Abstract
Purpose
To investigate the structural and functional reconstruction of the macula after autologous neurosensory retinal-free flap transplantation (ANRFFT) in eyes with large refractory idiopathic macular holes (IMHs).
Methods
Patients with refractory IMHs after multiple surgeries who underwent ANRFFT were retrospectively reviewed. The main outcomes were anatomic closure of MH, change in external limiting membrane (ELM) defect on optical coherence tomography (OCT) and best-corrected visual acuity (BCVA).
Results
A total of 7 patients (4 female and 3 male; mean age 60.6 ± 8.6 years) were included in the study. Mean preoperative largest basal diameter was 1146.7 ± 413.7 µm (range, 653–1768 µm), and mean narrowest inner-opening diameter was 788.9 ± 148.8 µm (range, 644–1100 µm). Mean BCVA (logarithm of the minimum angle of resolution [logMAR]) significantly improved from 1.53 ± 0.16 (range, 1.3–1.7) to 0.89 ± 0.23 (range, 0.6–1.3) at the final follow-up (P < 0.001). OCT revealed complete closure of MH in all eyes. Mean preoperative ELM defect significantly decreased from 1450.3 ± 306.5 µm (range, 1044–1908 mm) to 533.1 ± 399.2 µm (range, 0–1156 µm, P = 0.001). Postoperative complications included retinal detachment (n = 1), cystoid macular edema like changes in the graft (n = 1) and reactive pigment epithelial hyperplasia (n = 1).
Conclusion
Although some postoperative complications did occur, ANRFFT seems to be an effective treatment for large refractory IMHs, and can promote recovery of the outer retinal structure resulting in functional improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macular hole (MH) is a full thickness defect of the retinal tissue in the center of the fovea, with a prevalence rate ranging from 0.02% to 0.8% in persons older than 40 years [1, 2]. Recently, vitreoretinal surgeons have been focusing on refining and developing novel surgical techniques for MH. Although a high anatomical success rate can be achieved for idiopathic macular holes (IMHs) with pars plana vitrectomy (PPV) and internal limiting membrane (ILM) peeling, treatment of large and/or refractory IMHs possesses a challenge [3, 4]. It has been reported that patients with failed primary surgery for idiopathic MH have both poorer anatomic and visual prognosis after the second procedure [4]. Therefore, in the past decade, a variety of modified surgical techniques have been described in the treatment of recurrent macular holes such as autologous anterior or posterior lens capsule flap [5], arcuate retinotomy on the MH margin [6], macular hole hydrodissection [7], retina expansion technique for macular hole apposition [8], ILM flap transplantation (inverted or free flap) [9, 10], adjuvant blood components [11] and amniotic membrane transplantation [12]. Recently, Grewal et al. [13] reported a novel method for refractory myopic MH repair, which involved an autologous neurosensory retinal-free flap transplantation (ANRFFT). After the initial report of ANRFFT for closure of refractory myopic MH repair, Multicenter International Collaborative Study Group recently reported that ANRFFT for refractory MHs resulted in anatomic closure in approximately 88% of cases along with improvement in vision by at least 0.3 logarithm of the minimum angle of resolution (logMAR) unit in more than 36% of eyes [14].
Herein, we evaluated the effectiveness of ANRFFT for the management of refractory large IMHs after unsuccessful multiple surgeries including extended ILM peeling, free ILM flap transplantation or retina expansion technique for macular hole apposition, in addition to failed prior vitrectomy with ILM peeling and gas tamponade procedure.
Methods
Medical records of all patients undergoing 25 or 27-gauge PPV combined with ANRFFT for the management of refractory large IMHs after failed multiple surgeries at Ulucanlar Eye Training and Research Hospital from February 2016 to May 2019 were reviewed in this study. The study protocol was approved by the Ethics Committee of Ankara Training and Research Hospital, Ankara, Turkey, and conducted in accordance with the tenets of the Declaration of Helsinki. All patients provided written informed consent.
All patients underwent complete ophthalmic examination with fundus photography and swept-source optical coherence tomography (Spectralis HRA OCT; Heidelberg Engineering, Heidelberg, German). Age, gender, laterality, follow-up duration and history of previous ocular surgery were also recorded for analysis.
