Abstract
Purpose
To investigate long-term visual outcomes and factors associated with low vision in patients with childhood glaucoma.
Materials and methods
A retrospective review was conducted on the medical records of pediatric glaucoma patients at the Ondokuz Mayıs University Ophthalmology Clinic from 2005 to 2023. The patients were categorized into three groups: primary congenital glaucoma (PCG), secondary childhood glaucoma, and glaucoma following cataract surgery (GFCS). Groups were analyzed regarding visual acuity (VA), ocular conditions and comorbidities, and the cause of visual impairment. The study also investigated the potential risk factors associated with visual impairment.
Results
A total of 105 eyes of 60 patients with a mean age of 9.7 ± 5.5 years were included in the study. The mean VA in logMAR was 0.59 ± 0.52. At the final follow-up, 34.1% had good VA (≥ 20/50), 29.5% had moderate VA (20/50–20/200), and 36.4% had poor VA (< 20/200). The final mean intraocular pressure (IOP) was 16.2 ± 6.2 mmHg. Amblyopia was the leading cause of vision loss (38.2%), followed by glaucomatous damage (36.4%). Patients with GFCS had a higher rate of visual impairment (42.4%) and refractive error. The results of the regression analysis showed that low vision was associated with undergoing more than two surgeries, high IOP at baseline, high initial and final cup-to-disc (C/D) ratio, and high initial central corneal thickness (CCT) (CI 95%, p = 0.018, p= 0.017, p = 0.013, p = 0.003, p = 0.001, respectively).
Conclusion
Good VA can be achieved in 34.1% of childhood glaucoma cases. However, the VA prognosis may be worse in patients with GFCS. Achieving good visual outcomes in childhood glaucoma requires timely and effective treatment, consideration of risk factors, and management of amblyopia and ocular comorbidities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Childhood glaucoma is a heterogeneous group causing similar outcomes due to structural damage due to elevated intraocular pressure. It is responsible for approximately 5% of blindness in childhood [1,2,3]. The visual prognosis is more severe than adult glaucoma. In addition, optic nerve and visual field damage may result in axial elongation, leading to myopia, increased corneal diameter, corneal edema, and ophthalmia, especially below the age of 3 years [4].
The Childhood Glaucoma Research Network (CGRN) defined a classification system for childhood glaucoma [5]. Accordingly, primary childhood glaucoma includes PCG and juvenile open-angle glaucoma (JOAG). Secondary childhood glaucoma includes subgroups associated with non-acquired ocular anomalies, associated with non-acquired systemic anomalies, and associated with acquired anomalies. Patients who receive a diagnosis of glaucoma only after cataract surgery are considered to have glaucoma following cataract surgery.
In a child with the suspicion of glaucoma, accurate diagnosis, intraocular pressure control, and prevention of amblyopia are of great importance in terms of prognosis, and patient follow-up should be performed for many years to optimize the prognosis.
Visual prognosis of PCG cases has been reported in many studies, and risk factors associated with low vision have been investigated. Visual prognosis differs in different geographic regions, and the proportion of eyes achieving good VA ranges from 26.9% to 79% [6,7,8,9,10,11,12,13]. Young age at diagnosis, unilateral disease, multiple surgeries, glaucomatous optic atrophy, and corneal opacity have been associated with poor visual prognosis for PCG [11, 13].
The first-line management strategy in the treatment of PCG is surgical treatment. JOAG and secondary childhood glaucoma can initially be managed with medical treatment. Although the treatment of congenital glaucoma is aimed at normalizing IOP and preventing further damage to the optic nerve, visual rehabilitation remains integral to clinical management.
Although many studies have evaluated short and/or long-term visual outcomes and risk factors for PCG, few data are available on secondary childhood glaucoma. This study aimed to retrospectively determine the long-term visual prognosis and possible risk factors for vision loss in patients with PCG and secondary childhood glaucoma due to non-acquired anomalies and GFCS in Turkey.
Materials and methods
Subjects
Patients diagnosed and treated for glaucoma by the same clinician (NA) in Ondokuz Mayıs University Ophthalmology Clinic between 2005 and 2023 were retrospectively analyzed. All patients with glaucoma who were under 18 years of age at the time of diagnosis were reviewed for diagnosis and classification based on the CGRN guideline (Fig. 1). The classification was as follows: patients with primary congenital glaucoma (group 1), patients with a non-acquired systemic disease or syndrome or non-acquired ocular anomalies (group 2), and patients with GFSC (group 3). Patients with JOAG and acquired glaucoma were excluded from the study due to the insufficient sample size and lack of sufficient data for statistical analysis. Additionally, patients who were followed for the suspicion of glaucoma and did not receive treatment were excluded to avoid the potential for statistically biased results. All included patients received surgical or medical treatment.
