Abstract
Introduction
To describe the outcomes of Baerveldt glaucoma implants implanted via a modified technique with regard to early intraocular pressure (IOP) reduction in cases of uncontrolled glaucoma.
Methods
The medical records of patients who had Baerveldt glaucoma implants of 350 or 250 mm2 implanted via a modified technique and were followed up for a period of at least 6 months were reviewed. The primary outcome measures were the mean IOP and number of glaucoma medications at each visit. We evaluated complete success rates at 1 day, 1 week and 1 month, defined as IOP values \(\ge\) 5 mmHg and ≤ 21 mmHg prior to ligature rupture.
Results
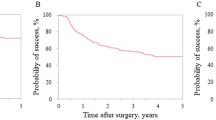
A total of 42 eyes had Baerveldt glaucoma implants and met the inclusion criteria. The mean preoperative intraocular pressure (IOP) was 34.2 ± 11.2 mmHg. The postoperative mean IOP values were 15.1 mmHg ± 8.8 (p < 0.05), 17.7 ± 7.1 mmHg (p < 0.05), 12.3 ± 4.0 mm Hg (p < 0.05) at 1 day, 1 month, and 6 months, respectively. The rate of complete success on the first day was 78%, at the first month was 69%, and at 6 months was 95.2%. The number of glaucoma medications used was significantly lower at 6 months (P = < 0.001).
Conclusion
The modified surgical technique using Baerveldt implants enables a safe, effective, and reliable IOP control in early postoperative patients with uncontrolled glaucomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaucoma is the second leading cause of visual impairment worldwide, and open-angle glaucoma is the most frequent diagnosis and can lead to blindness if left untreated [1,2,3,4]. Studies have demonstrated that lowering intraocular pressure is key to preventing the progression of visual field loss and can be achieved through the administration of glaucoma medications, laser, or surgery. Aqueous shunts are indicated in advanced glaucoma, which remains uncontrolled after failed conventional filtering surgery, or when a high rate of trabeculectomy failure is expected, including with regard to eyes having undergone previous surgery, with scarred conjunctiva, and in the case of uveitic, traumatic, neovascular, or refractory glaucomas [5,6,7,8]. For such high-risk glaucoma cases, aqueous shunts are usually the first-line treatment, given their success rate [9, 10].
The Ahmed glaucoma valve (AGV) (New World Medical Inc., CA, USA) and Baerveldt glaucoma implant (BGI) (Abbott Medical Optics, IL, USA) [10, 11] are the most commonly implanted drainage devices. The AGV includes a restrictive valve with an elastomer diaphragm that restricts flow when the pressure drops below 8–12 mmhg, preventing hypotonia, and a shallow anterior chamber during early postoperative periods, yielding high encapsulation rates and a hypertensive phase often requiring postoperative glaucoma medications [7]. On the other hand, BGI, a nonrestrictive or valveless implant, consists of a more flexible smooth silicone plate, which is biocompatible, easier to handle, bigger in size, and allows for a larger surface of reabsorption of aqueous humor, resulting in better IOP control, less encapsulation, and fewer medications [6, 8]. Mechanical flow restriction is temporarily needed with absorbable sutures, allowing a bleb to form in order to prevent hypotonia in early postoperative periods, with some cases reporting elevated intraocular pressures [1, 12].
We seek here to describe the midterm results of valveless implants via only a simple modified surgical technique consisting of stitches and fenestrations of the tube anterior to the ligature in order to avoid elevated intraocular pressures while minimizing suture rupture. In all previously published studies, ligature and fenestration, and therefore the final results, are carried out according to the discretion of the surgeon.
Materials and methods
In this retrospective cohort study, the medical records of 42 consecutive patients who underwent BGI of 350 or 250 mm2 surgery by the same glaucoma specialist via a modified technique and were followed up for a period of at least 6 months at the Oftalmosalud Instituto de Ojos were reviewed from July 2015 to October 2017. The study protocol complied with the principles of the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Institution. Informed consent was obtained from every patient.