Eyes with refractory IMHs treated with ANRFFT who had undergone at least two unsuccessful surgeries including other advanced surgical techniques such as extended ILM peeling, free ILM flap transplantation and retina expansion technique and had been followed up for at least 3 months after silicone oil extraction were included in the study. Regarding the pathologies of MH in this study, all eyes were classified as idiopathic MH and eyes with an existing ocular comorbidity such as myopia, glaucoma, diabetic retinopathy, trauma or macular degeneration were excluded.
Surgical techniques
All patients underwent a standard, 3-port, 25 or combined 25 and 27-gauge PPV (Constellation; Alcon Laboratories, Fort Worth, TX) with a 25-gauge chandelier endoilluminator to facilitate bimanual maneuvers, by the same surgeon (the author). Brilliant Blue G (Fluoron, Geuder, Germany) was applied to check the ILM remnants in the posterior pole. A neurosensory retina harvest site was selected in the mid-periphery, usually superior to the superotemporal arcade. A retinal detachment of 2–3 disk diameter area was created by using 41G subretinal injection needle, which was followed by endodiathermy to the blood vessels at the margin. The size of the harvest was selected according to the size of the MH which was approximately twice larger in diameter than the hole.
The edge of the graft was held using microforceps and cut using vertical scissors or 27 gauge cutter with 3D vertical scissor submode. The infusion was closed temporarily to prevent turbulent flow, and the graft was then gently moved toward the MH. The graft was carefully spread to cover the surface of the MH under perfluorocarbon liquid (PFCL), ensuring that photoreceptor layer of the graft faced toward the retinal pigment epithelium (RPE). Endolaser barricade was applied in a circular manner around the retinectomy site. Direct PFC–silicone oil (1000 centistokes) exchange was performed at the end of the surgery (Video, Supplemental Digital Content 1). All patients were positioned face down postoperatively for 1 week.
The primary outcome measure in this case series was anatomical closure of macular hole after ARNFFT, confirmed by OCT. Secondary outcomes were visual acuity (VA) improvement as logMAR, the percentage of patients achieving clinically significant gain in VA and restoration of the external limiting membrane (ELM) measured using OCT. Best-corrected visual acuity (BCVA) using a Snellen chart was converted to the logarithm of minimum angle of resolution (logMAR) for analysis purposes. Significant gain in VA was defined as an increase in VA from baseline of 0.3 ≥ logMAR unit. MH sizes and ELM measurements were recorded using one of the OCT scans where the hole presented with the largest diameter by measuring its aperture diameter from where the distance across the full-thickness defects was the largest or narrowest, its base diameter and its ELM defect diameter. The measurements were performed manually by using the in-built caliper system provided by the Spectralis Heidelberg software (DRI OCT-1 V.9.12.003.04 and Heidelberg Eye Explorer V.1.7.1.0).
Statistical analysis
Numerical data were summarized as mean ± standard deviation and range, whereas frequency and percentage were used for categorical data. The preoperative and postoperative data were compared using the paired sample t-test. All statistical analyses were performed using SPSS statistical software version 19.0 (IBM Corp., Armonk, NY, USA). A p value < 0.05 was considered statistically significant.
Results
Between 2016 and 2019, a total of 11 eyes of 11 patients underwent PPV combined with ANRFFT for the management of refractory large IMHs after failed multiple surgeries. Seven eyes of 7 patients (4 female, 3 male) who met the eligibility criteria were included in the study. Four eyes with an existing ocular comorbidity such as myopia, glaucoma or trauma history were excluded. The patients had a mean age of 60.6 ± 8.6 years (range, 48–74 years) and had undergone 2.42 ± 0.53 (range, 2–3) previous vitrectomies on average. Among the seven patients suffering from refractory IMHs after unsuccessful primary surgery (PPV combined with ILM peeling with gas tamponade), 3 underwent PPV with extended ILM peeling, 2 underwent PPV with free ILM flap transplantation, and 2 underwent PPV with retina expansion technique for macular hole apposition as a second or third surgical approach with unsatisfactory results. The transplanted retinal flaps covering the hole site were visible on fundus examination one day after ANRFFT in all eyes. Mean follow-up was 18.8 ± 9.6 months (range, 12–38 months). All eyes were pseudophakic. Figures 1, 2 and 3 demonstrate the clinical results of patients who underwent ANRFFT for large IMH after at least two failed previous vitrectomy surgeries. Preoperative and postoperative demographic and anatomic characteristics of patients are summarized in Table 1.
