Abstract
Children with autism spectrum disorder (ASD) are at considerable risk for difficulties with emotion regulation and related functioning. Although it is commonly accepted that parents contribute to adaptive child regulation, as indexed by observable child behavior, theory and recent evidence suggest that parenting may also influence relevant underlying child physiological tendencies. The current study examined concurrent associations between two elements of parental socialization of emotion and measures of both sympathetic and parasympathetic nervous system activity in 61 children with ASD aged 6 to 10 years. To index parental socialization, parents reported on their reactions to their children’s negative emotions, and parental scaffolding was coded from a dyadic problem-solving task. Children’s baseline respiratory sinus arrhythmia (RSA), electrodermal reactivity (EDA-R), and RSA reactivity in response to challenge were obtained as measures of the children’s physiological activity. Regression analyses indicated that supportive parent reactions were related to higher child baseline RSA, a biomarker of regulatory capacity. Fewer unsupportive parent reactions and higher quality scaffolding were associated with higher EDA-R, a physiological index of inhibition. The identification of these concurrent associations represents a first step in understanding the complex and likely bidirectional interplay between parent socialization and child physiological reactivity and regulation in this high-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Emotion regulation involves the ability to understand, monitor, and modulate emotion in the service of goal-directed activity (Thompson 1994). Adaptive emotion regulation has been linked to a host of positive outcomes across child populations (Baker et al. 2007; Eisenberg et al. 2005), whereas emotion dysregulation is a core feature of many forms of psychopathology (Cole et al. 2008; Mazefsky et al. 2013; Sheppes et al. 2015). Relative to other groups, children with autism spectrum disorder (ASD) exhibit heightened emotion regulation difficulties, including challenges with the selection and implementation of effective regulatory strategies (Jahromi et al. 2012; Mazefsky et al. 2014; Samson et al. 2015). In turn, problems with emotion regulation predict both internalizing and externalizing behavior problems as well as psychiatric comorbidities in children with ASD (Mazefsky et al. 2014; Samson et al. 2015). Growing evidence linking emotion dysregulation with mental health difficulties in children with ASD (Mazefsky and White 2014; White et al. 2014) underscores the importance of understanding factors that influence regulatory processes in this population.
Psychophysiological Processes in Emotion Regulation
Maturation of the autonomic nervous system (ANS) is believed to contribute directly to the development of emotion regulation (Beauchaine et al. 2007), and efforts to understand the psychophysiological underpinnings of emotion dysregulation have increasingly relied on ANS measures. The ANS subsumes the parasympathetic and sympathetic systems, which modulate reactivity and arousal. The sympathetic nervous system (SNS) is generally conceptualized as mobilizing resources to respond to threats or challenges (Beauchaine 2001; Sheppes et al. 2009), whereas the parasympathetic nervous system (PNS) is involved in restoration and reducing arousal (Beauchaine 2015a; Benevides and Lane 2015).
SNS activation is often measured through electrodermal activity (EDA), which refers to the strength of an electrical current applied to the skin that increases when sweating. EDA is thought to index the behavioral inhibition system, a neurophysiological motivational system that promotes caution in situations that involve potential negative consequences (Beauchaine 2001). EDA reactivity (i.e., increase in EDA) is a continuous measure: low EDA reactivity reflects low caution (sometimes termed fearlessness), moderate EDA reactivity reflects moderate caution, and high EDA reactivity reflects high caution (sometimes termed anxious arousal). Some studies have linked high EDA reactivity to social impairment and greater core symptom severity in children with ASD (Fenning et al. 2017; Kaartinen et al. 2012; Neuhaus et al. 2015), consistent with the conceptualization of high EDA reactivity as anxious arousal. However, a connection between low EDA reactivity and externalizing behavior problems is a more reliable finding across samples of children with ASD (Baker et al. 2018; Fenning et al. 2019) and without ASD (Beauchaine et al. 2007; Cappadocia et al. 2009; Raine 1993); these studies are more consistent with the conceptualization of low EDA reactivity as fearlessness. Of particular relevance to the present study, it has been suggested that children with lower EDA reactivity may be less sensitive to the negative consequences of their actions and therefore less open to socialization efforts (Baker et al. 2018; Beauchaine et al. 2007; Cappadocia et al. 2009; Raine 1993).
The PNS functions like a brake (via the vagus nerve) that decelerates heart rate during exhalation, reducing arousal (Porges 1997). The deceleration in heart rate produced by higher PNS output to the heart is reflected in respiratory sinus arrhythmia (RSA), heart rate variability at the frequency of respiration. Baseline RSA is considered a robust biomarker of emotion regulation (Beauchaine 2015b), with higher baseline levels of RSA generally indicating greater emotion regulation capacity (Beauchaine 2001; Beauchaine et al. 2007). However, the nature and implications of RSA reactivity are less clear and may depend upon study population, task demands, and contextual support (Baker et al. 2020; Beauchaine 2015a; Graziano and Derefinko 2013; Obradović et al. 2011). RSA reactivity is a measure of withdrawal of PNS influence on the heart in response to stress (i.e., withdrawal of the “vagal brake”); PNS withdrawal decreases RSA (and increases heart rate). In children presenting with clinical concerns (Beauchaine 2015a; 2015b; Beauchaine et al. 2007; Cole et al. 1996), including children with ASD (Fenning et al. 2019), greater RSA reactivity (i.e., greater PNS withdrawal, or greater decrease in RSA) may indicate loss of regulatory control, whereas low RSA reactivity may suggest problematic disengagement (Graziano and Derefinko 2013; Porges 1997).
Parental Socialization of Child Regulation
The development of emotion regulation involves the interplay between processes internal to the child and external environmental influences (Fox and Calkins 2003). It has long been recognized that parenting shapes the emergence of child emotion regulation (Baker et al. 2011; Cole et al. 1994; Eisenberg, Cumberland, et al. 1998a, b; Gottman et al. 1996; Morris et al. 2007; Thompson and Meyer 2007). Parents’ own emotional expressions, discussions of emotion, and reactions to child emotion are vehicles for socialization of child emotion in that they “affect the child’s experience, expression, understanding, or modulation of emotion” (Eisenberg, Spinrad et al. 1998a, b, p.317; see also Eisenberg, Cumberland, et al. 1998a, b and Morris et al. 2007). Evidence from studies of children with neurotypical development suggest that parent-reported unsupportive reactions to child negative emotion predict poorer child regulatory outcomes both concurrently (Morelen et al. 2016; Sanders et al. 2015; Shaffer et al. 2012) and over time (Eisenberg et al. 1999). Comparative studies examining parental reactions to child negative emotion in families of children with and without ASD suggest either a general lack of group differences (Mazzone and Nader-Grosbois 2017) or indicate that parents of children with ASD endorse more supportive, and fewer unsupportive, reactions to child negative emotions (Bougher-Muckian et al. 2016).