Patient selection
All selected patients were medically uncontrolled by defined as progression of visual field deficits over the course of two consecutive visits and/or decrease in the retinal nerve fiber layer in spectral domain optical coherence tomography (sdOCT). Patients were included if they were older than 18 years old, had a diagnosis of open-angle glaucoma (OAG), angle-closure glaucoma (ACG), or secondary glaucoma. Patients were excluded if they had had previous aqueous shunt surgeries, had a malignant glaucoma, had an absolute glaucoma (end-stage glaucoma that produces a complete loss of vision), congenital iridocorneal abnormalities, or had undergone retinal surgery with silicon oil. A BGI implant of 250 mm2 was used for patients with small eye orbits, conjunctival fibrosis, and scleral bands, while all others received a 350-mm2 BGI.
Data collection
Preoperatively, a complete medical record was obtained from all patients, including the number of glaucoma medications and ophthalmic examinations, visual acuity (VA) by Snellen testing, anterior and posterior slit-lamp biomicroscopy evaluations, and IOP measurements using a Goldman aplanation tonometer. Postoperative visits were conducted at 1 day, 1 week, and 1, 2, 3, and 6 months postoperatively. The primary outcome measures were the mean IOP and number of glaucoma medications at each visit; complete success was defined as an IOP \(\ge\) 5 mmHg and ≤ 21 mmHg prior to ligature rupture, with the main objective of early reduction of IOP in cases of uncontrolled glaucoma.
Data Synthesis and analysis
Statistical results were considered significant for p values under 0.05. Analyses were carried out in R statistical software, version 3.4.3 (https://www.r-project.org/).
Modified surgical technique
All BGI surgeries were performed under sedation and by the same surgeon (J.C.I). Before the surgery began, the tube was ligated 2 mm anterior to the plate with a Vicryl 7–0 suture (rupture usually after 4–6 weeks) and checked for complete occlusion. Vicryl 8.0 traction to the superior peripheral cornea at 50% thickness was performed, fornix-based peritomy 4 clock hours of superotemporal quadrant enough to expose lateral borders of superior and lateral rectus muscles with posterior dissection under Tenon’s capsule and completed with Weck-Cel sponge. Muscle hooks were used to isolate the superior and lateral rectus muscles and the implant was placed superotemporally under both, while being kept fairly mobile in all but anterior directions. The implant was sutured to the sclera 10–12 mm posterior to the limbus with a silk suture.
Before entering the anterior chamber, a paracentesis was performed with a 15-degree knife (Ophthalmic Knife, Alcon, Texas, USA), and viscoelastic (Healon GV® OVD, Abbott Medical Optics Inc., CA, USA) was injected into the anterior chamber.
A needle track with a 23-gauge bevel down 3 mm from the corneoscleral limbus was created, and the tube was cut to lie 2 mm into the anterior chamber, parallel to the iris plane and away from the cornea. Three double perforated cuts were made in the tube with a spatulated needle, and fenestrations were made with a Vicryl 7–0 suture, 1–3 mm posterior to the scleral needle track (Fig. 1a and b). The conjunctiva was finally sutured with Vicryl 7–0 (Fig. 2a and b).
All patients received a postoperative treatment consisting of tobramycin at a dose of 3 mg/ml, dexamethasone in the form of 1 mg/ml (Trazidex, Sophia, México) drops every 4 h for 7 days, atropine at a dose of 1 mg (Isopto Atropina al 1%, Alcon, Colombia) every 12 h for 7 seven days, and nepafenac (Nevanac, Alcon, Belgium) in suspension at a dose of 1 mg/ml every 6 h for 30 days.
Results
All 42 eyes from 42 patients who underwent the modified technique with occlusion and fenestrations of the tube were followed up for 6 months. This included a total of 16 females (38.1%) and 26 males (61.9%). The mean age was 42 ± 24.15 years old, and 37 (88%) eyes had had a BGI 350 mm2 model and 5 (11.9%) had had a BGI 250 mm2 model.
The most common diagnoses included 24 (57.1%) cases of secondary glaucoma, 11 (26.2%) cases of chronic open-angle glaucoma, and 5 (11.9%) cases of neovascular glaucoma. A total of 21 (50%) eyes had previously undergone cataract surgery (Table 1).
Intraocular pressure
The mean preoperative IOP was 34.2 ± 11.2 mmHg, and the mean postoperative IOP was 15.1 ± 8.8 mmHg at 1 day, 15.3 ± 9.4 mmHg at 1 week, 17.7 ± 7.1 at 1 month, 12.9 ± 4.5 at 2 months, and 12.3 ± 4.0 mm Hg at 6 months (64% reduction, p < 0.001). The rate of complete success in the first month was 69% and 95.2% at 6 months (Table 2).
