Abstract
Purpose
To determine normative data and reference ranges according to age groups by measuring the foveal avascular zone (FAZ), superficial capillary plexus vascular density (SCP-VD), deep capillary plexus vascular density (DVP-VD), radial peripapillary capillary plexus vessel density (RPC-VD), and peripapillary retinal nerve fiber layer (ppRNFL) in healthy children and to determine the age and sex-related changes of these values.
Methods
This prospective study included data from 370 eyes of 370 healthy children (202 girls, 168 boys) aged 7–18 years. Participants were divided into four groups according to their age. Optical coherence tomography angiography (OCTA) measurements were taken using AngioVue (Avanti; Optivue).
Results
No statistically significant difference was observed in terms of FAZ, SCP-VD, DCP-VD, RPC-VD, and ppRNFL thickness values according to the age groups (except the RPC-VD superior) (p > 0.05 for all). VDs in all deep parafoveal regions in groups 1 and 2 were higher in girls. While FAZ values were higher in girls in all age groups (statistically significant in groups 1, 3, and 4), ad SPD and DPD values were higher in boys in all age groups (statistically significant in group 1 and 2 for SPD, and group 1 and 3 for DPD).
Conclusions
We report normal reference ranges for macula and disk vessel density and ppRNFL parameters in healthy children aged 7–18 years using OCTA. These normative values could be useful in diagnosing retina and optic disk disease early in childhood.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introductıon
Retinal vascular imaging in the pediatric age group is of great importance in the diagnosis of retinal vascular diseases such as Coats disease, retinopathy of prematurity, choroidal neovascularization, diabetic retinopathy, and evaluating the retinal reflection of systemic disorders [1,2,3,4].
Conventionally, fluorescence angiography (FA) is still the gold standard for clinically evaluating the retinal and choroidal vasculature and their pathologies. It can locate abnormalities by providing dynamic visualization with a wide field of view. However, the main disadvantages of FA are that it is an invasive procedure that requires dye injection and offers only two-dimensional images [5]. Although superficial capillary structures can be visualized well, the deep capillary network is not well visualized in FA, probably due to light scattering in the retina [6]. In addition, dye-induced nausea and vomiting, and allergic reactions including anaphylactic shock may be observed [5].
Optical coherence tomography angiography (OCTA) is a current method used in retinal vascular imaging. OCTA produces vascular flow maps of the retina and choroid, thereby forming a three-dimensional image of the retinal microvasculature. Various studies have shown that OCTA can be a reliable tool for qualitative and quantitative assessment of the retinal choroidal or optic nerve vessels during ocular pathologic or physiologic changes [7,8,9].The advantages of OCTA over FA are that it is a noninvasive and rapid procedure, and provides quantitative data with high depth and resolution [10, 11]. Therefore, it can be easily used as a guide in the diagnosis, determination of severity, and follow-up of retinal pathologies in children.
The determination of normal values in retinal vascular diseases will help detect pathologic OCTA findings. Numerous studies have been conducted using OCTA in healthy adult individuals to examine normative values for macula and peripapillary vessel density [12,13,14,15]. There are also studies investigating normal vessel density values in healthy children [16,17,18,19]. These studies were conducted with a relatively low number of participants and the correlation of values with parameters such as age, sex, axial length, refractive errors, and body mass index (BMI) were examined. In this study, differently, we aimed to determine the mean values and reference intervals of normative values according to age groups by analyzing the vascular densities (VD) of the optic disk and macula, foveal avascular zone (FAZ) area, and ppRNFL thickness.
