. 1994).
The age of the children in both groups ranged from 8 to 14 (mean age: 10 for ASD and 11 for TD). In each group 18 children were male. The ASD and TD groups did not differ statistically in age and verbal performance as assessed by the Verbal intelligence quotient (VIQ) from the Wechsler Intelligence Scale for Children IV (WISC-IV2) (Table 1). They were distinct in ADI-R scores. The research project was approved by the Non-Interventional Research Ethics Committee at University of Toulouse (CERNI-2016-003). Parents signed an informed consent form and verbal assent was received from the children.
Material
Our measures of interest included psychological scores of social competence, of motor function, and of intellectual functioning, as captured by psychological questionnaires and the perceptual-motor coordination task.
Psychological Scores
All psychological scores and their (brief) interpretation are listed in Table 2. SAQ was measured through the Vineland Adaptive Behavior Scales Second Edition Vineland-II (Sparrow et al. 2012). The Vineland-II is a structured interview administered to primary caregiver/s to assess a child’s daily living skills. Three sub-scales of the Vineland-II were used: communication, autonomy, and socialization (ex: say “please”, “thanks”, or “excuse-me”), providing a total Socio-Adaptatif quotient (SAQ). For SAQ, possible item scores are 2, 1, or 0; lower scores indicate greater impairment in adaptive functioning and a total score of 70 or lower is normally taken as a sign of developmental delay.
Social skills were assessed by the Social Communication Questionnaire SCQ (Rutter et al. 2007) adapted to French by (Kruck et al. 2013). The SCQ is a standardized parent questionnaire with 40 yes-or-no items based on the revised ADI, intended to assess autistic-like symptoms and assist in the diagnosis of autism. It captures three areas of functioning: reciprocal social interaction (RSI) (ex: eye gaze, social smile, offering to share, interest in other children…), communication (ex: head shaking to mean no, imitation, pointing to express interest), and restricted, repetitive, and stereotyped patterns of behaviour (ex: spinning wheels, shaking objects…). The questionnaire typically takes less than 10 min to complete. Possible item scores are 0 or 1; the total SCQ score may thus vary from 0 to 39; a score of 15 or greater is used as an indication of possible ASD. We refer below to the reciprocal social interaction sub-score as RSI, specifically assessing sharing, reciprocity and exchanges in social interactions.
An indication of verbal and perceptual reasoning capacities was provided by two quotients drawn from the Wechsler Intelligence Scale for Children IV (WISC-IV2): the verbal intelligence quotient (VIQ) and the performance intelligence quotient (PIQ). An indication of VIQ was assessed through similarities tests requiring the examinee to describe in what way two given things (ex: tomatoes and carrots) are alike (ex: they are vegetables). An indication of PIQ, assessing perceptual reasoning, was assessed by a matrix test where the examinee is presented with incomplete abstract patterns and should select the logically fitting part from among some possibilities. For both quotients, a score below 70 is a sign of developmental delay.
Motor functioning was assessed by the Developmental Coordination Disorder Questionnaire DCDQ (Martini et al. 2011). The DCQD is a parent questionnaire designed to screen for coordination disorders in children aged 5–5 years. It consists of 15 items grouping into three distinct factors: motor control, fine motor skills/writing, and motor coordination (ex: “Your child throws a ball in a controlled and accurate fashion”). Possible item scores range from 1 to 5. The total DCQD score may vary from 15 to 75, and a score of 55 or lower warns about a risk of developmental coordination disorder.
Coordination Task
The child had to move a green circle left–right on a computer screen by oscillating a slider with his/her index. The finger motions were recorded by for further analysis by a data acquisition chain transforming the angular position of the slider into voltage by a linear potentiometer. The slider restricted the movement of the dominant index finger to the horizontal plane, allowing a 9 cm-range of friction-free flexion-extension motion about the metacarpo-phalangeal joint. The voltage value was read by an Arduino card connected to a 2.1 GHz computer by a serial port at a baud rate of 9600, thus providing a signal sampled at 200 Hz. The signal was used as a continuous input into the computer, which contained a custom-made program implemented on Processing.org. It controlled the horizontal position of a 60 × 60 pixels green circle on a grey × 244 pixels window displayed on a 14-inch computer screen, split in two halves by a vertical black line.
In most conditions, in addition to the green circle controlled by participant, a red circle animated by an equation of motion was displayed 200 pixels above the green one. The horizontal position of the red circle was controlled by the equation of motion simulating a self-sustained Rayleigh-van-der-Pol oscillator (Kay et al. 1987), according to:
The variables, x, \(\dot {x}\), and \(\ddot {x}\) refer to position, velocity and acceleration, respectively, and the constants α, β, γ, and ω to van der Pol, Rayleigh, and linear damping, and the oscillator’s eigen-frequency, respectively. All the parameters were fixed on the values used by (Kelso et al. 2009), α = 0.641, β = 0.00709, γ = 12.457 entailing self-sustained oscillations compatible with empirically measured properties of human oscillatory motion. The eigen-frequency was fixed at ω = 2π × 0.6. The differential equation returned instantaneous acceleration, which was integrated using a 4th order Runge–Kutta method to provide the red circle’s velocity and position. The circles’ positions were recorded for further analysis. The box containing the data acquisition chain was fastened to the table with Velcro strips and a cushion supported the child’s arm.
Procedure
In order to facilitate child collaboration, the whole experiment took place in the most familiar and reassuring place: at the child’s home. Only one experimental session was administered during which the Vineland-II was completed with the primary caregiver, most often the mother whereas the child realized the coordination task. The coordination task was administered in a quiet room without distractors in order to preserve the child’s attention. The child could ask for as many pauses as (s)he wanted, during which the subtests of Similarities and Matrix were administered. During the coordination task, the primary caregiver completed the SAQ, SCQ and DCQD questionnaires. The whole experiment lasted about 1.5 h. All data were collected between February 2016 to January 2017.