Glaucoma medications
The median number of preoperative glaucoma medications was 4, and the median number of postoperative glaucoma medications at 6 months was 3. Baseline values were reduced by 42.8% (P = < 0.001). A total of 8 (21.42%) eyes did not need any glaucoma medication at 6 months (Table 3).
Before surgery, all patients were under medical treatment with a topical drops of triple medication mixed (brimonidine 0,2%, timolol 0,5%, dorzolamide 2%) plus another medication as needed (travoprost 0.004%). Adverse effects, patient preferences, and cost of the medication were considered in the selection of medical treatment. Postoperatively, a topical drop of triple medication mixed (brimonidine 0,2%, timolol 0,5%, dorzolamide2%) was maintained if necessary.
Visual outcomes
The preoperative uncorrected visual acuity (UCVA) values showed an improvement from 1.46 to 1.34 LogMAR (p = 0.51) at 6 months postoperatively, and the best-corrected visual acuity (BCVA) values progressed from 1.17 to 1.14 LogMAR (p = 0.21). Even though there were improvements in visual acuity, the change was not statistically significant.
Baerveldt model types
Additional analyses were carried out on the different Baerveldt types. A total of 5 eyes had 250-mm2 implants and 37 eyes had 350-mm2 implants; there were no statistically significant differences in terms of the 1 week, 1 month, 6-month IOP values, and best-corrected visual acuity values (p = 0.32, p = 0.41, p = 0.28, respectively).
Postoperative complications
In the 6 months after surgery, six patients (14.2%) experienced complications. All complications arose 1 month after surgery and consisted of the following: 3 (7.14%) eyes had a tube occlusion, of which 1 had not had previous surgery, 1 had had a previous penetrating keratoplasty, and another had had a previous phacoemulsification surgery. All successfully underwent laser treatment. Other complications included focal choroidal hemorrhage, confirmed by an ultrasound scan in 1 (2.38%) eye, which resolved spontaneously with medical treatment. One eye (2.38%) experienced an anterior displacement of the tube, which needed to be cut through by a clear corneal incision, and 1 eye (2.38%) experienced a corneal decompensation that needed Descemet membrane endothelial keratoplasty (DMEK).
Discussion
This study documents the use of a modified surgical technique along with its safety profile, allowing for the better control of intraocular pressures in early postoperative periods, all the while allowing for ligature rupture to occur and the plate to be encapsulated, which is particularly important in patients with advanced glaucoma that warrants intraocular pressure lowering [3, 13, 14]. The use of a Vicryl suture through the fenestrations in the tube of nonrestrictive implants enables more control and sustains the aqueous flow, enabling more predictable outcomes and, apparently, fewer complications [3, 15,16,17]. Several methods have been described to limit the aqueous flow and prevent hypotony. A Molteno was used in the context of a two-stage insertion drainage implant in 1979. Rose EG et al. described an occlusive ligation and a venting stab into the drainage tube, which appeared to be the best design in their study [18]. Kee C et al. compared an external partial ligature in an Ahmed glaucoma valve with the absence of any ligature and found that a partial ligature was associated with a lower rate of hypotony and complications with no significant differences in terms of final success rates [19]. Valimaki J et al. used fibrin glue and absorbable ligatures to reduce peritubular filtration and prevent immediate postoperative hypotony with a single-plate Molteno [20]. El-sayyad F et al. described the use of a releasable suture in a Molteno glaucoma implant, with 18 of 19 patients having a normal anterior chamber depth [21]. GeoFFrey T et al. compared tube fenestrations with the absence of any fenestrations in patients with refractory glaucoma with BGI and found lower intraocular pressures in the fenestrations group at 1 day and 1 week, with no difference at 1 year [2].
The fenestrations will stop aqueous flow. According to the Poiseuille law, in the 4 weeks during which the ligature opens up by biodegradation, the tube light radius increases and resistance to flow is decreased, allowing for the aqueous flow to exit out through the plate and capsule, causing the aqueous flow through the anterior fenestrations to slow down or stop [11]. Another important issue described in the tube fenestration remodeling and closure is the elasticity of the silicon tube, which facilitates the collapse of the fenestration. With Vicryl sutures inside and through the fenestrations, flow filtration is controlled, and accordingly, no hypotony was detected in our study.
