Abstract
Purpose
To evaluate success rates in controlling intraocular pressure (IOP) after implantation of a second glaucoma drainage device (GDD) with a Baerveldt glaucoma implant in patients with refractory glaucoma, with a secondary aim of reducing the need for postoperative glaucoma medications.
Material and methods
This retrospective, noncomparative, interventional study included patients undergoing a second GDD for uncontrolled glaucoma from a tertiary care glaucoma service. Data were obtained from the medical records for the preoperative period and after the 1st, 15th, and 30th day, 3, 6, and 12 months, and then yearly until the last postoperative visit. Visual acuity, IOP, and number of glaucoma medications (NGM) from the follow-up visits were compared to baseline. Success and failure criteria were analyzed based on IOP level or need of glaucoma medications.
Results
Forty-nine patients were studied, with a mean follow-up time of 25 ± 21 months. The mean preoperative IOP was 23.7 ± 8.2 mmHg, and decreased to 14.8 ± 4.0 mmHg after 1 year, 14.4 ± 3.9 mmHg after 2 years, and 16.6 ± 8.5 mmHg after 3 years. The mean preoperative NGM was 3.4 ± 1.3, and decreased to 2.0 ± 1.8 after 1 year, 2.5 ± 1.6 after 2 years, and 2.8 ± 2.0 after 3 years. Absolute success was 9% after 1 year for a postoperative IOP between 5 and 18 mmHg, and 76% for a postoperative IOP between 5 and 21 mmHg. The qualified success was 88% at the first and second years and 83% at the third year.
Conclusion
With up to 3 years of follow-up, a second glaucoma drainage device was successful in reducing IOP to below 21 mmHg, but not as successful below 18 mmHg. The success rate is improved with the use of glaucoma medications with up to 3 years of follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concept of the glaucoma shunt device with a plate placed at the equator and away from the limbus was introduced by Molteno almost four decades ago [1–3]. This became the prevalent design of glaucoma drainage devices (GDD), with subsequent models from different companies. GDDs are normally indicated for the management of refractory glaucomas after a previous filtering surgery has failed [4–6]. However, their use has expanded to include primary surgery in patients with conjunctival scarring or with a high risk for trabeculectomy failure [4].
A number of studies have reported intraocular pressure (IOP) control and a decrease in the number of glaucoma medications after the implantation of GDDs [4, 7–12]. The advantages of a GDD are the possibility of implantation regardless of the state of the conjunctiva, previous failed surgeries or quadrant of the eye and also the fact that they are easily combined with other procedures without major effects on their efficacy [5, 6, 10, 13–18].
However, GDDs can fail to control glaucoma, and after a period of time may have their IOP-lowering effect decreased due to further scarring and thickening of the fibrous capsule around the plate [7, 19–22]. A second shunt implantation is a commonly entertained option for these challenging cases [23–27]. Several studies have reported the results of a second GDD, with variable success. However, it is unclear from prior studies as to the success of attaining certain IOP goals, and also if success is absolute (no glaucoma medications) or qualified (with the use of glaucoma medications).
The goal of this study was to evaluate the success rates of a second GDD in patients with uncontrolled glaucoma in the presence of a functional first GDD. In addition, we considered success rates depending on the level of IOP attained and whether there was a continued need for glaucoma medications.
Methods
This is a retrospective, non-randomized, medical chart review study, comprising consecutive patients with an existing glaucoma shunt, receiving the second shunt due to failure of the first shunt (IOP level incompatible with maintenance of optic nerve health despite maximum tolerated medication).
Records were obtained from the Doheny Eye Institute, Keck School of Medicine, University of Southern California (Los Angeles, CA, USA) after approval by the Los Angeles County/ University of Southern California Medical Center Institutional Review Board. Since this was a retrospective study, the requirement for informed consent was waived. The study was in compliance with the Health Insurance Protection and Portability Act (HIPPA) and the Declaration of Helsinki for research on human subjects.
All of the evaluations and surgeries were performed at the Doheny Eye Institute (DEI) by trained glaucoma specialists. The implants used were the Baerveldt implant 250 or 350 (Abbott Medical Optics, Santa Ana, CA, USA).