In the coordination task, the child was instructed to left–right oscillate the green circle around the black vertical line at his/her own pace. Four experimental conditions were administered sequentially: (1) spontaneous oscillation, (2) spontaneous coordination, (3) 0° intentional coordination and (4) 180° intentional coordination. In the spontaneous oscillation, only the green circle was displayed and the child was instructed to move it left–right at his/her own pace. In the spontaneous coordination, the oscillating red circle was displayed above the green one. The child was instructed to look at the screen and to ‘merely’ oscillate the green circle (s)he controlled. In the intentional 0° coordination the child was asked to keep the green circle below the red one, that is, to achieve an in-phase coordination. In the intentional 180° coordination (s)he was instructed to do the opposite movement to the red dot, that is, to produce an anti-phase relationship. Each block lasted 30 s and the whole coordination experiment 30 min. Overall, the experiment entailed six blocks of five trials (see Fig. 2). The first block corresponded to the spontaneous oscillation, the second block to the spontaneous coordination condition, the third and fifth blocks to the intentional 0° coordination condition, and the fourth and sixth blocks to the intentional 180° coordination condition.
Data Reduction and Analysis
The signal provided by the potentiometer, representing the displacement of the child-controlled circle, were mean-centered, linearly detrended, and low-pass filtered using a second order dual-pass Butterworth filter with a cut-off frequency of 20 Hz. After this pre-processing, the main features of the child’s produced oscillations, namely the periods and amplitudes of the oscillation cycles, were estimated. The period refers to cycle duration and the amplitude to the distance from the center of motion to either extreme of the oscillation’s cycles. For each trial, the averaged period, corresponding SD, and averaged amplitude were computed.
Coordination was captured by the zeroth-lag cross correlation and by the continuous RP between the positions of the human-controlled and model-controlled circles using the continuous Hilbert transform. To avoid transients, the first and last second of the time series were removed from the RP analysis. For all the circular variables, all means and variances were computed using circular statistics (Batschelet 1981). For each trail, four statistical variables were computed: the (zeroth-lag) cross correlation (CrossCorr), the absolute error of RP (AE), the variability of RP (SD), and the Apen of RP (see Table 1). The zeroth-lag CrossCorr captures the similarity between the child’s and the stimulus’ oscillation. For two identical times series CrossCorr amounts to one. Absolute error (AE), assessing coordination inaccuracy, was computed as the difference between the produced and the required RP (i.e., negative values indicate that the child lagged the stimulus). Low accuracy is related to high AE value. For the ‘spontaneous’ conditions, in which no required pattern was specified, the AE was computed with respect to 0° and 180° and the smallest discrepancy retained. Although the notion of error is senseless in this case, this measure informed us how far spontaneously produced RPs occurred from the spontaneously stable patterns. The RP variability, which inversely scales with coordination stability, was captured by the circular variability index and transformed to a SD.
The complexity of RP fluctuations were assessed by Apen. Apen captures the logarithmic likelihood that ‘similar’ vectors of observations will not be followed by additional ‘similar’ vectors in medium-sized (less than 1000 samples), stationary time series. It yields a value between 0 and 2 for full predictability and unpredictability, respectively. Apen calculation requires three parameters: the lengths of compared vectors (m), the lag between the vectors (τ) and a similarity threshold (r). As suggested in the literature (Restrepo et al. 2014), the parameters were fixed at m = 3, τ = {1, .., 10} and r at 0.2 times the SD of the raw signal. Unwrapped and, to comply with the stationarity requirement, differentiated RP time series were submitted to Apen analysis. As the results were similar for the various lags, we report here the data for τ = 1.
Statistical Analysis
The statistical analysis was fivefold. (1) We tested whether the kinematics of oscillation differed between ASD and TD children by performing t-tests on the period’s mean, corresponding SD, and on the oscillation amplitude as produced by the two groups of children in the spontaneous oscillation. (2) We checked whether the presentation of the red circle modified these kinematics using a 2 (Stimulus = {spontaneous oscillation, spontaneous coordination}) × 2 (Group = {ASD, TD}) ANOVA on all the above-mentioned variables. (3) We examined whether the mere presence of the oscillating red circle had spontaneously evoked a child—stimulus coordination by running a 2 (Stimulus = {spontaneous oscillation, spontaneous coordination}) × 2 (Group = {ASD, TD}) ANOVA on all coordination variables (SD and AE of RP, Apen of RP, and CrossCorr). If spontaneous coordination had arisen, AE and SD of RP should be lower in the spontaneous coordination as compared to the spontaneous oscillation. (4) We tested the effect of intentional constraint by carrying out a 3 (Coordination = {Spontaneous, 0°, 180°}) × 2 (Group = {ASD, TD}) ANOVA on all coordination variables. If the intention to coordinate improved the coordination, AE and SD of RP should be lower when the patterns (0° and 180°) are intentionally required as compared to when they arise spontaneously.
If appropriate, the ANOVAs were followed by post-hoc analyses. First, t-tests were performed to compare ASD and TD children for each Coordination condition separately. Second, orthogonal contrasts were designed to compare all Coordination conditions in ASD and in TD children separately. Both for the t-tests and the contrasts, Bonferroni correction was used for family-wise error rate. All ANOVAs were run with repeated measures on the Group factor. Homogeneity of variances was tested for by the Bartlett test; whenever it was violated, the Greenhouse-Geisser correction was applied. For all results, only significant effects at p < .05 are reported along with the corresponding estimates of the effect size (η2).
Our fifth goal was to capture the possible relationship between the psychological test z-scores and the experimental measures of coordination. A Spearman correlation matrix and a path analysis using Lavaan package in R® (Rosseel 2012) were undertaken. Because of high redundancy in our dataset, only four variables were selected for the path analysis: age, SAQ, RSI, and a composite coordination measure informing on RP accuracy and stability: (AE2 + SD2)1/2. In the to-be-tested model we assumed that age influences all other variables and that coordination influences all variables except age. For the sake of brevity, only path coefficients, corresponding to standardised β coefficients in regression analysis, and significance levels are reported. Standardised coefficients refers to estimates obtained when predictors and outcomes in regression analysis were standardized to have variance equal to one.