Patients who were over 18 years of age at the time of glaucoma diagnosis, who had glaucoma secondary to ocular trauma, and who had been treated in another center were excluded from the study. Also, patients with identifiable retinal pathology (e.g., Behcet's uveitis), active uveitis during the study (e.g., Herpes Simplex Uveitis), corneal abnormalities, associated refractive errors not attributable to childhood glaucoma, and patients with visual pathway pathologies were excluded from the study.
Participants underwent regular examinations every three months or more frequently as required. The study was approved by the Ondokuz Mayıs University Clinical Research Ethics Committee and conducted by the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
Ocular examinations and data collection
Personal data included age, sex, age at diagnosis, ocular and systemic comorbidities, mean follow-up, surgeries performed, number of surgeries performed, presence of visual impairment, spectacle, and contact lens wear (in patients with no filtration bleb). Ophthalmic findings included VA, the spherical equivalent of refractive error (SE), IOP, corneal diameter (HCD), corneal thickness (CCT), anterior segment abnormalities (e.g., aniridia, Axenfeld-Rieger anomaly), C/D ratio and other glaucomatous disc findings. Patients' corneal status was recorded: clear, peripheral or central (occluding the visual axis) band keratopathy and leukoma, and corneal edema. The recorded VA values were converted to logMAR equivalents. VA, evaluated as finger counting, hand movements, light perception, or absence of light perception, was recorded as logMAR values of 1.4, 2,7, 3,7, and 4.7, respectively. Patients who were uncooperative and could only be evaluated for light object tracking were excluded from the statistical analysis of VA. Cycloplegic refraction was performed for each patient at the first and last examination, and SE was recorded. In cooperative patients, IOP was measured with the Goldmann applanation tonometer. In non-compliant and underage patients, IOP was measured under general anesthesia using the Tono-Pen AVIA device (Reichert Technologies, Depew, NY, USA). Detailed slit lamp examination findings were recorded, including corneal comorbidities C/D ratio. CCT was measured using the UP-1000 ultrasonic pachymeter (Nidek Inc, Freemont, CA, USA). The corneal diameter was measured with a corneal caliper under general anesthesia in discordant and younger patients and under topical anesthesia (0.5% proparacaine) in cooperative patients under office conditions.
The visual field test was not included in the analysis due to the inability of most patients to perform an objective visual field test at the time of enrolment in the study and the lack of sufficient statistical data.
Evaluation of visual outcomes:
The best-corrected VA was classified as good (20/50 or better), moderate (20/50 to 20/200), or poor (less than 20/200). Additionally, patients were divided into two groups based on whether their VA was good (> 20/50) or low (< 20/50), with the aim of evaluating the causes of visual impairment in a comprehensive manner [11,14. At the final examination, the major contributing factor to the visual impairment was identified and recorded. The identified conditions were glaucomatous optic neuropathy, corneal opacity, amblyopia, and nystagmus. Regression analysis was also performed to investigate the contribution of gender, age, bilateral disease, high inital IOP, high intial CCT, high initial and final C/D ratio, high initial and final HCD, high initial refractive error, and more than 2 surgeries to a vision of less than 20/50 in childhood glaucoma patients.
Glaucomatous damage was considered in patients with focal notching of the disc, retinal nerve fiber defects, progressive growth of cupping, and asymmetric cupping. Participants whose VA was not age-expected and whose VA was significantly worse than the fellow eye in unilateral disease and eyes without corneal complications or significant glaucomatous disc damage that could explain the visual impairment and eyes with the following refractive errors were considered potentially amblyopia: hyperopia/myopia ≥ 4.00 diopters (D), astigmatism ≥ 2 D, or anisometropia ≥ 1.5 D. In addition, refraction between − 0.75 D and + 0.75 D was considered as emmetropia, − 1.00 D and − 6.00 D as mild-moderate myopia, above − 6.00 D as high myopia, and above + 1.00 D as hyperopia.