Patıents and methods
This prospective study was conducted at the ophthalmology clinic of a tertiary referral center in Turkey. The study protocol was approved by the institutional ethics committee and adhered to the principles of the Declaration of Helsinki for research involving human subjects. Written informed consent form was obtained from all participants and the parents of underage participants. The inclusion criteria were as follows: individuals aged 7–18 years, and best corrected visual acuity (BCVA) 20/20 or better, refractive error 4.0 diopters or less, axial length between 20 and 26 mm, and intraocular pressure less than 21 mm Hg. The exclusion criteria were any chorioretinal disease, optic disk pathology and glaucoma, strabismus and amblyopia, previous eye surgery and trauma, medical history of prematurity, history of any systemic disorder (e.g., diabetes mellitus, hypertension); refractive error of greater than 4.00 D or intraocular pressure readings of greater than 20 mm Hg, ocular media opacities that reduce image quality. In addition, children with BMI < 5th—> 95th percentile according to the percentile cards arranged by age and sex of the Centers for Disease Control and Prevention (CDC) were also excluded from the study [20].
The demographic data of the participants were recorded and they were divided into four groups according to their ages. Children aged 7–9 years were included in group 1, children aged 10–12 years in group 2, children aged 13–15 years in group 3, and children aged 16–18 years in group 4. All participants underwent a full ophthalmologic examination, including autorefraction (Carl Zeiss Meditec), BCVA with a Snellen chart, intraocular pressure measurements with an air-puff tonometer (Canon TX- 20P), slit-lamp examination, dilated funduscopy and axial length measurements using an IOLMaster (Carl Zeiss Meditec). All macular and peripapillary vessel densities and FAZ measurements were performed during the same daily time interval (between 9:00 AM and 12:00 PM) using the AngioVue software of the OCTA device (Optovue RTVue XR Avanti; Optovue Inc., Fremont, CA) (version 2017.1.0.151) after pupil dilation (1% tropicamide) and by the same technician who was trained in the use of the device. Only images with a signal strength index of 8 or greater were included in the study. Eyes with poor-quality images on OCTA due to eye movements, poor fixation, or media opacities were excluded.
AngioVue uses the Split-spectrum amplitude-decorrelation angiography (SS-ADA) algorithm to detect erythrocyte movement. This software enables noninvasive visualization of the retinal and choroidal vasculature via motion contrast. To calculate VD, the AngioVue Analytics software was used to extract a binary image of the blood vessels from the grayscale OCTA image and then the percentage of pixels with the flow signal greater than the threshold in the defined region was calculated. Each OCTA volume was acquired in 3 s, and two orthogonal OCTA volumes were acquired to perform motion correction to minimize motion artifacts due to microsaccades and fixation changes. FAZ area (mm2), superficial foveal and parafoveal VD (%), and deep foveal and parafoveal VD (%) were evaluated. AngioVue has an A-scan rate of 70,000 scans per second, uses a light source centered on 840 nm, a bandwidth of 45 nm, and an axial resolution of 5 mm.
OCTA examinations were performed using the standard macular and peripapillary protocol. All eye scans were of a 3 × 3-mm scanning area centered on the fovea, and a 4.5 × 4.5 mm scanning area centered on the papilla.
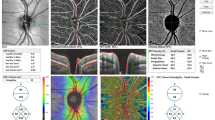
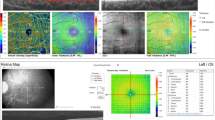
The FAZ is the retinal capillary-free area located in the central fovea. The FAZ area was measured using the ‘retina’ tool of the software, which delineates it automatically. FAZ measurement by OCTA are shown in Fig. 1. Parafoveal VD was calculated for the annular area between 0.3 and 1.25 mm radius from the center of the macula. The parafoveal region was divided into four sectors of 90 degrees each (temporal, superior, nasal, and inferior sectors) and the VD in each sector was calculated. The “en face” images of the SCP were segmented between an inner border 3 mm below the internal limiting membrane and an outer border 15 mm below the inner plexiform layer (IPL). The “en face” images of the DCP were segmented under the IPL with inner and outer boundaries at 15 and 70 mm, respectively. Süperficial and deep capillary vessel density measurements by OCTA are shown in Fig. 2.