Results
Visual Inspection of the Data
Figure 3 displays data from an individual trial for an ASD child (upper two panels) and a TD child (lower two panels) when 180° RP was required. The ASD child (dashed line) fails to sustain a specific phase lag with the oscillating stimulus (solid line). For TD child, in contrast, the lag between positions appears consistent and the corresponding RP less variable than for the ASD child. Figure 4, displaying the RP’s rate of change, illustrates that the corresponding fluctuations in the ASD child (top panel) is less complex as compared to TD child.
Spontaneous Oscillations and Coordination
The Kinematics of Oscillation
Period and Amplitude
In the spontaneous oscillation condition, t-tests revealed no statistically reliable difference between ASD and TD children on period, its SD, and amplitude (p < .05). A 2 (Stimulus = {spontaneous oscillation, spontaneous coordination}) × 2 (Group) ANOVA revealed a main effect of Stimulus on period (F(1, 39) = 8.98, p < .001, η2 = 0.23), its SD (F (1, 39) = 58.79, p < .001, η2 = 0.61), and amplitude (F(1, 39) = 6.21, p < .01, η2 = 0.16): the period and its SD were higher and the amplitude lower when the stimulus was absent (215.45 (SD = 78.14) vs. 184.72, (SD = 74.31) for the period, 67.54 (SD = 32.98) vs. 38.75 (SD = 22.61) its SD, 128.63 (SD = 62.72) vs. 133.15 (SD = 45.51)). There was no reliable difference between ASD and TD children in these kinematics features.
The presence of Spontaneous Coordination
AE and SD of RP
The same 2 (Stimulus) × 2 (Group) ANOVA carried out on the AE and the SD of the RP evidenced a main effect of Stimulus only (F(1, 39) = 4.88, p < .03, η2 = 0.26; F(1, 39) = 25.85, p < .001, η2 = 0.23, respectively): the coordination accuracy and variability were lower and higher, respectively, in the absence of the Stimulus.
Apen and CrossCorr
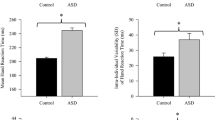
The same ANOVA carried out on Apen and on CrossCorr revealed a main effect of Group only (F(1, 39) = 8.75, p < .01, η2 = 0.17; F(1, 39) = 5.22, p < .03, η2 = 0.17, respectively). The complexity and similarity between the stimulus-oscillation and the child-oscillation was lower in ASD children than in TD controls (see Fig. 5).
Apen (a) and CrossCorr (b) of RP as a function of Group for the spontaneous oscillation and spontaneous coordination lumped together. Vertical bars depict between-subjects standard error, asterisks the statistical significance levels (* for 0.05, ** for 0.001 and *** for 0.0001). High Apen is indicative of high unpredictability
The effect of Intentional Coordination
Accuracy and Stability of Intentional Coordination
RP Absolute Error
AE as a function of Group and Coordination is displayed in Fig. 6a. A 3 (Coordination) × 2 (Group) ANOVA on AE revealed a main effect of Group (F(1,39) = 10.23, p < .003, η2 = 0.14), of Coordination (F(2, 78) = 130.71, p < .0001, η2 = 0.55), and a Coordination ⊆ Group interaction (F(2, 78) = 9.96, p < .001, η2 = 0.18). P-adjusted t-tests comparing ASD to TD children in each coordination condition showed that the groups were distinct in the 0° and in 180° coordination (p < .04, p < .001, respectively). Further contrasts evidenced that AE was highest in the spontaneous coordination, lower in the 180° condition and lowest in the 0° condition for the TD children (p < .001 for all). As for the ASD children, the AE in the spontaneous coordination was higher than in both the 0° and the 180° one (p < .001 for both) but the latter two conditions did not differ significantly.
RP Variability
The RP variability as a function of Group and Coordination is displayed in Fig. 6b. The same ANOVA on RP variability revealed a main effect of Group (F(1,39) = 10.44, p < .003, η2 = 0.14), of Coordination (F(2, 78) = 122.46, p < .001, η2 = 0.54), and a Coordination × Group interaction (F(2, 78) = 8.80, p < .001, η2 = 0.15). P-adjusted t-tests comparing the ASD with the TD children in each coordination condition showed that the groups were distinct in the 0° and 180° coordination (p < .001, p < .001, respectively). Further contrasts evidenced that the RP variability was highest in the spontaneous coordination, lower for the 180°, and lowest for the 0° coordination for the TD children (p < .001, p < .001 and p < .003). As for the ASD children, the RP variability in the spontaneous coordination was higher than in the 0° and 180° condition (p < .0001 for both), but as for the AE, the difference between the latter two conditions did not reach significance.
Complexity and Similarity of Intentional Coordination
RP Approximate Entropy
Apen as a function of Group and Coordination is displayed in Fig. 7. The ANOVA on Apen revealed a main effect of Group (F(2, 78) = 13.38, p < .001, η2 = 0.255) and a Coordination × Group interaction (F(2, 78) = 3.487, p < .035, η2 = 0.11). P-adjusted t-tests comparing ASD to TD children showed that the groups were significantly distinct in the spontaneous and 0° coordination (p < .05 and p < .02, respectively). Further contrasts evidenced that Apen was higher in the spontaneous condition as compared to the 180° coordination for the TD children (p < .037). No reliable difference between the three coordination conditions was found for the ASD children (p > .05).
CrossCorr
The ANOVA on the cross correlation revealed a main effect of Group (F(2, 78) = 14.27, p < .0005, η2 = 0.38) and of Coordination (F(2, 78) = 33.15, p < .0001, η2 = 0.873), indicating that the similarity between the model and child oscillation was higher in TD than in TSA children (0.39(SD = 0.27) vs. 0.61(SD = 0.24), respectively).
Psychological Scores and their Relationship to Coordination
As depicted in Table 3, ASD and TD children significantly differed in the RSI and SAQ scores as well as in their reasoning performance (QIP) and motor functioning score (DCQC).
The correlation matrix between the psychological and coordination variables in displayed in Table 4 in Appendix. For the sake of brevity and clarity, they are displayed in Fig. 8 in the form of a network of significant correlations. The width of each link illustrates the size of the correlation strength. Solid and dashed lines illustrates positive and negative correlations, respectively. All coordination variables significantly correlated with age, ADI, and SAQ, except for the SAQ—Apen correlation in the 180° condition. All SD and AE measures reliably correlated with the DCDC. RSI, is related to DCQC.