The surgical procedure consisted of a limbal conjuctival incision (one quadrant), and placement and suturing of the plate at 8–10 mm posterior to the limbus and posterior to the rectus muscle insertions. A 23-gauge needle was used to enter the anterior chamber near the limbus, and the tube inserted into the anterior chamber. The tube was covered with pericardium or sclera donor graft tissue, and the conjunctiva sutured.
All eyes with minimum of 6 months follow-up after the implantation of the second device were included in the study. One eye was randomly selected per patient if both met the inclusion criteria. The review consisted of gathering data from the preoperative visit when the surgery was planned, and follow-up data from the 1st, 15th and 30th day, 3, 6, and 12 months, and then yearly until the last visit.
Demographic data such as age, gender, and ethnicity were obtained. Preoperative data (baseline) consisted of diagnosis, visual acuity, Goldmann IOP, number of glaucoma medications, cup/disc ratio. When available, visual field mean deviation (MD) and pattern standard deviation (PSD) were described. In general, patients with visual acuity worse than 20/100 did not have visual field data. Postoperatively, visual acuity, IOP, and number of medications were compared to baseline.
Five definitions of surgical success were analyzed to assess IOP control. Criteria one and two defined success as a postoperative IOP between 5 and 18 mmHg or between 5 and 21 mmHg respectively, without the use of glaucoma medications. Criteria three and four were defined by IOP between 5 and 18 or between 5 and 21 mmHg respectively, with the use of medications. The last criteria, the “qualified success”, defined by IOP between 5 and 21 mmHg controlled with or without medications.
Failure was defined as an IOP less than 5 mmHg or more than 21 mmHg on two consecutive readings, less than 20% reduction of IOP from baseline, loss of light perception secondary to glaucoma, phthisis bulbi, additional glaucoma surgery, or a combination thereof. Hypotony was defined as an IOP of less than 6 mmHg on two consecutive measurements after the surgery, only if hypotony, maculopathy, and/or lens/IOL corneal touch were present.
Paired test was used to evaluate the IOP variation, and signed rank sum test for the variation in the glaucoma medications, between baseline and follow-ups. P values of less than 0.05 were considered statistically significant.
Visual acuity progression was evaluated based on the visual acuity conversion chart published by Holladay [28].
The Kaplan–Meier life-table (survival) analysis was used to determine cumulative success rates at specified time periods, based on the surgical success definitions and failure exclusion. SAS V9.2 (SAS Inst., Cary, NC, USA) programming language was used for all analyses.
Results
We reviewed the charts of 68 patients who had received a second GDD over the period 2006 to 2010. After eliminating those with inadequate follow-up, we examined 49 patients, age 60.6 ± 15.6 (mean ± SD), 20 females (40.8%) and 29 males (59.2%) (Table 1). In cases where both eyes met criteria, we randomly chose one eye per patient. Caucasian (51%) and Hispanic (26.5%) were the most prevalent ethnicities in the population studied.
The mean follow-up after the second GDD surgery was of 25 ± 21 months (range 6–72). The initial cohort of eyes were 30 with 1 year of follow-up, 20 with 2 years of follow-up, and 15 with 3 years of follow-up.
The most prevalent diagnosis was primary open-angle glaucoma (POAG), comprising 36.7% of all cases, followed by chronic angle-closure glaucoma (CACG) and inflammatory glaucoma, with 18.4%. All other diagnoses are represented with their respective percentage in Table 1. Prior surgeries, including previous glaucoma procedures, are listed in Table 2.
The cup/disc (C/D) ratio was evaluable in 40 patients and the baseline mean was 0.95 ± 0.66. Visual field (n= 23), mean deviation was −17.23 ± 8.68 with a pattern standard deviation of 6.75 ± 3.07.