The peripapillary images were acquired with a 4.5 × 4.5-mm scanning area centered on the optic disk. The eye-tracking function was activated. Motion correction to minimize motion artifacts arising from microsaccades and fixation changes was applied. Peripapillary capillary VD was measured in a 1.00-mm wide elliptical ring extending outward from the optic disk border in the radial peripapillary capillary (RPC) region. The RPC layer extends from the internal limiting membrane to the nerve fiber layer. For analyses, VD is automatically calculated for the inside-disk area, and the peripapillary area, respectively. The radial peripapillary capillary vessel density measurements by OCTA are shown in Fig. 3. PPRNFL thickness was also measured using the AngioVue (Optovue, Inc.). The PPRNFL thickness was assessed at a 3.45-mm-diameter circle around the optic disk in the ONH mode. PPRNFL thickness measurements by OCTA are shown in Fig. 3.
The PPRNFL thickness was assessed at a 3.45-mm-diameter circle around the optic disk in the optic nerve head (ONH) mode. Radial peripapillary capillary (RPC) vessel density was automatically calculated between two optic nerve head–centered concentric circles with a diameter of 2 mm (inner) and 4 mm (outer) (ring width, 1 mm) at eight equal sectors and two equal hemispheres (superior and inferior)
Image quality was assessed for all OCTA scans. Poor-quality images were defined as scans with quality index < 8 or images with residual motion artifacts. Segmentation errors were excluded from the analysis.
Statıstıcal analysıs
Statistical analysis of the data was performed using the SPSS 21.0 package program. Whether continuous variables showed normal distribution was examined using the Shapiro–Wilk test. Parametric tests were used for variables showing normal distribution, and nonparametric tests were used for those that did not show normal distribution. The Kruskal–Wallis test and one-way analysis of variance (ANOVA) were used for comparisons made according to age groups. For comparisons by sex, the Mann–Whitney U test and Student's t-test were used. Sex distribution according to the groups was checked with the Chi-Square test.
Results
Three hundred seventy eyes of 370 healthy children (202 girls, 168 boys) were enrolled in the study. The right eye of all participants was evaluated. There were 86 children in group 1, 82 children in group 2, 102 children in group 3, and 100 children in group 4. The number of participants and sex distribution in the groups did not differ significantly (Table 1).
The FAZ area, superficial foveal vessel density (SFD), superficial parafoveal density (SPD), superficial parafoveal temporal vessel density (STD), superficial parafoveal superior vessel density (SSD), superficial parafoveal nasal vessel density (SND), superficial parafoveal inferior vessel density (SID), deep foveal vessel density (DFD), deep parafoveal density (DPD), deep parafoveal temporal vessel density (DTD), deep parafoveal superior vessel density (DSD), deep parafoveal nasal vessel density (DND), and deep parafoveal inferior vessel density (DID) values were measured using OCTA. There was no statistically significant difference in terms of these values in all 4 groups according to age groups (p > 0.05 for all) (Table 2).
When the groups were compared in terms of ppRNFL (mean, superior, inferior) and RPC VD (e-image, peripapillary superior, peripapillary inferior, and inside disk) values, only group 1 had significantly lower RPC VD superior values than group 3 and group 4 (p = 0,037). There was no significant difference between the groups in terms of other values (Table 3). The changes in vessel density and retinal nerve fiber layer thickness parameters values between age groups in healthy children are shown in Fig. 4.
When looking at the change of OCTA parameters in age groups by sex in group 1, SFD and A DFD values were higher in boys, and DPD, DTD, DSD, DND, and DID values were higher in girls (p < 0.05). In group 2, SFD values were higher in boys, and DPD, DTD, DSD, DND, DID, RPCVD whole image and RPCVD inferior values were higher in girls (p < 0.05). In group 3, DFD and RPCID values were higher in boys, and SND and FAZ values were higher in girls (p < 0.05). The only difference between the sexes in group 4 was higher FAZ values in girls (p < 0.05) (Table 4).