Observed parental co-regulatory behavior has also been linked to child regulatory functioning, with less effective parental scaffolding associated with greater child dysregulation (Hoffman et al. 2006). Baker et al. (2007) found that higher quality scaffolding related to less observed emotion dysregulation for both young children with and without early developmental delays, with a stronger effect for the former group. For children with ASD, observed parent co-regulatory behaviors have been concurrently linked to the quality of child emotion regulation during dyadic parent–child tasks, but less so to child emotion regulation in independent contexts (Fenning et al. 2018; Gulsrud et al. 2010; Ting and Weiss 2017).
Parenting characterized by responsivity, sensitive structuring, and support for child autonomy is believed to facilitate children’s internalization of external, co-regulatory strategies (Grolnick and Farkas 2002). The influence of the social environment on child emotion regulation extends well beyond early childhood (Cole 2014; Morris et al. 2007) and could be especially influential for children with ASD who may experience a developmental lag in the internalization of regulatory processes (Baker, Fenning, and Moffitt 2019a, b).
Parental Socialization of Emotion and Child Psychophysiology
In addition to influencing observable regulatory behavior, parenting may interact with children’s underlying psychophysiological arousal tendencies to predict broader behavioral functioning in children with neurotypical development (Beauchaine et al. 2007; Dyer et al. 2016; Obradović et al. 2011) and those with ASD (e.g., Baker et al. 2018; 2020). However, the extent to which parenting influences child psychophysiology remains unclear. A few studies have examined associations between parenting and children’s baseline RSA in children without ASD. Kennedy et al. (2004) did not find supportive parenting to predict baseline RSA in toddlers; however, using a longitudinal design, Fox et al. (2018) reported that supportive parenting buffered the effects of stress on adolescents’ baseline RSA. Hinnant et al. (2015) found that harsh parenting predicted declines in baseline RSA from childhood to adolescence, though only among children who exhibited RSA reductions in response to challenge. Examining RSA levels during parent–child interaction rather than at baseline, Hane and Barrios (2011) reported that maternal benign framing during parent–child discussions about ambiguous threat scenarios was correlated with higher child RSA during the discussion.
Studies that examined RSA reactivity (i.e., PNS withdrawal, or decrease in RSA) from baseline to stressful or challenging conditions have revealed mixed findings in normative populations. Hastings et al. (2008) found that higher parental negative control related to greater RSA reactivity to a social task, and that greater RSA reactivity mediated the association between parental negative control and children’s behavioral maladjustment. In contrast, greater RSA reactivity has been positively associated with maternal sensitivity and was found to mediate associations between early sensitivity and later infant maternal-orientation behavior (Perry et al. 2014). Other studies have found no direct associations between parenting and children’s RSA reactivity (e.g., Perry et al. 2012; Scrimgeour et al. 2016). Discrepancies are likely due to unexplored differences in context and samples (Beauchaine 2015a, b; Obradović et al. 2011).
Studies linking EDA reactivity to parenting in families of individuals with neurotypical development have also demonstrated somewhat mixed findings. Wagner and Abaied (2016) reported a positive correlation between parental psychological control and EDA reactivity in emerging adults as measured by an interpersonal stress task. However, Erath et al. (2011) found that 8-year-old children’s report of harsh parenting was inversely related to their EDA reactivity during a star-tracing challenge task. Other studies have found no associations between children’s EDA reactivity and permissive parenting (Hinnant et al. 2016), or parent coping suggestions (Stanger et al. 2018).
Within clinical populations, recent studies have found participation in parenting interventions, supportive parenting, and reductions in unsupportive parenting to be related to increases in both baseline RSA (Bell et al. 2018) and EDA reactivity among children with ADHD (Beauchaine et al. 2015; Breaux et al. 2018). Tabachnick et al. (2019) reported that involvement in attachment-based intervention for maltreated infants predicted higher child baseline RSA, but not EDA reactivity.
The Current Study
To our knowledge, no investigation has examined associations between parenting and child SNS and PNS activity in families of children with ASD. We examined concurrent associations between parent socialization of emotion behaviors, including reported reactions to child negative emotion and observed parental scaffolding, and PNS and SNS responding in children with ASD between the ages of 6 and 10 years. Although the processes under consideration are often studied with younger individuals, children with ASD demonstrate substantial difficulty with regulatory competence well into middle childhood, with evidence suggesting a significant developmental lag in the internalization of emotion regulation skills in this population (see Baker, Fenning and Moffitt 2019a, b). The current study represents an important step toward elucidating biobehavioral processes critical to the development of emotion regulation in a population at high risk for challenges in this domain.
Consistent with existing theory and emerging literature, we expected that higher supportive and lower unsupportive parent behavior during a challenging activity and in response to children’s negative emotions would be related to children’s regulatory capacity, as indexed by higher baseline RSA (Hypothesis 1). We similarly expected more supportive and less unsupportive parent behavior to be related to higher child EDA reactivity. Although the literature is somewhat mixed when considered in different contexts and across development, we conceptualized EDA reactivity as adaptive activation of the behavioral inhibition system in a challenging situation (Baker et al. 2018; Beauchaine et al. 2007, 2015; Cappadocia et al. 2009; Fenning et al. 2019; Hypothesis 2). This prediction is supported by the existing empirical evidence from studies that involved children (as opposed to emerging adults), populations with neurodevelopmental conditions, and those utilizing non-social tasks (Beauchaine et al. 2015; Breaux et al. 2018; Erath et al. 2011). As noted, conceptualizations regarding RSA reactivity are even more complex, and interactive effects are likely present, making the investigation of this physiological index exploratory in nature. However, given theory and preliminary evidence that certain other forms of negative parenting (e.g., criticism, harsh discipline) may exhibit small, positive associations with RSA reactivity in children with ASD (Baker et al. 2020), we expected more supportive and less unsupportive parenting behavior to relate to lower child RSA reactivity (Hypothesis 3).
Method
Participants
Data were drawn from a larger study examining parent and child predictors of behavior problems in children with ASD (Baker et al. 2020; Fenning et al. 2019). An initial sample of 77 children with ASD, ages 6 to 10 years, and their primary caregivers participated in a laboratory visit that included child assessment, psychophysiological data collection, structured parent–child tasks, parent interview, and parent completion of questionnaires. Children with an existing ASD diagnosis provided by a physician or psychologist were recruited from the community and local service providers. Diagnoses were verified through laboratory administration of the Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al. 2012). Exclusionary criteria for the child included the presence of a genetic disorder of known etiology and motor impairment that would prevent independent ambulation. Of these 77 children, 11 refused the leads for physiological measurement, and both RSA and EDA data were considered invalid for 5 additional children due to problems with the physiological signal (e.g., noise due to touching electrodes or pulling on electrode leads). As noted in a previous report from this sample (Fenning et al. 2019), missing data analyses revealed no significant differences between the 16 children without usable physiological data and those included in the study except that missing data occurred more frequently for males (25% missing) as compared to females (0%), χ2 = 4.89, p = 0.03, and for children with higher ASD symptom scores, t = 2.43, p = 0.02, d = 0.63. These missing data were not significantly related to estimated IQ, t = –1.59, p = 0.18, d = 0.41.