The mean preoperative IOP was 23.7 ± 8.2 mmHg, with the use of 3.4 ± 1.3 glaucoma medications (range 1–5) (Table 3). In the post-op follow-up, IOP was 14.5 ± 5.7 mmHg after 3 months, 15.0 ± 6.5 mmHg after 6 months, 14.8 ± 4.0 mmHg after 1 year, 14.4 ± 3.9 mmHg after 2 years, and 16.6 ± 8.5 mmHg after 3 years. The percentage IOP reduction compared with the preoperative IOP was 38.8 ± 13.0% after 3 months, 36.7 ± 20.6% after 6 months, 37.6 ± 60.7% (p < 0.05) after 1 year, 39.2 ± 27.8% (p= 0.001) after 2 years, and 30.0 ± 33.4% (p < 0.01) after 3 years (Table 3).
The mean preoperative number of glaucoma medications was 3.4 ± 1.3, and decreased to 2.0 ± 1.8 after 1 year, 2.5 ± 1.6 after 2 years, and 2.8 ± 2.0 after 3 years.
The preoperative visual acuity ranged from 20/25 to no light perception (LP), with a mean of 20/300. The mean visual acuity at 3 months was 20/200 (range 20/25–LP), at 6 months 20/250 (range 20/25–LP), at 1 year counting fingers (CF) (range 20/20–LP), at 2 years CF (range 20/20–LP), and at 3 years CF [(range 20/20–hand motion (HM)].
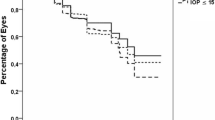
The cumulative success rate varied with the different criteria adopted (Table 4). Using the most stringent criteria of absolute success (1: IOP ≤ 18 mmHg without glaucoma medications), the success at 1, 2 , and 3 years is 9%, 0%, and 0% respectively. Using the most lenient criteria of qualified success (5: IOP ≤ 21 mmHg with or without medications), the success at 1, 2, and 3 years is 88%, 88%, and 82% respectively. The Kaplan–Meier plot is illustrated in Fig. 1.
Kaplan–Meier illustration plot for second shunt survival graph. Def 1: The success as a postoperative IOP between 5 and 18 mmHg without the use of glaucoma medications. Def 2: The success as a postoperative IOP between 5 and 21 mmHg without the use of glaucoma medications. Def 3: The success as a postoperative IOP between 5 and 18 with or without the use of medications. Def 4: The success as a postoperative IOP between 5 and 21 mmHg with the use of medications. Def 5: The “qualified success”, as a postoperative IOP between 5 and 21 mmHg controlled with or without medications.
The safety and complication data is contained within Table 5. High IOP was the most frequent postoperative complication in the first day, and at the first, third, and sixth months, present in 57% of our subjects in the first day. At 12 months, 13% of the eyes had corneal edema (Table 5).
Discussion
The results of this study agree with previous publications regarding the effectiveness of second GDD [23–27]. However, when comparing the results for different criteria that included or excluded the use of glaucoma medication, it became notable that in this series almost all patients required additional medical treatment to maintain IOP at acceptable levels. This finding indicates that the second GDD alone is not sufficient to provide glaucoma control. Surgeons should consider that these patients have a strong possibility of requiring glaucoma medications at some point of the postoperative period.
Several studies have reported the results of second GDD. Shah et al. [24] showed that after a failed tube shunt surgery, an additional tube shunt offers better IOP control than surgical revision by excision of an encapsulated bleb. They reported 42% of qualified success (25% IOP reduction with or without medication) in surgically revised patients, versus 62% in second GDD cases after a mean follow-up of 25.5 months. Burgoyne et al. [23] described that a second tube shunt did not cause “higher-than-expected complication rates” and provided satisfactory IOP control, but ten of 22 patients had corneal endothelial complications. Godfrey et al. [25] identified 18 patients who had a second GDD and reported 89%, 83%, 63%, and 37% surgical success at 6 months, 1 year, 2 years and 3 years respectively, with a mean follow-up of 19.6 ± 13.6 months. They considered success as an IOP below 21 mmHg regardless of the use of glaucoma medications, and at least 20% reduction in IOP without the need of additional procedures. Anand et al. [27] studied 43 eyes with mean follow-up of 32.6 ± 21.6 months. They found success rates of 93%, 89%, and 83% at 1, 2, and 3 years respectively (success was defined as IOP <21 mmHg with at least 25% IOP reduction with no prolonged hypotony) without any increase in number of medications or visual loss. When they considered a maximum IOP of 17 mmHg, success rates were 83% at 1 year, and 75% at 2 and 3 years.