Path coefficients (beta parameters), describing the effect of a variable on another variable is displayed in Fig. 9. The fit of the model was good (CFI = 0.99, NFI = 0.99, RMSEA = 0.00, SRMR = .00). Age directly influenced coordination (β = − 0.42, p < .01) and SAQ (β = 0.42, p < .001), the influence of coordination on SAQ was both direct (β = 0.54, p < .001) and mediated by age (β = − .42, β = − 0.54, p < .04). Multiple regressions involving SAQ, RP and age showed that the β parameter for age was higher when SAQ was the outcome and RP and age the predictors (βRP = 0.44, βage = − 0.55, r2 = .27), as compared to when RP was the outcome with SAQ and age as predictors (βQA = − 0.56, βage = − 0.43, r2 = 0.241).
Path diagram with standardized estimates of path coefficients for four variables: age, SAQ, RSI, and a composite coordination measure informing on RP inaccuracy and instability: (AE2 + SD2)1/2. Arrows indicate directed influence, asterisks the statistical significance levels (* for 0.05, ** for 0.001 and *** for 0.0001)
Discussion
Deviant perceptual-motor coordination is thought to be involved in the cascade of typical social deficits of ASD. To test this hypothesis, high functioning 8–14 years old ASD children matched in age, verbal and reasoning competence to the typical control group passed a coordination task together with social competence and SAQ tests. We anticipated that (1) coordination in ASD children is less accurate, less stable, and less complex as compared to TD controls and that (2) there is a relationship between coordination variables and social competence measures.
Perceptual-motor Coordination in ASD Children
Our data showed that the oscillation kinematics and the spontaneous coordination in ASD were similar to the TD controls. In the latter condition, both groups showed a smaller variability and absolute error of RP than the highest possible values (360° and 180°, respectively). In this simple, perceptual-motor coordination task, ASD children thus displayed no notable spontaneous coordination deficit, in contrast to reports in more demanding inter-personal coordination tasks (Fitzpatrick et al. 2016; Marsh et al. 2013). When coordination patterns were intentionally required in our study, ASD children complied less well with the demand. A twofold interpretation may be suggested. First, ASD children may be handicapped by a deficit in the process of error perception and its subsequent reduction and correction. Second, they may be challenged by the demand raised by experimenter expectation: the social-adaptive quotient warn us that ASD children adjust less well to social expectations than typical controls. Regardless, older children produced patterns of higher stability, accuracy and complexity than younger ones, signalling that coordination develops between 8 and 14 years. Coordination indeed is a life-span developing feature (Leinen et al. 2016), warning that motor abnormalities may not be similar at all ages.
Intentional control raises a special challenge to children about eight years old. Sport educators indeed noted that between the seventh or eighth year, typical children enter a “transition stage”, where they start using acquired motor skills at the service of specific tasks (Gallahue et al. 2012). In principle, the role of intention is to stabilize a task-required pattern, and simultaneously destabilize the competing task-irrelevant ones (Schöner and Kelso 1988). This requires integrating the information about the current required and all competing coordination states to act on the produced pattern. The process is thought to involve the basal ganglia (De Luca et al. 2010), which in ASD are supposed to fail in selecting and routing relevant sensory signals to the frontal area, overloading the latter with irrelevant information (Prat et al. 2016). Confused by the sensory overload, ASD children may fail in giving priority to experimenter requirements and expectations.
Whereas in TD controls the 0° pattern was more stable and accurate than the 180° one, the difference was lower, and statistically not significant in ASD children. The distinction between the 0° and 180° patterns in terms of their stability is one of the most robust experimental observations, though. It is evidenced in healthy adults (Kelso 2012), in aged persons (Temprado et al. 2010), in (TD) children (Volman and Geuze 2000; Leinen et al. 2016), and in adult Parkinsonians (Song et al. 2010). It increases when coordination is challenged as movement frequency rises (Kelso et al. 1986). Although this result is to be taken with caution, it is useful here to draw attention to the relationship between stability and pattern selection. When one pattern is spontaneously more stable than the other at a low movement frequency, the (healthy) individual readily interacts with the environment using the most stable state. When both patterns are of similar stability, the (ASD) individual should struggle to select one of them, which may laid ground for in initiative deficit.
The only variables differentiating ASD and TD children in all experimental situations were coordination complexity and the overall similarity between the oscillation produced by the child and the stimulus. Reduced complexity (lowered Apen) was previously observed in the resting state EEG of ASD children (Bosl et al. 2011) and envisioned as a possible mathematical marker for the diagnosis of the autism similar to biological markers. One interpretation of complexity is that it reflects a system’s functional connectivity: mathematical simulations show that ApEn increases (i.e., complexity increases) with greater system coupling and feedback and greater external influences (Pincus 1994). Lowered Apen values in ASD children may be indicative of weaker coupling between the coordination system composed of the child and of the oscillating environmental stimulus.
Coordination and SAQ
In line with the hypothesis of embodied social cognition, we found an age-dependent relationship between intentional (0° and 180°) coordination and SAQ. Coordination difficulties do affect the daily utilization of skills required for social and personal autonomy, such as dressing, washing, locomotion in space, asking information and help—all the skills requiring executive functions known as being deficient in ASD (Pennington and Ozonoff 1996). Perceptual-motor abnormalities seems to be involved in the development of SAQ. There was also a relationship between the severity of ASD symptoms, as assessed by SCQ and ADI-R, in conformity with the Motor Hypothesis of Autism (Donnellan et al. 2012). Such result, in line with those reported by (Dziuk et al. 2007; Fitzpatrick et al. 2013, 2017), suggest that motor disorder are involved in ASD. Note, there was no reliable direct relationship between intentional coordination and RSI. The orofacial communicative movements involved in RSI recruits probably more elaborated skills than those captured by our manual coordination motions.