The current study sample of 61 children (74% male) was diverse with regard to intellectual ability and ASD symptom levels (see Table 1), with estimated IQ ranging from 47 to 121. The majority of the families identified their children as Hispanic (47%), 33% were Caucasian non-Hispanic, 5% were Asian American, 5% were African American, 3% identified as “other,” and 8% identified as “multi-ethnic/racial.” The median annual family income was between US$50,000 and US$70,000. The majority of primary caregivers were married (71%), and 3% of the primary caregivers were fathers. Nineteen (31%) of the children were taking medication, most commonly for attention problems/hyperactivity (13%), asthma (8%), allergies (7%), or seizures (5%). No children were reported to be taking selective serotonin reuptake inhibitors (SSRIs), which may be related to RSA measurements (Beauchaine et al. 2019).
Of the current sample of 61 children with some valid psychophysiological data, 11 families (18%) did not return the questionnaires measuring parent reactions. These families did not differ on any demographic factor or variable of interest considered. EDA data for nine children (15%) were determined to be artifactual (e.g., noise or loss of signal due to pulling on electrode wires). Children missing EDA data differed significantly from those with valid EDA data only in that the former scored lower on the IQ estimate (M = 66.89, SD = 15.89, n = 9) as compared to the latter (M = 82.29, SD = 20.69, n = 52), t = 2.12, p = 0.038, d = 0.83 (see also Fenning et al. 2019).
Procedures
All procedures were approved by the institutional review board of California State University, Fullerton. Parents provided written consent for themselves and for their children, and verbal assent was obtained from children prior to participation in any study procedures.
RSA and EDA data collection. Children were seated at a table that faced a small television on a stand in front of them. A wall was to the children’s left and a temporary partition was placed to the children’s right, behind which the parent was eventually seated. The electrodes were placed on the child by a female research assistant with the help of the parent.
Electrodes were placed on the lower ribs and on the right clavicle for RSA, and on the lower palm of the non-dominant hand for EDA. A short adjustment period occurred during which the data acquisition systems were checked for appropriate signal and then a three-minute baseline procedure was performed. This baseline involved viewing a series of slides on the television that included images of trees, water, mountains, and other nature scenes (Erath et al. 2016; Fenning et al. 2019). Parents were asked beforehand if they perceived their children to have any particular interests in, or fears of, these types of stimuli; none were reported. A video camera mounted high above the television recorded the child for later data assurance and allowed the parent to view the child from behind the partition. The child then engaged in a three-minute challenging task. As in previous studies utilizing this task to elicit physiological arousal (e.g., El-Sheikh 2005; Fenning et al. 2019), the child was provided with a pencil and instructions to trace an image of a star using a structure that permitted only an indirect, mirror-image view of the child’s hand and paper. The reversed directionality of the image makes this a difficult task to perform. Despite the large range of cognitive functioning present in our sample, every child was judged to have understood the request for basic tracing.
Scaffolding task. Following the psychophysiological data collection, the parents and children were asked to engage in a series of interactive tasks, including one from which the measure of parental scaffolding was obtained. In this task, which has been used several times by our laboratory and others with children with ASD and related disabilities (Baker et al. 2007; Fenning et al. 2018) the dyad was provided with colorful block tiles and a photo of a completed puzzle. The child was instructed to make the structure depicted in the photo. The parent was asked to let the child try to build the structure, providing any help that the parent deemed necessary. The experimenter returned after 5 min.
Parent interview and forms. Parents reported on their reactions to children’s emotion and demographic information through questionnaires.
Measures
ASD diagnostic confirmation and symptom level. Diagnostic confirmation was primarily based upon the existence of an ASD diagnosis by a community physician or psychologist and evidence that the child met the criterion for an autism spectrum classification on our laboratory administration of the ADOS-2 (Lord et al. 2012). The ADOS-2 is a semi-structured assessment that facilitates observation and recording of child behaviors related to language, social communication, play, repetitive behaviors, and restricted interests and was performed by assessors certified as research reliable in the system. Most children (66%) received Module 3, 26% were tested with Module 2, and 8% received Module 1. The ADOS-2 comparison score was used to characterize the sample according to overall ASD symptom severity and to provide a robust measure of ASD symptom levels for consideration as a covariate. The comparison score allows for examination of symptom levels across different modules, with 1 indicative of minimal to no evidence of ASD-related symptoms and 10 reflecting a high level of symptoms.
As described in previous publications with this sample (e.g., Fenning et al. 2019), five children did not meet the ADOS-2 criterion for an ASD classification, but were retained following completion of an in-depth, multi-method clinical best estimate by a licensed clinical psychologist with research reliability in the ADOS-2 and significant expertise in ASD assessment. All five children met clinical criteria on the Social Responsiveness Scale–2 (SRS-2; Constantino and Gruber 2012), a widely used parent report measure of ASD symptoms, and all but one also met criteria on the Social Communication Questionnaire, Lifetime Version (SCQ; Rutter et al. 2003a, b), a screening instrument based on the Autism Diagnostic Interview–Revised (ADI-R; Rutter et al. 2003a, b).
Child IQ. An estimate of child IQ was obtained using the Stanford-Binet 5 ABIQ (Roid 2003). The ABIQ is comprised of two subscales with high loading on the general intelligence factor: a Matrix Reasoning task that assesses non-verbal fluid reasoning and a Vocabulary task that evaluates expressive word knowledge. ABIQ and FSIQ were highly related in the standardization sample for individuals age 6 and above, r = 0.87, and for a subsample of individuals with intellectual disability, r = 0.87 (Roid 2003). Studies of children with ASD have revealed the ABIQ and FSIQ to be similarly strongly correlated (e.g., r = 0.89; Twomey et al. 2018).
Parental reactions to child emotion. The Coping with Children’s Negative Emotion Scale (CCNES; Fabes et al. 2002) features 12 vignettes depicting situations in which the child is experiencing different negative emotions. Parents are asked to rate how likely they are to react in a specific way. These responses related to six subscales that have often been combined as they were in the current study—into ‘supportive’ (expressive-encouragement, emotion-focused, and problem-focused subscales), and ‘unsupportive’ reactions (punitive, minimizing, and distress subscales; Baker et al. 2011; Fabes et al. 2002). The original psychometric study reported acceptable internal consistency (alphas 0.69 to 0.85), test–retest reliability (rs 0.56 to 0.83), and construct validity of the CCNES in relation to reported parenting behaviors and child emotional competence (Fabes et al. 2002). Bougher-Muckian et al. (2016) utilized a variant of the CCNES in families of children with ASD, with internal consistencies for more specific subscales ranging from alpha 0.53 to 0.97. Paczkowski and Baker (2007) reported good internal consistencies for the CCNES supportive (alpha: 0.90) and unsupportive scales (0.84) for their sample that included children with and without intellectual/developmental disabilities. Internal consistency was acceptable in the current study (alpha for supportive = 0.87 and unsupportive reactions = 0.86).