Dıscussıon
In recent years, OCTA has become increasingly important in detecting vascular abnormalities of the retina, choroid and ocular findings of systemic diseases. The short duration of the procedure is also important for children because it is noninvasive without allergic adverse effects owing to it not requiring dye injection. There are several studies showing FAZ and vascular density abnormalities in children with various diseases [21,22,23]. However, normative data must be available for OCTA before it can be used clinically in children. Several studies in the literature have aimed to determine the normative values for the FAZ area, macula, and optic disk vascular densities in children 16–19]. Our study aimed to determine these quantitative values in the form of mean values and reference intervals according to age groups.
It has been reported that both vessel density and FAZ can be affected by image quality/signal strength [24, 25].Therefore, while presenting normative data in our study, we tried to obtain optimal results by using only measurements with high image quality/signal strength (≥ 8). We found no statistically significant difference in terms of FAZ, SCP-VD, DCP-VD, RPC-VD values according to age groups (P > 0.05 for all). Only the RPC VD superior values of group 1 were found to be significantly lower than in group 3 and group 4. Similarly, in a study conducted in 71 eyes of 71 healthy children aged 5–18 years, no significant correlation was found in the FAZ area, macula, and optic disk density parameters, except for the positive correlation in the RPC density of the inside disk [16]. Again, in another study conducted with 92 eyes of 92 healthy children aged 7–18 years, no significant change was found in FAZ and vessel density parameters depending on age [17]. In a study conducted with 77 eyes of 52 healthy children aged 5–17 years, no age-related changes were found in the perfusion density values of the macular and optic disk, but they reported a positive correlation between FAZ area and age [18]. In a study conducted with 146 eyes of 146 healthy children aged between 6 and 16 years, no age-related changes in FAZ, SCP-VD, and RCP-VD values were detected, but DCP-VD values were shown to increase with age [19]. Considering our results and similar studies, we can say that the VD values of the optic disk and macula in children aged 7–18 years do not differ significantly according to age. When we look at changes in ppRNFL thickness in children, in contrast to an optical coherence angiography study [26] that found a positive correlation with age, no significant age-related changes were found in our study and many other studies [19, 27,28,29].
In our study, when we looked at the change of FAZ, SCP-VD, DCP-VD values according to age groups, we found significant differences. In groups 1 and 2, we consistently found that VDs in all deep parafoveal regions were higher in girls. In all age groups, the FAZ value was higher in girls (statistically significant for groups 1, 3, and 4), and SPD and DPD values were higher in boys (statistically significant for SPD in groups 1 and 2, for DPD in groups 1 and 3). Changes related to sex are not significant, except for the FAZ area in adolescents aged 15–18 years. Although the difference between the parameters in boys and girls disappears with the increase of age, we have seen that the rate of development in different layers is different in the development process. Similarly, in two different OCTA studies conducted with healthy children [16, 17], DFD and SPD values were found to be significantly higher in boys and FAZ values in girls, but no sex difference was observed in parafoveal densities. In another study [19], it was shown that there was no difference between the sexes in terms of FAZ, SCP-VD, DCP-VD, and RPC-VD values in children, whereas another study [18] found higher SCP perfusion density values in boys. However, the parameters that were evaluated according to sex were not classified according to age groups in any of these studies, unlike our study. The different results obtained in the studies may be due to the different measurement techniques used and the different ethnicities of the participants. In this study, 3 × 3-mm macular scanning was performed, and in some studies, 6 × 6-mm macular scanning was used. In our study, these changes in vascular density, which were more pronounced in girls than in boys, might be due to hormonal factors. We cannot explain the increase observed in deep parafoveal vascular densities in girls compared with boys found in younger age groups. Containing estrogen receptors of the ocular tissues may have been effective. Future studies may clarify the role of these receptors or other possible influencing factors on retinal microvascular structures.