Corneal opacities were defined as band keratopathy and leukoma that occluded the optic axis and reduced vision. Patients with VA less than 20/50 at the last examination and who did not have other ocular comorbidities such as optic nerve damage, corneal opacity, and nystagmus were considered amblyopia.
Statistical analysis
Statistical analyses were performed with SPSS software (IBM Corp. 2019. IBM SPSS Statistics for Windows, version 26.0. Armonk, NY: IBM Corp). Group differences were analyzed with the Kruskal–Wallis, Chi-square, one-way ANOVA, T-test, and Mann–Whitney U tests. Good and poor vision scores were compared with T-test, Chi-square and Mann–Whitney U-test. Potential risk factors for low vision were evaluated by logistic regression analysis, and a possible risk model was constructed. A p-value of < 0.05 was considered statistically significant.
Results
A total of 105 eyes of 60 patients were included in the study. At the time of participation in the study, the mean age of the cases was 9.7 ± 5.5 years, the mean age at diagnosis was 15.9 ± 5.8 months, and the mean follow-up time was 84.2 ± 49.2 months. Of the participants, 24 were females and 36 were males. 15 patients had glaucoma in one eye, and 45 had glaucoma in both eyes. Of all cases, 47 (44.8%) were diagnosed with PCG, 23 (21.9%) with secondary childhood glaucoma, and 35 (33.3%) with GFCS. In all PCG patients, surgery was the initial treatment approach, with medical therapy employed to regulate IOP until surgical intervention. In the secondary childhood glaucoma and GFCS group, the initial treatment approach was medical therapy. The surgical treatments included angle surgeries (goniotomy, trabeculotomy) and filtration surgeries (trabeculectomy, combined trabeculotomy and trabeculectomy, drainage implantation surgery). All trabeculectomies were performed with the administration of Mitomycin-C. Combined trabeculotomy-trabeculectomy (CTT) was the first-choice surgery in PCG patients without corneal edema and opacity allowing the visualization of the angle. Otherwise, patients underwent trabeculectomy. Ahmed glaucoma valve implantation has been performed in a select few cases of PCG with buphthalmos, high risk of intraoperative anterior chamber shallowing, and inability to visualize the anterior segment due to significant corneal edema. Secondary childhood glaucoma and GFCS patients who did not respond to medical therapy underwent surgery: trabeculectomy in patients with corneal conditions that did not allow angle visualization, and Ahmed Glaucoma Valve implantation was the first-choice surgery in patients with special conditions such as Sturge-Weber syndrome. Ahmed Glaucoma Valve implantation or cyclodestruction was performed after failed glaucoma surgery. Surgical intervention was performed in 43,5% of secondary childhood glaucoma patients and in 42.9% of GFCS patients who did not respond to medical treatment. A total of 31.4% of the patients were undergoing medical treatment. Cyclodestruction was performed in seven eyes in which intraocular pressure (IOP) could not be controlled despite surgery and maximum medical treatment. Demographic and clinical data are summarized in Table 1.
Group 1 had the highest number of glaucoma surgeries per eye. (1.34 ± 0.56, p = 0.001). The initial C/D ratio was highest in Group 1 and lowest in Group 3 (0.54 ± 0.24 vs. 0.26 ± 0.19, p = 0.001). The C/D ratio increased in all groups, and the final C/D ratio was highest in Group 2 (initial C/D: 0.43 ± 0.37 vs final C/D: 0.69 ± 0.38, p = 0.024). Mean IOP was 27,4 ± 6,6 mmHg at the initial follow-up and 16,2 ± 6,2 mmHg at the final follow-up, and there was no significant difference between the groups (p = 0.794, p = 0.663, respectively).
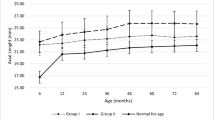
At both the initial and final examination, Group 1 had a significantly larger HCD (p = 0.001, p = 0.001, respectively). At baseline, the groups had similar CCT (p = 0.194). However, at the last follow-up, significant differences in CCT were observed between the groups (p = 0.011). CCT regressed in groups 1 and 2 but progressed in group 3.