Parental scaffolding. The Parental Scaffolding Observation System (Hoffman et al. 2006) was applied to videotapes of the dyadic problem-solving task. This system considers parents’ ability to provide motivational, emotional, and technical support to their children while approaching and engaging in a challenging activity. Motivational scaffolding includes the ability of the parent to recruit the child’s attention to the task, foster enthusiasm for the task, and refocus the child should he or she become distracted. Emotional scaffolding scores reflect a parent’s ability to provide co-regulatory emotional support to the child and to contribute to the child’s feelings of accomplishment. Technical scaffolding evaluates the parent’s skill in providing structure and support for the child with regard to the task through instruction, guidance, prompting, and/or modification of the task or goal. Each of these subscales is rated from 1 (very low or absent support) to 5 (characteristically high support). These subscales are highly positively correlated (rs 0.66 to 0.76) and the measure is most commonly used as an average overall score (alpha 0.88; Baker et al. 2007). In the current study, interscale alpha was 0.83 and interrater reliability (ICC) based on 25% of cases was 0.73.
Psychophysiological indices. RSA and EDA were measured with a MindWare data acquisition system (MindWare Technologies, Inc.) following developer guidelines.
RSA. Electrocardiography data were collected through disposable Ag–AgCl electrodes placed on participants’ right clavicle and lower left and right ribs. Data were sampled at 500 Hz. RSA scores were quantified using spectral analysis (Berntson et al. 1997) with MindWare HRV analysis software (version 3.0.22) as the natural log of the variance in heart period within age-adjusted respiratory frequency bands (e.g. 0.27–0.50 Hz for 9-year-olds, 0.25–0.50 Hz for 10-year-olds; see Shader et al. 2018, for additional ranges). RSA was expressed in units of ln(ms2). Possible artifacts were flagged by an algorithm that detects improbable interbeat intervals, allowing visual inspection and editing when necessary; relatively few artifacts were detected, and these were corrected manually (Berntson et al. 1997). RSA reactivity (RSA-R) was calculated as the residual of the regression of RSA during the star-tracing period on RSA during the baseline period (Burt and Obradović 2013; Baker et al. 2020). Residualized change scores were multiplied by − 1 so that higher RSA reactivity scores indicated greater reductions in RSA (i.e., greater withdrawal) from the baseline to the star-tracing period.
Electrodermal reactivity (EDA-R). EDA (units = microsiemens or µS) was measured with two disposable Ag–AgCl electrodes placed on the palm of the non-dominant hand. Participants were seated throughout the physiological assessment. Data were sampled at 500 Hz. EDA scores were computed with MindWare EDA analysis software (version 3.0.22). EDA-R was calculated as the residual of the regression of EDA during the star-tracing period on EDA during the baseline period (Burt and Obradović 2013; Fenning et al. 2019). Higher EDA-R scores reflect greater increases in EDA level from baseline to star-tracing.
Data Analysis
Separate regressions predicting each child psychophysiological index were performed, with the three parenting behaviors entered as predictors. Missing parent reactions data were estimated for the regressions using multiple imputation across 10 datasets. Given that EDA reactivity was the sole outcome variable in the second regression, EDA data were included in the imputation of reaction data but were not estimated in that regression. Regressions were also performed with either no data estimated (complete case analysis) or with all data (including EDA reactivity) estimated; the pattern of significant findings did not change across the three methods.
Results
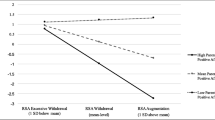
Descriptive statistics and correlations among study variables are shown in Table 1. Supportive parent reactions to negative emotion were related to higher child baseline RSA. Child baseline RSA was related to child age and was associated with child IQ at the level of a trend, so these variables were controlled in subsequent regressions. It was not necessary to control for any other demographic variable or relevant child variable (e.g., gender, ASD symptom level).
Results of the regression analyses are shown in Table 2. In support of our first hypothesis, regression analyses confirmed the bivariate finding that more supportive parental reactions were related to higher child baseline RSA. This association, along with child age, accounted for approximately a quarter of the variance in baseline RSA. Fewer unsupportive parent reactions and higher quality parental scaffolding were each significantly related to higher child EDA reactivity, with medium effect sizes, suggesting support for our second hypothesis. Parenting variables, along with child age and child IQ, accounted for 31% of the variance in EDA reactivity. With respect to the third hypothesis, regression analyses did not indicate a relationship between RSA reactivity and either supportive or unsupportive parenting.
Discussion
Findings from the present study identified concurrent associations between two facets of parental socialization of child emotion (reactions to child negative emotion and co-regulatory scaffolding) and children’s PNS and SNS profiles. Investigations aimed at understanding how parenting behavior may influence psychophysiological processes thought to underlie effective child emotion regulation in populations with neurodevelopmental disorders are only recently emerging (Bell et al. 2018; Breaux et al. 2018) and, to our knowledge, this study is the first to do so in families of children with ASD—a population at extreme risk for emotion dysregulation (Fenning et al. 2018; Mazefsky et al. 2013).
Findings were largely consistent with our first hypothesis in revealing associations between parent-reported supportive reactions to child negative emotion and higher baseline child RSA, a putative biomarker for regulatory capacity (Beauchaine 2015b). However, observed parental scaffolding was essentially unrelated to child baseline RSA, and unsupportive reactions were only correlated due to inverse overlap with supportive reactions. The specificity of these results suggests that socialization processes captured by our measure of supportive reactions may uniquely benefit children’s biological regulatory competence. For example, the encouragement of emotional expression and active problem-solving around emotion may be critical strategies for increasing adaptive development in this area (Eisenberg, Cumberland, et al. 1998a, b; Morris et al. 2007). In contrast, these results suggest that the absence of unsupportive reactions, and parental scaffolding quality—which considered both supportive and unsupportive behaviors—may be less powerful in promoting these processes. Findings underscore the importance of moving beyond broad parenting dimensions to further address the role of specific “supportive” socialization of emotion behaviors.