Our study had some limitations. Children aged under 7 years could not be included in the study due to their low cooperation. This study was conducted on the Turkish population and the results and normal range may vary for other ethnic groups.
Studies with larger numbers of patients and groups with ethnic heterogeneity may contribute more to the determination of normative FAZ, macula, and optic disk vessel densities in children. As a result, the FAZ, SCP-VD, DCP-VD, RPC-VD, and pRNFL values we obtained using OCTA were important in terms of determining normative values and reference ranges according to age in children. These will help to detect pathologic changes in the evaluation of retinal disorders and the ocular reflection of systemic diseases.
References
Hautz W, Gołębiewska J, Kocyła-Karczmarewicz B (2017) Optical coherence tomography and optical coherence tomography angiography in monitoring Coats’ disease. J Ophthalmol 2017:7849243. https://doi.org/10.1155/2017/7849243
Rezar-Dreindl S, Eibenberger K, Told R et al (2021) Retinal vessel architecture in retinopathy of prematurity and healthy controls using swept-source optical coherence tomography angiography. Acta Ophthalmol 99(2):e232–e239. https://doi.org/10.1111/aos.14557
Niestrata-Ortiz M, Fichna P, Stankiewicz W et al (2019) Enlargement of the foveal avascular zone detected by optical coherence tomography angiography in diabetic children without diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 257(4):689–697. https://doi.org/10.1007/s00417-019-04264-8
Ong SS, Hsu ST, Grewal D et al (2020) Appearance of pediatric choroidal neovascular membranes on optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol 258(1):89–98. https://doi.org/10.1007/s00417-019-04535-4
Johnson RN, Fu AD, McDonald HR, Jumper JM, et al. 2012 Fluorescein angiography: Basic principles and ınterpretation. In Retina Fifth Edition; (Vol. 1, pp. 2–50.e1). Elsevier Inc. https://doi.org/10.1016/B978-1-4557-0737-9.00001-1
Mendis KR, Balaratnasingam C, Yu P et al (2010) Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying capillary detail. Invest Ophthalmol Vis Sci 51:5864–5859
Jia Y, Bailey ST, Wilson DJ, et al. 2014 Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 121(7):1435–1444
Couturier A, Mané V, Bonnin S et al (2015) Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina 35(11):2384–2391. https://doi.org/10.1097/IAE.0000000000000859
Coscas F, Glacet-Bernard A, Miere A, et al. Optical coherence tomography angiography in retinal vein occlusion: Evaluation of superficial and deep capillary plexa. Am J Ophthalmol. 2016 Jan;161:160–71.e1–2. doi: https://doi.org/10.1016/j.ajo.2015.10.008.
Lei J, Durbin MK, Shi Y et al (2017) Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol 135(10):1092–1098. https://doi.org/10.1001/jamaophthalmol.2017.3431
Rodríguez FJ, Staurenghi G, Gale R; Vision Academy Steering Committee 2018 The role of OCT-A in retinal disease management. Graefes Arch Clin Exp Ophthalmol. 256(11):2019–2026. doi: https://doi.org/10.1007/s00417-018-4109-3.