Mean VA was similar in groups 1 and 2 (p = 0.651) but was poorer in group 3. The initial mean SE was 4.06 ± 4.65, and the final mean SE was 0.86 ± 1.88. The SE was significantly hyperopic in the GFCS group (10.49 ± 4.27, p = 0.001). It was observed that the initial hyperopia values of all groups decreased and shifted towards myopia. In Groups 1 and 2, patients with myopia and high myopia, respectively, constituted the majority, with values of 36,4% and 55%. In the GFCS group, the majority of patients were hyperopic, accounting for 71% of cases (p = 0.002).
Clear corneas constituted the majority in all groups (%70.2). In group 3, 42,9% of the participants had corneal complications, proportionally different from the other two groups (p = 0.009).
The most common cause of low vision was amblyopia (38.2%), followed by glaucomatous optic nerve damage (36.4%). The groups showed similar characteristics regarding the causes of low vision (p = 0.510). In Group 3, amblyopia constituted the majority of visual impairment, with a rate of 41.7%. The clinical data and statistical comparisons of primary congenital glaucoma, secondary childhood glaucoma, and GFCS groups are summarized in Table 2.
Initial IOP, number of surgeries per eye, initial and final C/D ratio, initial and final CCT values were significantly higher in participants with low vision (VA < 20/50) compared to those with good vision (VA ≥ 20/50) (p = 0.001, p = 0.015, p = 0.008, p = 0.002, p = 0.001, p = 0.024, p = 0.001, respectively). Furthermore, corneal complications were relatively higher for the low vision group (53.7% vs. 12.2%). Both groups had a similar distribution of demographic characteristics. There were no significant differences between the groups in the type of surgery performed, initial and final SE, type of refractive correction, initial and final HCD. Table 3 summarises the clinical characteristics and statistical comparisons of participants with good and low vision.
Logistic regression analysis showed that high initial IOP, high initial CCT, high initial and final C/D ratio, and more than 2 operations were significantly associated with poor VA. Table 4 summarises the logistic regression analysis for low VA in childhood glaucoma.
Discussion
Our study aimed to determine the visual prognosis in cases with childhood glaucoma and investigate possible risk factors for low vision. In addition, we tried to assess the effect of etiology on visual prognosis and its relation with ocular comorbidities in childhood glaucoma cases. After a mean follow-up of 84.2 months, 34.1% of participants had a VA of 20/50 or better. Good final VA was achieved in 48,8% of PCG cases compared to 31.3% in the secondary childhood glaucoma group and 27,3% in the GFCS group. This difference may be attributed to the older age at diagnosis, higher final IOP, and shorter follow-up period in the secondary childhood glaucoma group. The secondary childhood glaucoma group underwent less glaucoma surgery than the PCG group, which may highlight the importance of filtration surgery and strict IOP control. The GFCS group had the lowest final IOP. Still, the higher rate of corneal comorbidities, high refractive errors, aphakia, and late age at diagnosis may explain the lack of good final VA.
Different visual results have been reported in many studies of PCG patients. Mandal et al. [15] found that 20/60 VA was achieved in 7 of 19 PCG patients (26,9%) with HCD ≥ 14 mm. Yassin SA [11] reported a VA of 20/50 or better in 27 of 53 eyes (51%) that underwent successful surgical treatment. Fang et al. [7] reported that a VA of 20/50 and above was achieved in 56% of 95 eyes under IOP control. Our study found that 48,8% of the eyes had a VA of 20/50 or better in PCG. Few studies have linked secondary childhood glaucomas and GFSC with VA. The differences between VA levels can be explained by the fact that our study was conducted on a heterogeneous group with different demographic characteristics, number of operations, and inclusion criteria. Lopes et al. reported that patients in these two groups had worse visual outcomes compared to PCG, which is consistent with our findings [16].
Lee et al. [8] suggested that multiple surgeries would have a negative impact on visual prognosis. In our study, the number of glaucoma surgeries per eye was 0.7 ± 0.64 in cases with VA ≥ 20/50 and 1.13 ± 0.82 in cases with VA < 20/50 (p = 0.015). Multiple surgeries are a harbinger of uncontrolled IOP and, therefore, progression of glaucomatous disc damage. It can lead to ocular comorbidities such as corneal opacity, nystagmus, strabismus, and cataracts. Multiple surgeries are also associated with a certain postoperative recovery time, which can cause amblyopia. In parallel, logistic regression analysis showed that multiple surgeries were a risk factor for low vision.