Although supportive parent reactions were uniquely associated with child baseline RSA, higher child EDA reactivity was linked with both fewer parent-reported unsupportive reactions and higher quality parental scaffolding. Both unsupportive reactions and poorer scaffolding involve behaviors considered deleterious to the development of child regulation, as scaffolding included unsupportive behaviors that were reverse coded. Unsupportive parent reactions include dismissive and punitive responses to children’s vulnerable emotions, and lower-quality scaffolding ratings may indicate similar behaviors observed during frustrating challenges. These parenting behaviors may undermine activation of children’s behavioral inhibition system in challenging situations, consistent with lower child EDA reactivity. In contrast, fewer unsupportive parent reactions and higher-quality scaffolding may involve encouraging children to engage actively with the challenge and to inhibit impulsive responses, as potentially reflected in higher EDA reactivity to the challenge task. As the meaning of a physiological response depends in part on the conditions that elicit the response, future research with similar parenting measures and physiological assessments during tasks more specifically designed to elicit either caution or active engagement could test our interpretation of the results.
Low child EDA reactivity appears to be a risk factor for externalizing problems in children with neurotypical development (Beauchaine et al. 2015; Erath et al. 2009) and those with ASD (Baker et al. 2018; Fenning et al. 2019), with parent–child coercive exchanges posited as a primary mechanism through which this EDA risk eventuates in child externalizing problems (e.g., Beauchaine and Zalewski 2016). Children with low EDA reactivity, who present as more fearless and less concerned with environmental contingencies for problem behavior (Beauchaine et al. 2015), may not only elicit more unsupportive parenting but may also trigger and maintain more hostile, coercive exchanges. In turn, increases in unsupportive parenting behavior may exacerbate child biological and behavioral dysregulation (Beauchaine and Zalewski 2016). The centrality of the SNS to these parent–child processes is highlighted by empirical evidence that parenting interventions may increase EDA responsivity in children with ADHD (Beauchaine et al. 2015), and that reductions in negative parenting may mediate treatment effects on related indices of sympathetic arousal (pre-ejection period—PEP; Bell et al. 2018).
Reduced sensitivity to consequences is not the only hypothesis that has been proposed to explain the risk for externalizing problems conferred by low EDA reactivity, as it is also the case that persistent low sympathetic arousal may be experienced by the child as aversive boredom, urging the child to find ways to increase this response (i.e., the sensation-seeking hypothesis, Cappadocia et al. 2009; Raine et al. 1997). Recent evidence suggests that high-quality parental scaffolding may attenuate the association between low EDA reactivity and externalizing problems in children with ASD (Baker et al. 2018). In this context, higher quality parental scaffolding, which includes up-regulation of child motivation, emotion coaching, and task-specific assistance, may have provided children with low EDA reactivity more adaptive methods for increasing arousal in the service of goal-directed behavior. Thus, it is likely that certain relevant positive parenting behaviors may also shape and increase child sympathetic responding. The scaffolding system used in the current study is a global coding system that considers a host of supportive and unsupportive parent behaviors; future studies that involve more micro-level or sequential coding might clarify which particular behaviors may be driving the present findings and could potentially provide more evidence with regard to direction of parent–child effects. Further work may also consider parental scaffolding across a variety of situational contexts.
The lack of significant findings related to children’s RSA reactivity was not particularly surprising given the complexity of this index. RSA reactivity may be adaptive or maladaptive depending on the nature of the task and the population considered (Beauchaine 2015a; Graziano and Derefinko 2013; Obradović et al. 2011). Indeed, in our previous work with this sample, RSA reactivity interacted with both parenting quality and the child’s concurrent EDA reactivity in the prediction of child externalizing behavior problems (Baker et al. 2020; Fenning et al. 2019). Similarly, studies of children with ADHD have not found direct assocations between RSA reactivity and parent emotion socialization practices even though these parenting behaviors were related to other measures of child psychophysiology (Bell et al. 2018; Breaux et al. 2018). The present study did not have sufficient power to confidently examine complex interactions. Future studies are needed with larger sample sizes and sufficient power to investigate highly complex interactive effects (e.g., RSA reactivity x EDA reactivity x parenting) in order to more thoroughly consider the role of this biological index.
Current results converge with findings from previous investigations of parenting and the development of children with ASD. This work to date suggests that parents may influence children’s behavioral and biological regulatory competence but that careful attention needs to be devoted to considerations of regulation as a state versus a trait (i.e., the internalization of regulatory competence; Baker, Fenning and Moffitt 2019a, b) and the degree to which children’s physiology may moderate socialization effects (Baker et al. 2018; 2020; Baker, Fenning, Howland et al. 2019a, b). Although carefully-controlled, longitudinal investigations are clearly needed, links between specific socialization of emotion behaviors and children’s concurrent physiology suggest the potential for meaningful parenting effects in a population experiencing both delays and differences in regulatory development.
In this paper, we have presented a commonly-accepted perspective that associations between parent socialization of emotion and children’s development are transactional (e.g., Morris et al. 2007), and we have highlighted theory and evidence focusing on the potential effects of parenting on children’s physiological development. However, our findings may also represent parent reactions to children’s behavior resulting from, or correlated with, the physiological indices of interest (i.e., reactive gene-environment correlations). Although issues of causality will be important to clarify in future research, given the lack of study in this area, evidence that children’s psychophysiological tendencies may influence parent socialization of emotion is also valuable and represents an important contribution. Longitudinal studies are needed to address these questions in families of children with neurotypical development as well as families of children with clinical needs at high risk for physiological dysregulation. It is also possible that the present findings could be at least partially explained through passive gene-environment correlations, whereby the parenting behaviors considered might be behavioral consequences or correlates of the parents’ own physiological tendencies based upon genetic profiles shared with their children. Parallel EDA and RSA measurement of the parents was beyond the scope of the present study but could provide important information to address this possibility.
Despite the limitations, the present study has many strengths. First, studies including psychophysiological measurement in children with disabilities have historically excluded children with lower cognitive functioning, which is problematic from an inclusion perspective and may also limit external validity. No such exclusionary criterion was present in this study. Although children with lower estimated IQ were more likely to have missing EDA data, IQ was not related to missingness for RSA. The present sample was also diverse with regard to ethnicity, socio-economic status, and child ASD symptom levels, but our sample was not sufficiently large to investigate differences by ethnicity or by gender. Future studies would benefit from inclusion of children with greater racial diversity as well. Findings identified associations between parent and child factors across multiple measurement methods, including questionnaires, physiological laboratory tasks, and an independent observation of parent–child interaction. This initial study will ideally contribute to a foundation for continued work examining the complex interplay of parental socialization of emotion and relevant child physiological processes over time.
References
Baker, J. K., Fenning, R. M., & Crnic, K. A. (2011). Emotion socialization by mothers and fathers: Coherence among behaviors and associations with parent attitudes and children’s social functioning. Social Development, 20, 412–430. https://doi.org/10.1111/j.1467-9507.2010.00585.x.
Baker, J. K., Fenning, R. M., Crnic, K. A., Baker, B., & Blacher, J. (2007). Prediction of social skills in 6-year-old children with and without developmental delays: Contributions of early regulation and maternal scaffolding. American Journal on Mental Retardation, 112, 375–391. https://doi.org/10.1352/0895-8017(2007)112[0375:POSSIY]2.0.CO;2.