Evan J. Allan et al. compared the long-term efficacy of Baerveldt 250-mm2 to 350-mm2 implants in 89 eyes for 40 months, showing no differences in terms of surgical success, visual acuity, intraocular pressure, or the number of glaucoma medications [15]. Our study using 250-mm2 and 350-mm2 implants revealed similar results [8, 22].
Shiming Wang et al.’s [1] meta-analysis, comparing the efficacy of the Ahmed valve to the Baerveldt implant with ligature and fenestrations, carried out at the surgeon’s discretion for the treatment of refractory glaucoma, suggests that the latter had higher success rates and reduced the need for glaucoma medications, without incurring any differences in IOP reduction [1]. Panos G.Chirstakis et al.’s study either used a polyglactin ligation or a rip cord, depending on the surgeon’s preference, and tube fenestrations, at the surgeon’s discretion, finding in the BGI group a 58% reduction in IOP and a 55% reduction in the use of glaucoma medications at 5 years [3]. In contrast, in our study, we found a 64% reduction in IOP and a 42.8% reduction in the number of glaucoma medications at 6 months.
In the tube versus trabeculectomy (TVT) study [23, 24], after 5 years, the BGI group was found to have higher success rates, despite a similar IOP reduction in both groups. The failure rate was 5%, compared to 9%. With the lower follow-up period in our study (6 months) and no control group, we found a 95.2% success rate [5, 6, 25, 26].
The Ahmed versus Baerveldt (ABC) study [11] showed that the mean postoperative use of glaucoma medications was lower for the BGI group (65% reduction), with 50% of patients not requiring any medication at 3 years [3, 5, 7, 25]. This study showed a 41.2% reduction in the number of glaucoma medications. The lower rate found in our study may have been related to the presence of more severe glaucomas, as 57.1% had secondary glaucomas compared to the ABC study in which 50% had open-angle glaucomas.
Potential limitations of our study included its retrospective nature, the fact that it did not include any control group to compare outcomes with, and its short period follow-up.
Our study based on the Baerveldt modified surgery technique implant shows that small changes in the surgical procedure can make significant differences in clinical outcomes [9, 10]. We found no cases of hypotony at the conclusion of the study [6, 15]. More studies need to be carried out with this technique using different types of valveless implants.
Conclusion
A Baerveldt drainage implant, implanted by a modified surgical technique, with fenestrations and Vicryl piercings, is a safe, efficient and robust method for the control of early postoperative IOP, allowing for the successful control of IOP at 6 months.
Data availability
The datasets used and analyzed during this study are available via the corresponding author at reasonable request.
References
Wang S, Gao X, Qian N (2016) The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma: a meta-analysis. BMC Ophthalmol 8(16):83
Emerick GT, Gedde SJ, Budenz DL (2002) Tube fenestrations in Baerveldt Glaucoma Implant surgery: 1-year results compared with standard implant surgery. J Glaucoma 11(4):340–346
Christakis PG, Tsai JC, Kalenak JW, Zurakowski D, Cantor LB, Kammer JA et al (2013) The Ahmed versus Baerveldt study: three-year treatment outcomes. Ophthalmology 120(11):2232–2240
Budenz DL, Feuer WJ, Barton K, Schiffman J, Costa VP, Godfrey DG et al (2016) Ahmed Baerveldt comparison study group. Postoperative complications in the Ahmed Baerveldt comparison study during five years of follow-up. Am J Ophthalmol 163:75–82
Poels MMF, Niessen AGJE, de Waard PWT, Lemij HG (2013) Surgical outcomes of the Baerveldt glaucoma implant: differences between surgical techniques in the Rotterdam eye hospital. J Glaucoma 22(5):363–368
Zuo W, Lesk MR (2018) Surgical outcome of replacing a failed Ahmed glaucoma valve by a Baerveldt glaucoma implant in the same quadrant in refractory glaucoma. J Glaucoma 27:421–428
Tseng VL, Coleman AL, Chang MY, Caprioli J (2017) Aqueous shunts for glaucoma. Cochrane Database Syst Rev 28(7):CD004918
El Gendy NMS, Song JC (2012) Long term comparison between single stage Baerveldt and Ahmed glaucoma implants in pediatric glaucoma. Saudi J Ophthalmol 26(3):323–326