Lim CW, Cheng J, Tay ELT et al (2018) Optical coherence tomography angiography of the macula and optic nerve head: microvascular density and test-retest repeatability in normal subjects. BMC Ophthalmol 18(1):315. https://doi.org/10.1186/s12886-018-0976-y
Falavarjani KG, Shenazandi H, Naseri D et al (2018) Foveal avascular zone and vessel density in healthy subjects: An optical coherence tomography angiography study. J Ophthalmic Vis Res 13(3):260–265. https://doi.org/10.4103/jovr.jovr_173_17
Lee MW, Nam KY, Lim HB et al (2020) Long-term repeatability of optical coherence tomography angiography parameters in healthy eyes. Acta Ophthalmol 98(1):e36–e42. https://doi.org/10.1111/aos.14203
Yilmaz H, Karakurt Y, Icel E et al (2019) Normative data assessment of vessel density and foveal avascular zone metrics using AngioScan software. Curr Eye Res 44(12):1345–1352. https://doi.org/10.1080/02713683.2019.1639769
Zhang Y, Zhang B, Fan M et al (2020) The vascular densities of the macula and optic disc in normal eyes from children by optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol 258(2):437–444. https://doi.org/10.1007/s00417-019-04466-0
Kiziltoprak H, Tekin K, Cevik S et al (2020) Normative data assessment of peripapillary and macular vessel density and foveal avascular zone metrics using optical coherence tomography angiography in children. J Pediatr Ophthalmol Strabismus 57(6):388–398. https://doi.org/10.3928/01913913-20200903-01
Borrelli E, Lonngi M, Balasubramanian S et al (2019) macular microvascular networks in healthy pediatric subjects. Retina 39(6):1216–1224. https://doi.org/10.1097/IAE.0000000000002123
İçel E, Yılmaz H, Uçak T et al (2020) Evaluation of the optic disc and macula in healthy children using optical coherence tomography angiography. Turk J Ophthalmol 50(4):228–233
The CDC BMI-for-age growth charts are available at: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html
Takagi M, Maruko I, Yamaguchi A et al (2019) Foveal abnormalities determined by optical coherence tomography angiography in children with history of retinopathy of prematurity. Eye (Lond) 33(12):1890–1896. https://doi.org/10.1038/s41433-019-0500-5
Takase N, Nozaki M, Kato A et al (2015) Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence angiography. Retina 35(11):2377–2383. https://doi.org/10.1097/IAE.0000000000000849
Gołębiewska J, Biała-Gosek K, Czeszyk A et al (2019) Optical coherence tomography angiography of superficial retinal vessel density and foveal avascular zone in myopic children. PLoS ONE 14(7):e0219785. https://doi.org/10.1371/journal.pone.0219785
Zhang J, Tang FY, Cheung C et al (2021) Different effect of media opacity on automated and manual measurement of foveal avascular zone of optical coherence tomography angiographies. Br J Ophthalmol 105(6):812–818. https://doi.org/10.1136/bjophthalmol-2019-315780
Zhang J, Tang FY, Cheung CY et al (2020) Different Effect of Media Opacity on Vessel Density Measured by Different Optical Coherence Tomography Angiography Algorithms. Transl Vis Sci Technol 9(8):19. https://doi.org/10.1167/tvst.9.8.19
Yanni SE, Wang J, Cheng CS et al (2013) Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am J Ophthalmol 155(2):354-360.e1. https://doi.org/10.1016/j.ajo.2012.08.010
Erkan Turan K, Taylan Şekeroğlu H, Baytaroğlu, et al (2018) Normative values for optical coherence tomography parameters in healthy children and interexaminer agreement for choroidal thickness measurements. Arquıvos Brasıleıros de Oftalmologıa. https://doi.org/10.5935/0004-2749.20180003
Al-Haddad C, Barikian A, Jaroudi M et al (2014) Spectral-domain optical coherence tomography in children: normative data and biometric correlations. BMC Ophthalmol 14:53. https://doi.org/10.1186/1471-2415-14-53
Krumova S, Sivkova N, Marinov V et al (2020) Normal reference ranges of optical coherence tomography parameters in children. Folia Med (Plovdiv). 62(2):338–344. https://doi.org/10.3897/folmed.62.e46678
Acknowledgements
This work was done at University of Health Sciences Adana City Training and Research Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article. The authors (all) meet all four criteria of the ICMJE.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurumoğlu İncekalan, T., Naz Şimdivar, G.H., Çelik, Ü. et al. Optical coherence tomography angiography in healthy children: normative data and age–related changes in microvascular structure of the optic disk and macula. Int Ophthalmol 42, 2373–2383 (2022). https://doi.org/10.1007/s10792-022-02236-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02236-2