Baker, J. K., Fenning, R. M., Erath, S. A., Baucom, B. R., Moffitt, J. M., & Howland, M. A. (2018). Sympathetic under-arousal and externalizing behavior problems in children with autism spectrum disorder. Journal of Abnormal Child Psychology, 46, 895–906. https://doi.org/10.1007/s10802-017-0332-3.
Baker, J. K., Fenning, R. M., Erath, S. A., Baucom, B., Messinger, D. S., Moffitt, J. M., et al. (2020). Respiratory sinus arrhythmia, parenting, and externalizing behavior in children with autism spectrum disorder. Autism, 24, 109–120. https://doi.org/10.1177/1362361319848525.
Baker, J. K., Fenning, R. M., Howland, M. A., & Huynh, D. (2019). Parental criticism and behavior problems in children with autism spectrum disorder. Autism, 23, 1249–1261. https://doi.org/10.1177/1362361318804190.
Baker, J. K., Fenning, R. M., & Moffitt, J. (2019). Brief report: A cross-sectional examination of the internalization of emotion co-regulatory support in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 49, 4332–4338. https://doi.org/10.1007/s10803-019-04091-0.
Beauchaine, T. P. (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214. https://doi.org/10.1017/s0954579401002012.
Beauchaine, T. P. (2015a). Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 44, 875–896. https://doi.org/10.1080/15374416.2015.1038827.
Beauchaine, T. P. (2015b). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinions in Psychology, 3, 43–73. https://doi.org/10.1016/j.copsyc2015.01.017.
Beauchaine, T.P., Bell, Z., Knapton, E., McDonough-Caplan, H., Shader, T., & Zisner, A. (2019). Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology. Online ahead of print. https://doi.org/10.1111/psyp.13329.
Beauchaine, T. P., Gatzke-Kopp, L., & Mead, H. K. (2007). Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology, 74, 174–184. https://doi.org/10.1016/j.biopsycho.2005.08.008.
Beauchaine, T. P., Neuhaus, E., Gatzke-Kopp, L. M., Reid, M. J., Chipman, J., Brekke, A., et al. (2015). Electrodermal responding predicts responses to, and may be altered by, preschool intervention for ADHD. Journal of Consulting and Clinical Psychology, 83, 293–303. https://doi.org/10.1037/a0038405.
Beauchaine, T. P., & Zalewski, M. (2016). Physiological and developmental mechanisms of emotional lability in coercive relationships. In T. J. Dishion & J. J. Snyder (Eds.), Oxford handbook of coercive relationship dynamics. New York, NY: Oxford University Press.
Bell, Z., Shader, T., Webster-Stratton, C., Reid, M. J., & Beauchaine, T. P. (2018). Improvements in negative parenting mediate changes in children’s autonomic responding following a preschool intervention for ADHD. Clinical Psychological Science, 6, 134–144.
Benevides, T., & Lane, S. (2015). A review of cardiac autonomic measures: Considerations for examination of physiological response in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 560–575. https://doi.org/10.1007/s10803-013-1971-z.
Berntson, G., Bigger, J., Eckberg, D., Grossman, P., Kaufmann, P., Malik, M., & van der Molen, M. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34, 623–648. https://doi.org/10.1111/j.1469.8986.1997.tb02140.x.
Bougher-Muckian, H. R., Root, A. E., Coogle, C. G., & Floyd, K. K. (2016). The importance of emotions: The socialization of emotion in parents of children with autism spectrum disorder. Early Child Development and Care, 186, 1584–1593. https://doi.org/10.1080/03004430.2015.1112799.
Breaux, R. P., McQuade, J. D., Harvey, E. A., & Zakarian, R. J. (2018). Longitudinal associations of parental emotion socialization and children’s emotion regulation: The moderating role of ADHD symptomatology. Journal of Abnormal Child Psychology, 46, 671–683. https://doi.org/10.1007/s10802-017-0327-0.
Burt, K. B., & Obradović, J. (2013). The construct of psychophysiological reactivity: Statistical And psychometric issues. Developmental Review, 33, 29–57. https://doi.org/10.1037/t05570-000.
Cappadocia, M. C., Desrocher, M., Pepler, D., & Schroeder, J. H. (2009). Contextualizing the neurobiology of conduct disorder in an emotion dysregulation framework. Clinical Psychology Review, 29, 506–518. https://doi.org/10.1016/j.cpr.2009.06.001.
Cole, P. M. (2014). Moving ahead in the study of the development of emotion regulation. International Journal of Behavioral Development, 38, 203–207. https://doi.org/10.1177/0165025414522170.
Cole, P. M., Hall, S. E., & Hajal, N. J. (2008). Emotion dysregulation as a risk factor for psychopathology. Child and adolescent psychopathology, 2, 341–373.
Cole, P. M., Michel, M. K., & Teti, L. O. (1994). The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the Society for Research in Child Development, 59(2/3), 73–100.
Cole, P. M., Zahn-Waxler, C., Fox, N. A., Usher, B. A., & Welsh, J. D. (1996). Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology, 105(4), 518.
Constantino, J. N. & Gruber, C.P. (2012). Social Responsiveness Scale, Second Edition (SRS-2). Manual. Los Angeles, CA: Western Psychological Services.
Dyer, W., Blocker, D., Day, R., & Bean, R. (2016). Parenting style and adolescent externalizing behaviors: The moderating role of respiratory sinus arrhythmia. Journal of Marriage and Family, 78, 1149–1165. https://doi.org/10.1111/jomf.12316.
Eisenberg, N., Cumberland, A., & Spinrad, T. L. (1998). Parental socialization of emotion. Psychological Inquiry, 9, 241–273. https://doi.org/10.1207/s15327965pli0904_1.
Eisenberg, M., Spinrad, T. L., & Cumberland, A. (1998). Parental socialization of emotion: Reply to commentaries. Psychological Inquiry, 9, 317–333.
Eisenberg, N., Fabes, R. A., Shepard, S. A., Guthrie, I. K., Murphy, B. C., & Reiser, M. (1999). Parental reactions to children’s negative emotions: Longitudinal relations to quality of children’s social functioning. Child development, 70(2), 513–534.
Eisenberg, N., Sadovsky, A., & Spinrad, T. L. (2005). Associations of emotion-related regulation with language skills, emotion knowledge, and academic outcomes. New directions for child and adolescent development, 2005(109), 109–118.
El-Sheikh, M. (2005). Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology, 46, 66–74. https://doi.org/10.1002/dev.20036.
Erath, S. A., Bub, K., & Tu, K. (2016). Responses to peer stress predict academic outcomes across the transition to middle school. Journal of Early Adolescence, 36, 5–28. https://doi.org/10.1177/0272431614556350.