Wang J, Barton K (2017) Aqueous shunt implantation in glaucoma. Taiwan J Ophthalmol 7(3):130–137
Akil H, Vu PQ, Nguyen AH, Nugent A, Chopra V, Francis BA et al (2017) Reversible venting stitch for fenestrating valve-less glaucoma shunts. J Glaucoma 26(12):1081–1085
Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP et al (2015) Ahmed Baerveldt comparison study group. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology 122(2):308–16
Pakravan M, Yazdani S, Shahabi C, Yaseri M (2009) Superior versus inferior Ahmed glaucoma valve implantation. Ophthalmology 116(2):208–213
Elhefney E, Mokbel T, Abou Samra W, Kishk H, Mohsen T, El-Kannishy A (2018) Long-term results of Ahmed glaucoma valve implantation in Egyptian population. Int J Ophthalmol 11(3):416–421
Alasbali T, Alghamdi AA, Khandekar R (2015) Outcomes of Ahmed valve surgery for refractory glaucoma in Dhahran. Saudi Arab Int J Ophthalmol 8(3):560–564
Allan EJ, Khaimi MA, Jones JM, Ding K, Skuta GL (2015) Long-term efficacy of the Baerveldt 250 mm2 compared with the Baerveldt 350 mm2 implant. Ophthalmology 122(3):486–493
Tan AN, De Witte PM, Webers CAB, Berendschot TTJM, De Brabander J, Schouten JSAG et al (2014) Baerveldt drainage tube motility in the anterior chamber. Eur J Ophthalmol 24(3):364–370
Kawamorita S, Hamanaka T, Sakurai T (2014) The early postoperative complications of two different tube ligation methods in Baerveldt implant surgery. J Curr Glaucoma Pract 8(3):96–100
Rose GE, Lavin MJ, Hitchings RA (1989) Silicone tubes in glaucoma surgery: the effect of technical modifications on early postoperative intraocular pressures and complications. Eye (Lond) 3(Pt 5):553–561
Kee C (2001) Prevention of early postoperative hypotony by partial ligation of silicone tube in Ahmed glaucoma valve implantation. J Glaucoma 10(6):466–469
Välimäki J (2006) Fibrin glue for preventing immediate postoperative hypotony following glaucoma drainage implant surgery. Acta Ophthalmol Scand 84(3):372–374
El-Sayyad F, El-Maghraby A, Helal M, Amayem A (1991) The use of releasable sutures in Molteno glaucoma implant procedures to reduce postoperative hypotony. Ophthalmic Surg 22(2):82–4
Tai AX, Song JC (2014) Surgical outcomes of Baerveldt implants in pediatric glaucoma patients. J AAPOS 18(6):550–553
Barton K, Gedde SJ, Budenz DL, Feuer WJ, Schiffman J, Ahmed Baerveldt Comparison Study Group (2011) The Ahmed Baerveldt comparison study methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology 118(3):435–42
Panarelli JF, Banitt MR, Gedde SJ, Shi W, Schiffman JC, Feuer WJ (2016) A retrospective comparison of primary Baerveldt implantation versus trabeculectomy with mitomycin C. Ophthalmology 123(4):789–795
Rockwood EJ (2016) The Ahmed Baerveldt comparison (ABC) study: Long-term results, successes, failures, and complications. Am J Ophthalmol 163:xii–xiv
Rittenbach TL (2014) Proptosis from a Baerveldt tube shunt implant. Optom Vis Sci 91(6):e145-148
Acknowledgements
We thank Jose Chauca, PhD, for helping with the statistical analysis and providing technical support.
Funding
No funding or grant support.
Author information
Authors and Affiliations
Contributions
JC.I drafted the work, revised it critically, interpreted the data, performed conception and study design, and approved the final version to be published. J.M, N.A, and B.R drafted the work, revised it critically, interpreted the data, performed conception and study design.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no competing interests: JCI; LCR; JMS; NA; BRL.
Ethical approval
The study was approved by the Ethics Committee of the Instituto de Ojos Oftalmosalud. All research was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent forms prior to enrolment. This report does not contain any personal information that could lead to the identification of the patient. We do not require any administrative permissions and/or licenses to access the data used in our research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izquierdo-villavicencio, J.C., Rubio-Lastra, B., Mejías-Smith, J.A. et al. Primary outcomes of Baerveldt glaucoma implants with a modified technique to control intraocular pressure in different cases of glaucoma. Int Ophthalmol 41, 2547–2554 (2021). https://doi.org/10.1007/s10792-021-01813-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01813-1