In our study, the cumulative success rate can vary depending on which criterion is used. Using more strict criteria, such as IOP < 18 mmHg with no medications, the success rate is considered low, going from 18.2% in the first 6 months, 9.1% at 1 year, and zero in the following years. On the other hand, if the target IOP is 21 mmHg, with or without medications, the success rates can increase up to 82.7%, even 3 years after the surgery. Previous studies report success rates up of 73.7% when using this last criterion [26]. As with our study, these were all retrospective analyses with limited numbers.
The number of medications used increased in the first year of follow-up, and then decreased after that. Nevertheless, the second shunt in our study was associated with lower IOP in the follow-ups. The IOP decrease ranged from 36% at 6 months to 33% at the second year. The patients undergoing a second implant in this study had very advanced glaucoma, with a very advanced C/D ratio and visual field damage. Despite achieving lower IOP with this second intervention, the mean visual acuity decreased in the follow-up period.
Previous studies have described corneal decompensation as the most significant complication following sequential tubes. Earlier series report a 25% to 45% incidence of corneal decompensation following second aqueous shunts [25, 29–31]. Considering the number of previous surgical procedures, laser procedures, and poor IOP control, it is not surprising that a substantial number of corneas decompensated. In our study, cornea decompensation was also the most prevalent complication following a second GDD. Interestingly, the highest incidence in our study was seen at 12 months and then decreased. This could be explained by the use of hyperosmolar drops or be due to loss to follow-up.
The limitations of this study are its retrospective design and possible selection bias. The treating surgeons may have chosen patients with better prognosis for a second GDD rather than other procedures such as transscleral cyclophotocoagulation. The subjects that were excluded due to insufficient follow up may have not returned due to poor results. Finally, the complications were based on observations in the medical record but were not necessarily looked for at each visit.
In conclusion, a second GDD procedure can help control refractory glaucoma without hypotony and phthisis over the long term in highly complicated glaucomatous eyes. Although corneal decompensation and vision loss occured in this study, a sequential tube shunt procedure can be considered a viable approach for refractory glaucoma when an initial tube shunt has failed to control IOP. Patients should be counseled about the risk of corneal decompensation and vision loss. According to our results, surgeons should expect that glaucoma medications will be required even after a second GDD.
References
Lieberman MF, Ewing RH (1990) Drainage implant surgery for refractory glaucoma. Int Ophthalmol Clin 30:198–208
Molteno AC, Straughan JL, Ancker E (1976) Long tube implants in the management of glaucoma. S Afr Med J 50:1062–1066
Molteno AC, Van Biljon G, Ancker E (1979) Two-stage insertion of glaucoma drainage implants. Trans Ophthalmol Soc N Z 31:17–26
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube versus Trabectome study group (2012) Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 153:789–803
Minckler DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD, Singh K (2008) Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology 115:1089–1098
Patel S, Pasquale LR (2010) Glaucoma drainage devices: a review of the past, present, and future. Semin Ophthalmol 25:265–270
Tran DH, Souza C, Ang MJ, Loman J, Law SK, Coleman AL, Capriolli J (2009) Comparison of long-term surgical success of Ahmed Valve implant versus trabeculectomy in open-angle glaucoma. Br J Ophthalmol 93:1504–1509