The relationship between coordination difficulties and ADI-R score evidenced here posits the key question of why all children displaying coordination troubles, and often the consecutives social difficulties (Missiuna et al. 2007), do not develop autism. Motor anomalies are a common characteristic in numerous disorders—developmental coordination disorder, ADHD, and mental retardation (“American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV),” 2011)—and does not explain ASD symptoms on its own. This opens the key question behavioural comorbidities. A possibility, raised by (Kaplan et al. 1998), is that an early disruption in brain development creates a general vulnerability to trouble, but the specific full-blown syndrome depends on the extent of the underlying neurological abnormality in combination with environmental factors.
Limitation and Perspectives
The present study presents two serious limitations. First, we found intentional coordination and SAQ to be related. It remains to be seen, however, whether they are casually linked, and how. In healthy individuals, it has been repeatedly shown that coordination stability lawfully depends on the state of environmental parameters, such as movement frequency, component inertia or attentional load (Schmidt et al. 1990; Kostrubiec et al. 2013). This leaves the hypothetical and to-be-tested possibility that by manipulating these parameters in ASD children, their coordination abnormalities could be reduced, and their social deficits, perhaps, alleviated. A second limitation of our current study, and of the established coordination approach more in general, is that it assesses steady state motor behaviour, but has little to say about the switching between distinct (motor) behaviours: how patterns appear and disappear as behaviours are knit together is not addressed (but see Huys et al. 2014 for an inroad thereto). This issue, likened to the topics of behavioural initiative and resistance to change, calls for experimental investigation in ASD children, though.
Conclusion
These data provides further support to the Motor Hypothesis of Autism (Donnellan et al. 2012) seeking for the motor underpinnings of ASD. Whereas no significant differences between the ASD and TD groups appeared in spontaneous coordination, discrepancies arose when the 0° and 180° patterns were intentionally required: the coordination accuracy, variability, and complexity were lower for the ASD children as compared to the typical controls. Coordination variables were influenced by age, and affected SAQ. Our data indicates that coordination evolves with age and its age-dependent state affects SAQ skills in ASD children.
Appendix
Correlation Matrix
See Table 4.
References
Akar, S. A., Kara, S., Latifoğlu, F., & Bilgiç, V. (2016). Analysis of the complexity measures in the EEG of schizophrenia patients. International Journal of Neural Systems, 26(02), 1650008. doi:10.1142/S0129065716500088.
Amazeen, P. G., Schmidt, R. C., & Turvey, M. T. (1995). Frequency detuning of the phase entrainment dynamics of visually coupled rhythmic movements. Biological Cybernetics, 72(6), 511–518. doi:10.1007/BF00199893.
American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). (2011). In SpringerReference. Berlin/Heidelberg: Springer. Retrieved from http://www.springerreference.com/index/doi/10.1007/SpringerReference_179660.
Banerjee, A., Tognoli, E., Kelso, J. A. S., & Jirsa, V. K. (2012). Spatiotemporal re-organization of large-scale neural assemblies underlies bimanual coordination. NeuroImage, 62(3), 1582–1592. doi:10.1016/j.neuroimage.2012.05.046.
Batschelet, E. (1981). Circular statistics in biology. London, New York: Academic Press.
Belmonte, M. K., Allen, G., Beckel-Mitchener, A., Boulanger, L. M., Carper, R. A., & Webb, S. J. (2004). Autism and abnormal development of brain connectivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(42), 9228–9231. doi:10.1523/JNEUROSCI.3340-04.2004.
Bogdašina, O. (2005). Sensory perceptual issues in autism and Asperger syndrome: Different sensory experiences—Different perceptual worlds ((4). impression). London: Kingsley.
Bosl, W., Tierney, A., Tager-Flusberg, H., & Nelson, C. (2011). EEG complexity as a biomarker for autism spectrum disorder risk. BMC Medicine, 9(1). doi:10.1186/1741-7015-9-18.
CDC (2016). Autism spectrum disorder: Data & statistic. Center for disease and control prevention. Retrieved from https://www.cdc.gov/ncbddd/autism/data.html.
Chamak, B., Bonniau, B., Oudaya, L., & Ehrenberg, A. (2011). The autism diagnostic experiences of French parents. Autism: The International Journal of Research and Practice, 15(1), 83–97. doi:10.1177/1362361309354756.
Cirelli, L. K., Wan, S. J., & Trainor, L. J. (2014). Fourteen-month-old infants use interpersonal synchrony as a cue to direct helpfulness. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1658), 20130400–20130400. 10.1098/rstb.2013.0400.
Cook, J. L., Blakemore, S. -J., & Press, C. (2013). Atypical basic movement kinematics in autism spectrum conditions. Brain: A Journal of Neurology, 136(9), 2816–2824. doi:10.1093/brain/awt208.
Dawson, G., Hill, D., Spencer, A., Galpert, L., & Watson, L. (1991). Affective exchanges between young autistic children and their mothers. Journal of Abnormal Child Psychology, 19(1), 115–115. doi:10.1007/BF00910569.
De Coster, L., Verschuere, B., Goubert, L., Tsakiris, M., & Brass, M. (2013). I suffer more from your pain when you act like me: Being imitated enhances affective responses to seeing someone else in pain. Cognitive, Affective, & Behavioral Neuroscience, 13(3), 519–532. doi:10.3758/s13415-013-0168-4.
De Luca, C., Jantzen, K. J., Comani, S., Bertollo, M., & Kelso, J. A. S. (2010). Striatal activity during intentional switching depends on pattern stability. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(9), 3167–3174. doi:10.1523/JNEUROSCI.2673-09.2010.
de Marchena, A., & Eigsti, I. -M. (2010). Conversational gestures in autism spectrum disorders: Asynchrony but not decreased frequency. Autism Research, 3(6), 311–322. doi:10.1002/aur.159.
Dessing, J. C., Daffertshofer, A., Peper, C. E., & Beek, P. J. (2007). Pattern stability and error correction during in-phase and antiphase four-ball juggling. Journal of Motor Behavior, 39(5), 433–446. doi:10.3200/JMBR.39.5.433-448.