Erath, S. A., El-Sheikh, M., & Cummings, E. M. (2009). Harsh parenting and child externalizing behavior: Skin conductance level reactivity as a moderator. Child Development, 80, 578–592. https://doi.org/10.1111/j.1467-8624.2009.01280.x.
Erath, S. A., El-Sheikh, M., Hinnant, J., & Cummings, M. (2011). Skin conductance level reactivity moderates the association between harsh parenting and growth in child externalizing behavior. Developmental Psychology, 47, 693–706. https://doi.org/10.1037/a0021909.
Fabes, R. A., Poulin, R. E., Eisenberg, N., & Madden-Derdich, D. A. (2002). The coping with Children’s negative emotions scale (CCNES): Psychometric properties and relations with children’s emotional competence. Marriage & Family Review, 34, 285–310. https://doi.org/10.1300/J002v34n03_05.
Fenning, R. M., Baker, J. K., Baucom, B., Erath, S., Howland, M., & Moffitt, J. (2017). Electrodermal activity and symptom severity in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 1062–1072. https://doi.org/10.1007/s10803-016-3021-0.
Fenning, R.M., Baker, J.K., & Moffitt, J. (2018). Instrinsic and extrinsic predictors of emotion regulation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. Online ahead of print. https://doi.org/10.1007/s10803-018-3647-1.
Fenning, R. M., Erath, S. A., Baker, J. K., Messinger, D. S., Baucom, B., Moffitt, J., & Kaeppler, A. (2019). Sympathetic-parasympathetic interaction and externalizing problems in children with autism spectrum disorder. Autism Research, 12, 1805–1816. https://doi.org/10.1002/aur.2187.
Fox, A. R., Aldrich, J. T., Ahles, J. J., & Mezulis, A. H. (2018). Stress and parenting predict changes in adolescent respiratory sinus arrythmia. Developmental Psychobiology, 61, 1214–1224. https://doi.org/10.1002/dev.21863.
Fox, N. A., & Calkins, S. D. (2003). The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion, 27, 7–26. https://doi.org/10.1023/A:1023622324898.
Gottman, J. M., Katz, L. F., & Hooven, C. (1996). Parental meta-emotion philosophy and the emotional life of families: Theoretical models and preliminary data. Journal of Family Psychology, 10, 243–268. https://doi.org/10.1037/0893-3200.10.3.243.
Graziano, P., & Derefinko, K. (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94, 22–37. https://doi.org/10.1016/j.biopsycho.2013.04.011.
Grolnick, W. S., & Farkas, M. (2002). Parenting and the development of children’s self-regulation. In M. H. Bornstein (Ed.), Handbook of parenting: Practical issues in parenting (pp. 89–110). Mahwah, NJ: Lawrence Erlbaum Associates Publishers.
Gulsrud, A. C., Jahromi, L. B., & Kasari, C. (2010). The co-regulation of emotions between mothers and their children with autism. Journal of Autism and Developmental Disorders, 40, 227–237. https://doi.org/10.1007/s10803-009-0861-x.
Hane, A. A., & Barrios, E. S. (2011). Mother and child interpretations of threat in ambiguous situations: Relations with child anxiety and autonomic responding.Journal of Family Psychology, 25(5), 644–652.https://doi.org/10.1037/a0024149
Hastings, P. D., Nuselovici, J. N., Utendale, W. T., Coutya, J., McShane, K. E., & Sullivan, C. (2008). Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biological psychology, 79(3), 299–306. https://doi.org/10.1016/j.biopsycho.2008.07.005.
Hinnant, J. B., Erath, S. A., & El-Sheikh, M. (2015). Harsh parenting, parasympathetic activity, and development of delinquency and substance use.Journal of Abnormal Psychology, 124(1), 137–151.https://doi.org/10.1037/abn0000026
Hinnant, J. B., Erath, S. A., Tu, K. M., & El-Sheikh, M. (2016). Permissive parenting, deviant peer affiliations, and delinquent behavior in adolescence: The moderating role of sympathetic nervous system reactivity. Journal of Abnormal Child Psychology, 44, 1071–1081. https://doi.org/10.1007/s10802-015-0114-8.
Hoffman, C., Crnic, K. & Baker, J.K. (2006). Maternal depression and parenting: Implications for children’s emergent emotion regulation and behavioral functioning. Parenting: Science and Practice, 6, 271–295. https://doi.org/10.1207/s15327922par0604_1.
Jahromi, L., Meek, S., & Ober-Reynolds, S. (2012). Emotion regulation in the context of frustration in children with high functioning autism and their typical peers. Journal of Child Psychology and Psychiatry, 53, 1250–1258. https://doi.org/10.1002/aur.1366.
Kaartinen, M., Puura, K., Makela, T., Rannisto, M., Lemponen, R., Helminen, M., et al. (2012). Autonomic arousal to direct gaze correlates with social impairments among children with ASD. Journal of Autism and Developmental Disorders, 42, 1917–1927. https://doi.org/10.1007/s10803-011-1435-2.
Kennedy, A. E., Rubin, K. H., Hastings, P. D., & Maisel, B. (2004). Longitudinal relations between child vagal tone and parenting behavior: 2 to 4 years. Developmental Psychobiology, 45, 10–21. https://doi.org/10.1002/dev.20013.
Lord, C., Rutter, M., DiLavore, P., Risi, R., Gotham, K., & Bishop, S. (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Manual. Torrance, CA: WPS.
Mazefsky, C. A., Borue, X., Day, T. N., & Minshew, N. J. (2014). Emotion regulation patterns in adolescents with high-functioning autism spectrum disorder: Comparison to typically developing adolescents and association with psychiatric symptoms. Autism Research, 7(3), 344–354.
Mazefsky, C. A., Herrington, J., Siegel, M., Scarpa, A., Maddox, B. B., Scahill, L., & White, S. W. (2013). The role of emotion regulation in autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 52(7), 679–688. https://doi.org/10.1016/j.jaac.2013.05.006.
Mazefsky, C., & White, S. (2014). Emotion regulation concepts & practice in autism spectrum disorder. Child and Adolescent Psychiatric Clinics of North America, 23, 15–24. https://doi.org/10.1016/j.chc.2013.07.002.
Mazzone, S., & Nader-Grosbois, N. (2017). How are parental reactions to children’s emotions linked with theory of mind in children with autism spectrum disorder? Research in Autism Spectrum Disorders, 40, 41–53. https://doi.org/10.1016/j.rasd.2017.05.003.
Morelen, D., Shaffer, A., & Suveg, C. (2016). Maternal emotion regulation: Links to emotion parenting and child emotion regulation. Journal of Family Issues, 37(13), 1891–1916.
Morris, A. S., Silk, J., Steinberg, L., Myers, S., & Robinson, L. R. (2007). The role of family context in the development of emotion regulation. Social Development, 16, 361–388. https://doi.org/10.1111/j.1467-9507.2007.00389.x.