Nassiri N, Kamali G, Rahnavardi M, Mohammadi B, Nassiri S, Rahmani L, Nassiri N (2010) Ahmed glaucoma valve and single-plate Molteno implants in treatment of refractory glaucoma: a comparative study. Am J Ophthalmol 149:893–902
Christakis PG, Tsai JC, Kalenak JW, Zurakowski D, Cantor LB, Kammer JA (2011) The Ahmed Versus Baerveldt study: one-year treatment outcomes. Ophthalmology 118:2180–2189
Aref AA, Gedde SJ, Budenz DL (2012) Glaucoma drainage implant surgery. Dev Ophthalmol 50:37–47
Poels MM, Niessen AG, de Waard PW, Lemij HG (2013) Surgical outcomes of the Baerveldt glaucoma implant: differences between surgical techniques in the Rotterdam Eye Hospital. J Glaucoma 22:363–368
Teixeira SH, Doi LM, Freitas Silva AL, Silva KD, Paes ÂT, Higa FS, Mendonça M, Prata JA, Paranhos A (2012) Silicone Ahmed glaucoma valve with and without intravitreal triamcinolone acetonide for neovascular glaucoma: randomized clinical trial. J Glaucoma 21:342–348
Assaad MH, Baerveldt G, Rockwood EJ (1999) Glaucoma drainage devices: pros and cons. Curr Opin Ophthalmol 10:147–153
Budenz DL, Scott IU, Nguyen QH, Feuer W, Singh K, Nicolela MT, Bueche M, Palmberg PF (2002) Combined Baerveldt glaucoma drainage implant and trabeculectomy with mitomycin C for refractory glaucoma. J Glaucoma 11:439–445
Hoffman KB, Feldman RM, Budenz DL, Gedde SJ, Chacra GA, Schiffman JC (2002) Combined cataract extraction and Baerveldt glaucoma drainage implant: indications and outcomes. Ophthalmology 109:1916–1920
Minckler DS, Vedula SS, Li TJ, Mathew MC, Ayyala RS, Francis BA (2006) Aqueous shunts for glaucoma. Cochrane Database Syst Rev CD004918
Schwartz KS, Lee RK, Gedde SJ (2006) Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol 17:181–189
Yu DY, Morgan WH, Sun X, Su EN, Cringle SJ, Yu PK, House P, Guo W, Yu X (2009) The critical role of the conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res 28:303–328
Molteno AC, Bevin TH, Dempster AG, Sarris M, McCluskey P (2009) Otago Glaucoma Surgery Outcome Study: tissue matrix breakdown by apoptotic cells in capsules surrounding molteno implants. Invest Ophthalmol Vis Sci 50:1187–1197
Sarkisian SR Jr (2009) Tube shunt complications and their prevention. Curr Opin Ophthalmol 20:126–130
Budenz DL, Barton K, Feuer WJ (2011) Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology 118:443–452
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube versus Trabeculectomy study group (2012) Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol 153:804–814
Burgoyne JK, WuDunn D, Lakhani V, Cantor LB (2000) Outcomes of sequential tube shunts in complicated glaucoma. Ophthalmology 107:309–314
Shah AA, WuDunn D, Cantor LB (2000) Shunt revision versus additional tube shunt implantation after failed tube shunt surgery in refractory glaucoma. Am J Ophthalmol 129:455–460
Godfrey DG, Krishna R, Greenfield DS, Budenz DL, Gedde SJ, Scott IU (2002) Implantation of second glaucoma drainage devices after failure of primary devices. Ophthalmic Surg Lasers 33:37–43
Smith M, Buys YM, Trope GE (2009) Second Ahmed valve insertion in the same eye. J Glaucoma 18:336–340
Anand A, Tello C, Sidoti PA, Ritch R, Liebmann JM (2010) Sequential glaucoma implants in refractory glaucoma. Am J Ophthalmol 149:95–101
Holladay JT (2004) Visual acuity measurements. J Cataract Refract Surg 30:287–290
Al-Torbak A (2003) Graft survival and glaucoma outcome after simultaneous penetrating keratoplasty and ahmed glaucoma valve implant. Cornea 22:194–197
Lee EK, Yun YJ, Lee JE, Yim JH, Kim CS (2009) Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol 148:361–367
Nassiri N, Nassiri N, Majdi-N M, Salehi M, Panahi N, Djalilian AR, Peyman GA (2011) Corneal endothelial cell changes after Ahmed valve and Molteno glaucoma implants. Ophthalmic Surg Lasers Imaging 42:394–399
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Francis, B.A., Fernandes, R.A.B., Akil, H. et al. Implantation of a second glaucoma drainage device. Graefes Arch Clin Exp Ophthalmol 255, 1019–1025 (2017). https://doi.org/10.1007/s00417-017-3596-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3596-y