Donnellan, A. M., Hill, D. A., & Leary, M. R. (2012). Rethinking autism: Implications of sensory and movement differences for understanding and support. Frontiers in Integrative Neuroscience, 6, 124. doi:10.3389/fnint.2012.00124.
Dziuk, M. A., Gidley Larson, J. C., Apostu, A., Mahone, E. M., Denckla, M. B., & Mostofsky, S. H. (2007). Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology, 49(10), 734–739. doi:10.1111/j.1469-8749.2007.00734.x.
Elsabbagh, M., Divan, G., Koh, Y.-J., Kim, Y. S., Kauchali, S., Marcín, C., et al. (2012). Global prevalence of autism and other pervasive developmental disorders: Global epidemiology of autism. Autism Research, 5(3), 160–179. doi:10.1002/aur.239.
Esposito, G., Venuti, P., Apicella, F., & Muratori, F. (2011). Analysis of unsupported gait in toddlers with autism. Brain and Development, 33(5), 367–373. doi:10.1016/j.braindev.2010.07.006.
Fischer, R., Callander, R., Reddish, P., & Bulbulia, J. (2013). How do rituals affect cooperation?: An experimental field study comparing nine ritual types. Human Nature, 24(2), 115–125. doi:10.1007/s12110-013-9167-y.L
Fitzpatrick, P., Diorio, R., Richardson, M. J., & Schmidt, R. C. (2013). Dynamical methods for evaluating the time-dependent unfolding of social coordination in children with autism. Frontiers in Integrative Neuroscience. doi:10.3389/fnint.2013.00021.
Fitzpatrick, P., Frazier, J. A., Cochran, D. M., Mitchell, T., Coleman, C., & Schmidt, R. C. (2016). Impairments of social motor synchrony evident in autism spectrum disorder. Frontiers in Psychology. doi:10.3389/fpsyg.2016.01323.
Fitzpatrick, P., Romero, V., Amaral, J. L., Duncan, A., Barnard, H., Richardson, M. J., & Schmidt, R. C. (2017). Social motor synchronization: Insights for understanding social behavior in autism. Journal of Autism and Developmental Disorders. doi:10.1007/s10803-017-3124-2.
Fournier, K. A., Hass, C. J., Naik, S. K., Lodha, N., & Cauraugh, J. H. (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. doi:10.1007/s10803-010-0981-3.
Gallagher, S. (2008). Understanding others: Embodied social cognition. In P. Calvo & T. Gomila (Eds.), Elsevier handbook of cognitive science: An embodied approach (pp. 439–452). London: Elsevier.
Gallahue, D. L., Ozmun, J. C., & Goodway, J. (2012). Understanding motor development: Infants, children, adolescents, adults (7th edn.). New York: McGraw-Hill.
Guinchat, V., Chamak, B., Bonniau, B., Bodeau, N., Perisse, D., Cohen, D., & Danion, A. (2012). Very early signs of autism reported by parents include many concerns not specific to autism criteria. Research in Autism Spectrum Disorders, 6(2), 589–601. doi:10.1016/j.rasd.2011.10.005.
Haken, H. (1983). Synergetik. Berlin, Heidelberg: Springer. doi:10.1007/978-3-642-96775-7.
Hove, M. J., & Risen, J. L. (2009). It’s all in the timing: Interpersonal synchrony increases affiliation. Social Cognition, 27(6), 949–960. doi:10.1521/soco.2009.27.6.949.
Huys, R., Daffertshofer, A., & Beek, P. J. (2004). Multiple time scales and multiform dynamics in learning to juggle. Motor control, 8(2), 188–212. 10.1123/mcj.8.2.188.
Huys, R., Perdikis, D., & Jirsa, V. K. (2014). Functional architectures and structured flows on manifolds: A dynamical framework for motor behavior. Psychological Review, 121, 302–336.
Isenhower, R. W., Marsh, K. L., Richardson, M. J., Helt, M., Schmidt, R. C., & Fein, D. (2012). Rhythmic bimanual coordination is impaired in young children with autism spectrum disorder. Research in Autism Spectrum Disorders, 6(1), 25–31. doi:10.1016/j.rasd.2011.08.005.
Jantzen, K. J., & Kelso, J. A. S. (2007). Neural coordination dynamics of human sensorimotor behavior: A review. In V. Jirsa & A. R. McIntosh (Eds.), Handbook of brain connectivity understanding complex systems (pp. 421–461). Berlin: Springer.
Kanner, L. (1968). Autistic disturbances of affective contact. Acta Paedopsychiatrica, 35(4), 100–136.
Kaplan, B. J., Wilson, B. N., Dewey, D., & Crawford, S. G. (1998). DCD may not be a discrete disorder. Human Movement Science, 17(4–5), 471–490. doi:10.1016/S0167-9457(98)00010-4.
Kay, B. A., Kelso, J. A., Saltzman, E. L., & Schöner, G. (1987). Space-time behavior of single and bimanual rhythmical movements: Data and limit cycle model. Journal of Experimental Psychology. Human Perception and Performance, 13(2), 178–192.
Kelso, J. A. (1984). Phase transitions and critical behavior in human bimanual coordination. The American Journal of Physiology, 246(6 Pt 2), R1000–R1004.
Kelso, J. A. S. (1995). Dynamic patterns: The self-organization of brain and behavior. Cambridge, Mass.: MIT Press.
Kelso, J. A. S. (2012). Multistability and metastability: Understanding dynamic coordination in the brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1591), 906–918. 10.1098/rstb.2011.0351.
Kelso, J. A. S., de Guzman, G. C., Reveley, C., & Tognoli, E. (2009). Virtual Partner Interaction (VPI): Exploring Novel Behaviors via Coordination Dynamics. PLoS ONE, 4(6), e5749. doi:10.1371/journal.pone.0005749.
Kelso, J. A. S., Dumas, G., & Tognoli, E. (2013). Outline of a general theory of behavior and brain coordination. Neural Networks, 37, 120–131.doi:10.1016/j.neunet.2012.09.003.
Kelso, J. A. S., Scholz, J. P., & Schöner, G. (1986). Nonequilibrium phase transitions in coordinated biological motion: Critical fluctuations. Physics Letters A, 118(6), 279–284.doi:10.1016/0375-9601(86)90359-2.