Neuhaus, E., Bernier, R., & Beauchaine, T. P. (2015). Electrodermal response to reward and non-reward among children with autism. Autism Research. Online ahead of print. https://doi.org/10.1002/aur.1451.
Obradović, J., Bush, N., & Boyce, W. (2011). The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology, 23, 101–114. https://doi.org/10.1017/S094579410000672.
Paczkowski, E., & Baker, B. L. (2007). Parenting children with and without developmental delay: The role of self-mastery. Journal of Intellectual Disability Research, 51, 435–446. https://doi.org/10.1111/j.1365-2788.2006.00894.x.
Perry, N. B., Calkins, S. D., Nelson, J. A., Leerkes, E. M., & Marcovitch, S. (2012). Mothers’ responses to children’s negative emotions and child emotion regulation: The moderating role of vagal suppression. Developmental Psychobiology, 54, 503–513. https://doi.org/10.1002/dev.20608.
Perry, N. B., Mackler, J. S., Calkins, S. D., & Keane, S. P. (2014). A transactional analysis of the relation between maternal sensitivity and child vagal regulation. Developmental Psychology, 50(3), 784. https://doi.org/10.1037/a0033819.
Porges, S. W. (1997). Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of the New York Academy of Sciences, 807, 62–77.
Raine, A. (1993). The psychopathology of crime: Criminal behavior as a clinical disorder. San Diego: Academic Press.
Raine, A., Venables, P. H., & Mednick, S. A. (1997). Low resting heart rate at age 3 predisposes to aggression at age 11 years: Evidence from the Mauritius child health project.Journal of the American Academy of Child and Adolescent Psychiatry, 36(10), 1457–1464.
Roid, G. H. (2003). Stanford-Binet Intelligence Scales (5th ed.). Itasca, IL: Riverside.
Rutter, M., Bailey, A., & Lord, C. (2003). The social communication questionnaire. Manual. Torrance, CA: Western Psychological Services.
Rutter, M., LeCouteur, A., & Lord, C. (2003). The autism diagnostic interview-revised (ADI-R). Los Angeles, CA: Western Psychological Services.
Samson, A. C., Hardan, A. Y., Lee, I. A., Phillips, J. M., & Gross, J. J. (2015). Maladaptive behavior in autism spectrum disorder: The role of emotion experience and emotion regulation. Journal of Autism and Developmental Disorders, 45(11), 3424–3432. https://doi.org/10.1007/s10803-015-2388-7.
Sanders, W., Zeman, J., Poon, J., & Miller, R. (2015). Child regulation of negative emotions and depressive symptoms: The moderating role of parental emotion socialization. Journal of Child and Family Studies, 24(2), 402–415.
Scrimgeour, M. B., Davis, E. L., & Buss, K. A. (2016). You get what you get and you don’t throw a fit!: Emotion socialization and child physiology jointly predict early prosocial development. Developmental Psychology, 52, 102–116. https://doi.org/10.1037/dev0000071.
Shader, T., Gatzke-Kopp, L., Crowell, S., Jamila Reid, M., Thayer, J. F., Vasey, M., & Beauchaine, T. P. (2018). Quantifying respiratory sinus arrhythmia: Effects of misspecifying breathing frequencies across development. Development and Psychopathology, 30, 351–366. https://doi.org/10.1017/S0954579417000669.
Shaffer, A., Suveg, C., Thomassin, K., & Bradbury, L. L. (2012). Emotion socialization in the context of family risks: Links to child emotion regulation. Journal of Child and Family Studies, 21(6), 917–924.
Sheppes, G., Catran, E., & Meiran, N. (2009). Reappraisal (but not distraction) is going to make you sweat: Physiological evidence for self-control effort. International Journal of Psychophysiology, 71, 91–96. https://doi.org/10.1016/j.ijpsycho.2008.06.006.
Sheppes, G., Suri, G., & Gross, J. J. (2015). Emotion regulation and psychopathology. Annual Review of Clinical Psychology, 11, 379–405.
Stanger, S., Abaied, J., Wagner, C., & Sanders, W. (2018). Contributions of observed parent socialization of coping and skin conductance level reactivity to childhood adjustment. Family Process, 57, 181–194. https://doi.org/10.1111/famp.12272.
Tabachnick, A. R., Raby, K. L., Goldstein, A., Zajac, L., & Dozier, M. (2019). Effects of an attachment-based intervention in infancy on children’s autonomic regulation during middle childhood. Biological Psychology, 143, 22–31. https://doi.org/10.1016/j.biopsycho.2019.01.006.
Thompson, R. A. (1994). Emotion regulation: A theme in search of definition. Monographs of the society for research in child development, 59, 25–52. https://doi.org/10.1111/j.15405834.1994.tb01276.x.
Thompson, R. A., & Meyer, S. (2007). Socialization of emotion regulation in the family. In J. J. Gross (Ed.), Handbook of emotion regulation (pp. 249–268).
Ting, V., & Weiss, J.A. (2017). Emotion regulation and parent co-regulation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. Online ahead of print. https://doi.org/10.1007/s10803-016-3009-9.
Twomey, C., O’Connell, H., Lillis, M., Tarpey, S. L., & O’Reilly, G. (2018). Utility of an abbreviated version of the stanford-binet intelligence scales (5th ed.) in estimating ‘full scale’ IQ for young children with autism spectrum disorder. Autism Research, 11(3), 503–508. https://doi.org/10.1002/aur.1911.
Wagner, C. R., & Abaied, J. (2016). Skin conductance level reactivity moderates the association between parental psychological control and relational aggression in emerging adulthood. Journal of Youth and Adolescence, 45, 687–700. https://doi.org/10.1007/x10964-016-0422-5.
White, S. W., Mazefsky, C. A., Dichter, G. S., Chiu, P. H., Richey, J. A., & Ollendick, T. H. (2014). Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: understanding anxiety in autism spectrum disorder. International Journal of Developmental Neuroscience, 39, 22–36. https://doi.org/10.1016/j.ijdevneu.2014.05.012.
Acknowledgements
We thank, Meghan Orr, Alyssa Bailey, Caitlyn Kelleher, Kerensa Jorgenson, Marison Lee, Arielle Garcia, Alex Gant, Shivani Patel, Karrie Tran, Jasmine Gonzalez, Kyra Da Silva Colaco, Lauren Richardson, Alexander Kaeppler, and the staff and families of the Autism Emotion and the Family Project. These findings were presented at the 2020 Convention for the American Psychological Association. Jacquelyn Moffitt is now at the University of Miami.
Funding
This project was funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R15HD087877) awarded to the second and third authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moffitt, J.M., Baker, J.K., Fenning, R.M. et al. Parental Socialization of Emotion and Psychophysiological Arousal Patterns in Children with Autism Spectrum Disorder. Res Child Adolesc Psychopathol 49, 401–412 (2021). https://doi.org/10.1007/s10802-020-00745-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-020-00745-1