Our study observed an increase in HCD for all groups at the end of the follow-up period, consistent with previous findings for cases with PCG [17. The increase in HCD can be attributed to the elevated IOP and the high plasticity of the cornea and sclera in children. Kun et al. [18] suggested horizontal corneal diameter (HCD) is lower in eyes with congenital cataracts. Similarly, in our study, HCD was lower in patients with glaucoma after cataract surgery at the initial and final examination. Factors such as age at glaucoma diagnosis, differences in glaucoma etiology, and IOP control may explain this difference (GFCS participants had lower IOP at the initial and final follow-up). Fang et al. [7] suggested that preoperative HCD is related to low vision. However, we could not establish a relationship between HCD and low vision.
In cases of PCG without corneal edema, it has been suggested that the CCT is reduced due to the stretching of the tissues [19] The CCT decreased at the final examination in both the PCG and secondary childhood glaucoma groups compared to the initial examination. The initial high IOP likely caused corneal edema and increased CCT. However, it has been reported that individuals with congenital cataracts may have a high CCT [18,20] Similarly, in our study, the CCT increased at the last visit in the group with GFCS compared to the initial visit. Furthermore, it was found that the group with VA < 20/50 had significantly higher CCT both at the initial and the final visit. Regression analysis revealed that higher CCT was associated with a poorer visual outcome. Therefore, it can be concluded that CCT is a visual prognosis marker.
Many studies suggest that myopia is the most common refractive error in PCG patients [7,11,21]. Increased IOP is thought to cause axial elongation of the eye [21]. In our study, we found that myopia was the most common refractive error in PCG and secondary childhood cases (36.4% and 55%, respectively). However, GFCS cases shifted the overall refractive error to hyperopia. The groups differed significantly in terms of refractive error correction. This may be due to the inability to use contact lenses in patients with filtration blebs, the presence of emmetropic patients in the PCG and secondary childhood group, and the high refractive errors in the GFCS group. There was no significant effect of the type of refractive correction in the good and poor vision groups.
Amblyopia is a significant cause of vision loss in childhood glaucoma patients with controlled IOP. The study found that amblyopia was a significant cause of visual impairment, followed by glaucomatous damage. These findings were consistent with previous literature [7,11,22] Kargı et al. [6] suggested that long-term control of IOP and keeping it below 19 mmHg may help preserve vision in patients with childhood glaucoma. Similar to our study, the authors suggested that amblyopia is the most common cause of low vision, and low vision was significantly higher in cases of aphakic glaucoma. In addition, ocular comorbidities such as corneal opacities, cataract, and nystagmus also cause poor visual outcomes, both directly and by causing amblyopia. This situation highlights the importance of the treatment of amblyopia and ocular comorbidities in childhood glaucoma cases.
A limitation of our study was the large number of patients who were unable to comply with the visual examination. As a result, the VA values of these patients could not be included in the study, which limited the sample size. Additionally, we were unable to examine the effect of the retinal nerve fiber layer on visual prognosis due to the poor measurement cooperation of most of the pediatric patients at the time of the study. Visual field tests were not included in the analysis due to an insufficient number of patients who were compatible with the visual field. Axial length follow-up was not possible in most patients due to examination difficulties and therefore could not be included in the analysis. The inclusion of patients with GFCS and the heterogeneity of the study groups may affect the overall refractive error. Some patients may experience a minimization effect due to high refractive error, which could lead to a poor VA prognosis.
Conclusion
Numerous studies in the literature investigate the visual prognosis of PCG. However, limited studies are reporting visual outcomes in patients with secondary congenital glaucoma and GFCS. Our study aimed to determine the factors associated with visual impairment in childhood glaucoma. Regression analysis revealed that high initial IOP, high CCT, more than two surgeries, and high initial and final C/D ratio were associated with visual impairment. Achieving vision above 20/50 in 34.1% of childhood glaucoma patients with controlled IOP is possible. Early and accurate diagnosis, successful treatment, including IOP control, and prevention of amblyopia are crucial for a positive prognosis. These results may be a potential step towards early diagnosis, timely and effective treatment, and management of amblyopia and ocular comorbidities in order to achieve good visual outcomes in childhood glaucoma.
Data availability
No datasets were generated or analysed during the current study.
References:
Gilbert C, Rahi J, Quinn G. Visual impairment and blindness in children. 2003.