Kirschner, S., & Tomasello, M. (2010). Joint music making promotes prosocial behavior in 4-year-old children☆☆☆. Evolution and Human Behavior, 31(5), 354–364. doi:10.1016/j.evolhumbehav.2010.04.004.
Kostrubiec, V., Danna, J., & Zanone, P.-G. (2013). Co-variation between graphic pattern stability and attentional cost: A clue for the difficulty to produce handwritten traces. Human Movement Science, 32(5), 1010–1025. doi:10.1016/j.humov.2012.12.005.
Kostrubiec, V., Zanone, P. G., Fuchs, A., & Kelso, J. A. S. (2012). Beyond the blank slate: Routes to learning new coordination patterns depend on the intrinsic dynamics of the learner—experimental evidence and theoretical model. Frontiers in Human Neuroscience, 6, 222. doi:10.3389/fnhum.2012.00222.
Kruck, J., Baudel, S., & Rogé, B. (2013). Validation française du SCQ, In J. Kruck, S. Baduel & Roge (Eds.), Questionnaire de Communication Sociale pour le Dépistage des Troubles du Spectre Autistique. Boston: Hogrefe.
Lakens, D. (2010). Movement synchrony and perceived entitativity. Journal of Experimental Social Psychology, 46(5), 701–708. doi:10.1016/j.jesp.2010.03.015.
Launay, J., Tarr, B., & Dunbar, R. I. M. (2016). Synchrony as an Adaptive Mechanism for Large-Scale Human Social Bonding. Ethology, 122(10), 779–789. doi:10.1111/eth.12528.
Le Callenec, S., & Chapel, F. (2016). Autisme la grande enquête. Paris: Arènes.
Leary, M. R., & Hill, D. A. (1996). Moving on: Autism and movement disturbance. Mental Retardation, 34(1), 39–53.
Leinen, P., Vieluf, S., Kennedy, D., Aschersleben, G., Shea, C. H., & Panzer, S. (2016). Life span changes: Performing a continuous 1:2 bimanual coordination task. Human Movement Science, 46, 209–220. 10.1016/j.humov.2016.01.004.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685.
Mari, M., Castiello, U., Marks, D., Marraffa, C., & Prior, M. (2003). The reach-to-grasp movement in children with autism spectrum disorder. Philosophical Transactions of the Royal Society B: Biological Sciences, 358(1430), 393–403. 10.1098/rstb.2002.1205.
Marsh, K. L., Isenhower, R. W., Richardson, M. J., Helt, M., Verbalis, A. D., Schmidt, R. C., & Fein, D. (2013). Autism and social disconnection in interpersonal rocking. Frontiers in Integrative Neuroscience, doi:10.3389/fnint.2013.00004.
Martini, R., St-Pierre, M. -F., & Wilson, B. N. (2011). French Canadian Cross-Cultural Adaptation of the Developmental Coordination Disorder Questionnaire’07: DCDQ-FC. Canadian Journal of Occupational Therapy, 78(5), 318–327. doi:10.2182/cjot.2011.78.5.7.
Maximo, J. O., Cadena, E. J., & Kana, R. K. (2014). The implications of brain connectivity in the neuropsychology of autism. Neuropsychology Review, 24(1), 16–31. doi:10.1007/s11065-014-9250-0.
McNeill, V. H. (1995). Keeping together in time: Dance and drill in human history. Cambridge, MA: Harvard University Press.
Missiuna, C., Moll, S., King, S., King, G., & Law, M. (2007). A Trajectory of troubles: Parents’ impressions of the impact of developmental coordination disorder. Physical & Occupational Therapy in Pediatrics, 27(1), 81–101. doi:10.1080/J006v27n01_06.
Moran, M. F., Foley, J. T., Parker, M. E., & Weiss, M. J. (2013). Two-legged hopping in autism spectrum disorders. Frontiers in Integrative Neuroscience. doi:10.3389/fnint.2013.00014.
Pennington, B. F., & Ozonoff, S. (1996). Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, 37(1), 51–87. doi:10.1111/j.1469-7610.1996.tb01380.x.
Pikovsky, A., Rosenblum, M., & Kurths, J. (2001). Synchronization: A Universal Concept in Nonlinear Sciences. Cambridge, MA: Cambridge University Press. Retrieved from http://ebooks.cambridge.org/ref/id/CBO9780511755743.
Pincus, S. M. (1991). Approximate entropy as a measure of system complexity. Proceedings of the National Academy of Sciences, 88, 2297–2301.
Pincus, S. M. (1994). Greater signal regularity may indicate increased system isolation. Mathematical Biosciences, 122(2), 161–181. doi:10.1016/0025-5564(94)90056-6.
Pincus, S. M., & Goldberger, A. L. (1994). Physiological time-series analysis: What does regularity quantify? The American Journal of Physiology, 266(4 Pt 2), H1643–H1656.
Powell, D. W., Muthumani, A., & Xia, R. (2014). Parkinson’s disease is associated with greater regularity of repetitive voluntary movements. Motor Control, 18(3), 263–277. doi:10.1123/mc.2013-0025.
Prat, C. S., Stocco, A., Neuhaus, E., & Kleinhans, N. M. (2016). Basal ganglia impairments in autism spectrum disorder are related to abnormal signal gating to prefrontal cortex. Neuropsychologia, 91, 268–281. doi:10.1016/j.neuropsychologia.2016.08.007.
Provost, B., Lopez, B. R., & Heimerl, S. (2007). A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders, 37(2), 321–328. doi:10.1007/s10803-006-0170-6.
Restrepo, J. F., Schlotthauer, G., & Torres, M. E. (2014). Maximum approximate entropy and threshold: A new approach for regularity changes detection. Physica A: Statistical Mechanics and Its Applications, 409, 97–109. doi:10.1016/j.physa.2014.04.041.
Richardson, M. J., Marsh, K. L., Isenhower, R. W., Goodman, J. R. L., & Schmidt, R. C. (2007). Rocking together: Dynamics of intentional and unintentional interpersonal coordination. Human Movement Science, 26(6), 867–891. doi:10.1016/j.humov.2007.07.002.
Rosseel, Y. (2012). lavaan : An R Package for Structural Equation Modeling. Journal of Statistical Software. doi:10.18637/jss.v048.i02.
Rutter, M., Bailey, A., Lord, C., Cianchetti, C., & Fancello, G. S. (2007). SCQ : Social Communication Questionnaire : Manuale. Florence: Giunti OS.
Schmidt, R. C., Bienvenu, M., Fitzpatrick, P. A., & Amazeen, P. G. (1998). A comparison of intra- and interpersonal interlimb coordination: Coordination breakdowns and coupling strength. Journal of Experimental Psychology: Human Perception and Performance, 24(3), 884–900. doi:10.1037/0096-1523.24.3.884.
Schmidt, R. C., Carello, C., & Turvey, M. T. (1990). Phase transitions and critical fluctuations in the visual coordination of rhythmic movements between people. Journal of Experimental Psychology: Human Perception and Performance, 16(2), 227–247. doi:10.1037/0096-1523.16.2.227.
Schmidt, R. C., & Fitzpatrick, P. A. (2016). The origin of the ideas of interpersonal synchrony and synergies. In P. Passos, K. Davids & C. J. Yi (Eds.), Interpersonal coordination and performance in social systems. London: Routledge.
Schmitz, C., Martineau, J., Barthélémy, C., & Assaiante, C. (2003). Motor control and children with autism: Deficit of anticipatory function? Neuroscience Letters, 348(1), 17–20.
Schöner, G., Haken, H., & Kelso, J. A. (1986). A stochastic theory of phase transitions in human hand movement. Biological Cybernetics, 53(4), 247–257.
Schöner, G., & Kelso, J. A. S. (1988). A dynamic pattern theory of behavioral change. Journal of Theoretical Biology, 135(4), 501–524. doi:10.1016/S0022-5193(88)80273-X.
Slifkin, A. B., & Newell, K. M. (1999). Noise, information transmission, and force variability. Journal of Experimental Psychology: Human Perception and Performance, 25(3), 837–851. doi:10.1037/0096-1523.25.3.837.
Song, Y. -G., Yoo, K. -S., Park, K. -W., & Park, J. -H. (2010). Coordinative and limb-specific control of bimanual movements in patients with Parkinson’s disease and cerebellar degeneration. Neuroscience Letters, 482(2), 146–150. doi:10.1016/j.neulet.2010.07.021.
Sparrow, S. S., Cicchetti, D., & Balla, D. A. (2012). Vineland Adaptive Behavior Scales, Second Edition. Retrieved from http://doi.apa.org/getdoi.cfm?doi=10.1037/t15164-000.
Sutera, S., Pandey, J., Esser, E. L., Rosenthal, M. A., Wilson, L. B., Barton, M., et al. (2007). Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(1), 98–107. doi:10.1007/s10803-006-0340-6.
Teitelbaum, P., Teitelbaum, O., Nye, J., Fryman, J., & Maurer, R. G. (1998). Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences, 95(23), 13982–13987. doi:10.1073/pnas.95.23.13982.
Temprado, J. J., Monno, A., Laurent, M., & Zanone, P. G. (2001). A dynamical framework to understand performance trade-offs and interference in dual tasks. Journal of Experimental Psychology. Human Perception and Performance, 27(6), 1303–1313.
Temprado, J. -J., Vercruysse, S., Salesse, R., & Berton, E. (2010). A dynamic systems approach to the effects of aging on bimanual coordination. Gerontology, 56(3), 335–344. doi:10.1159/000262445.
Thelen, E., & Smith, L. B. (1996). A dynamic systems approach to the development of cognition and action. Cambridge, MA: MIT Press. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=1712.
Thelen, E., Schöner, G., Scheier, C., & Smith, L. B. (2001). The dynamics of embodiment: A field theory of infant perseverative reaching. Behavioral and Brain Science, 24(1), 34–86.
Turvey, M. T. (1990). Coordination. The American Psychologist, 45(8), 938–953.
Uddin, L. Q., Supekar, K., & Menon, V. (2013). Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in Human Neuroscience. doi:10.3389/fnhum.2013.00458.
Valdesolo, P., & DeSteno, D. (2011). Synchrony and the social tuning of compassion. Emotion (Washington, D. C.), 11(2), 262–266. doi:10.1037/a0021302.
van Ulzen, N. R., Lamoth, C. J. C., Daffertshofer, A., Semin, G. R., & Beek, P. J. (2008). Characteristics of instructed and uninstructed interpersonal coordination while walking side-by-side. Neuroscience Letters, 432(2), 88–93. doi:10.1016/j.neulet.2007.11.070.
Volman, M. C. J., & Geuze, R. H. (2000). Temporal stability of rhythmic tapping “on” and “off the beat”: A developmental study. Psychological Research Psychologische Forschung, 63(1), 62–69.
Whyatt, C. P., & Craig, C. M. (2012). Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42(9), 1799–1809. doi:10.1007/s10803-011-1421-8.
Wiltermuth, S. S., & Heath, C. (2009). Synchrony and cooperation. Psychological Science, 20(1), 1–5. doi:10.1111/j.1467-9280.2008.02253.x.
Zwaigenbaum, L., Bryson, S., Rogers, T., Roberts, W., Brian, J., & Szatmari, P. (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23(2–3), 143–152. doi:10.1016/j.ijdevneu.2004.05.001.
Acknowledgments
The authors are grateful to all children, their parents, to the participating centers, and to Aurélie Peltier and Naïma Hilal for their help with data collection.
Author information
Authors and Affiliations
Contributions
VK, RH and JK participated in the design of the study. BJ collected the data. VK analysed the results and drafted the manuscript. All authors critically reviewed and edited the manuscript for important intellectual content, and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee CERNI and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kostrubiec, V., Huys, R., Jas, B. et al. Age-dependent Relationship Between Socio-adaptability and Motor Coordination in High Functioning Children with Autism Spectrum Disorder. J Autism Dev Disord 48, 209–224 (2018). https://doi.org/10.1007/s10803-017-3326-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-017-3326-7