Senthil S, Badakere S, Ganesh J et al (2019) Profile of childhood glaucoma at a tertiary center in South India. Indian J Ophthalmol 67(3):358–365
Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG (2012) An update on progress and the changing epidemiology of causes of childhood blindness worldwide. Journal of American Association for Pediatric Ophthalmology and Strabismus 16(6):501–507
Brito MC, Bentinjane A, Dietlein T. 1. DEFINITION, CLASSIFICATION, DIFFERENTIAL DIAGNOSIS. Childhood glaucoma. 2013;9:1.
Karaconji T, Zagora S, Grigg JR (2022) Approach to childhood glaucoma: A review. Clin Experiment Ophthalmol 50(2):232–246
Kargi SH, Koc F, Biglan AW, Davis JS (2006) Visual acuity in children with glaucoma. Ophthalmology 113(2):229–238
Fang L, Hu Y, Zhong Y et al (2022) Long-Term Visual Outcomes of Primary Congenital Glaucoma in China. Ophthalmic Res 65(3):342–350
Lee H-J, Kim YK, Jeoung JW, Park KH, Kim S-J (2021) Visual outcomes and associated factors of primary congenital glaucoma in children. Graefes Arch Clin Exp Ophthalmol 259(11):3445–3451
Esfandiari H, Prager A, Hassanpour K et al (2020) The Long-term Visual Outcomes of Primary Congenital Glaucoma. J Ophthalmic Vis Res 15(3):326–330
Gusson E, Chemello F, Longo R et al (2021) Primary congenital glaucoma surgery: outcomes and visual function. Int Ophthalmol 41(11):3861–3867
Yassin SA (2017) Long-Term Visual Outcomes in Children with Primary Congenital Glaucoma. Eur J Ophthalmol 27(6):705–710
Mendicino ME, Lynch MG, Drack A et al (2000) Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. J aapos 4(4):205–210
Khitri MR, Mills MD, Ying GS, Davidson SL, Quinn GE (2012) Visual acuity outcomes in pediatric glaucomas. J aapos 16(4):376–381
Tansuebchueasai N, Kiddee W, Wangsupadilok B (2020) Clinical Characteristics and Prognostic Factors of Visual Outcomes in Childhood Glaucoma. J Pediatr Ophthalmol Strabismus 57(5):283–291
Mandal AK, Matalia JH, Nutheti R, Krishnaiah S (2006) Combined trabeculotomy and trabeculectomy in advanced primary developmental glaucoma with corneal diameter of 14 mm or more. Eye (Lond) 20(2):135–143
Lopes NL, Gracitelli CPB, Rolim-de-Moura C (2021) Childhood Glaucoma Profile in a Brazilian Tertiary Care Center Using Childhood Glaucoma Research Network Classification. J Glaucoma 30(2):129–133
Yu Chan JY, Choy BN, Ng AL, Shum JW (2015) Review on the Management of Primary Congenital Glaucoma. J Curr Glaucoma Pract 9(3):92–99
Kun L, Szigeti A, Bausz M, Nagy ZZ, Maka E (2018) Preoperative biometry data of eyes with unilateral congenital cataract. J Cataract Refract Surg 44(10):1198–1202
Henriques MJ, Vessani RM, Reis FA, de Almeida GV, Betinjane AJ, Susanna R Jr (2004) Corneal thickness in congenital glaucoma. J Glaucoma 13(3):185–188
Chattannavar G, Badakere A, Mohamed A, Kekunnaya R (2022) Visual outcomes and complications in infantile cataract surgery: a real - world scenario. BMJ Open Ophthalmol 7(1):e000744
MacKinnon JR, Giubilato A, Elder JE, Craig JE, Mackey DA (2004) Primary infantile glaucoma in an Australian population. Clin Exp Ophthalmol 32(1):14–18
Chaudhary RS, Gupta A, Sharma A et al (2020) Long-term functional outcomes of different subtypes of primary congenital glaucoma. Br J Ophthalmol 104(9):1288–1292
Funding
No funding was provided for this manuscript.
Author information
Authors and Affiliations
Contributions
A.G. and L.N. collected patients data, wrote the main manuscript text A.G. prepared Fig. 1 A.G. prepared Table 1,2, and 3 L.N. prepared Table 4 All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gurpinar, A., Niyaz, L. & Ariturk, N. Long-term follow-up results and visual outcomes of childhood glaucoma in the black sea region of turkey. Int Ophthalmol 44, 360 (2024). https://doi.org/10.1007/s10792-024-03275-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03275-7