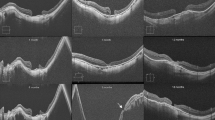
Preoperative and postoperative color fundus and SD-OCT images of a 59-year-old man who underwent ANRFFT. a Fundus photograph after PPV with ILM peeling and additional retina expansion technique for MH apposition shows persistent large MH. b Preoperative OCT scan shows a full thickness MH with ELM defect of 1044 μm (inset) c One month after ANRFFT, OCT scan showing closure of the MH with the graft, which is hyperreflective, and the boundary between the graft and the MH edge seen as hyporeflective cracks (yellow arrow) d Three months after ANRFFT, OCT scan showing integrated retinal graft with the surrounding retinal tissue and the boundary is not obvious e Three months after SO removal, cystic changes (yellow arrow) on OCT with partial restoration of ELM (inset) f OCTA showing no perfusion within the graft and a very limited vascularization along the margin of the graft at level of superficial capillary plexus (white arrowhead). g Cystoid spaces (yellow arrow) located at the graft clearly seen on the enface OCT image of the corresponding OCTA image. h Thirty-two months after SO removal, fundus photograph revealing the presence of a closed MH with retinal graft well located in situ (white dotted circle), and showing scar and laser spots (white arrow) at the superior site in which the graft retina was harvested
An eye with large refractory MH with 25 months follow-up after ANRFFT. a Despite 2 pars plana vitrectomies with ILM peeling and additional autologous ILM free flaps transplantation, OCT image shows the hole persisting with ELM defect of 1411 μm (inset). b Intraoperative fundus image showing the retinal graft harvest site (white arrow) and the retinal graft placed over the macular hole (black dotted circle). (c and d) 12 months after the SO removal, OCT showing integrated retinal graft with the surrounding retinal tissue with a partial restoration of ELM (inset). The hyperreflective and thick RPE line (yellow arrowheads) is corresponding to the area of pigment clumping secondary to pigment epithelial hyperplasia (black arrow) on the fundus photo. Fundus photograph exhibiting the retinal flap well located in situ (white dotted circle)
Preoperative and postoperative color fundus and SD-OCT images of a 65-year-old woman who underwent ANRFFT for the management of refractory MH after failed multiple surgeries including retina expansion technique for MH apposition. (a and b) Preoperative fundus photograph and OCT scan showing large persistent MH after failed previous ILM peeling. The length of the ELM defect is 1571 μm (inset) c OCT image showing persistent macular hole after applying retina expansion technique for MH apposition. A defect 1509 μm in diameter persists in the ELM (inset). d 1 month after ANRFFT, OCT showing the hyperreflective retinal flap visualized in place over the MH. Hyperreflective line (yellow arrows) presenting the boundary between the graft and the MH edge is obvious. (e and f) 10 months after the SO removal, OCT scan and fundus photograph showing retinal flap in situ (white dotted circle). The boundary between the graft and the surrounding tissue disappear on OCT scan. Total restoration of the ELM (yellow arrow) is seen with improvement of VA from 20/800 to 20/80
Silicone oil (SO) removal was performed 3 to 6 months after the surgery in all eyes. Retinal detachment occurred in one eye 1 month after SO removal. This eye underwent repeat vitrectomy with SO injection and was successfully reattached with complete anatomic closure of MH after SO extraction. At the final follow-up, there were no cases with recurrence of MH or retinal detachment after silicone oil extraction. The graft tissue was well located in situ and tightly adhered to surrounding retinal tissue in all eyes after the SO removal on fundus and OCT examination (Figs. 1, 2, 3).
The mean BCVA [logMAR] significantly improved from 1.53 ± 0.16 (range, 1.3–1.7) to 0.89 ± 0.23 (range, 0.6–1.3) at the final final-follow-up (t = 7.643, P < 0.001), with vision improving more than 0.3 logMAR unit in all eyes. Overall, the final visual acuity was higher than 20/200 in 57% (4/7) of eyes with a closed hole after ANRFFT.
Optical coherence tomography findings
Mean preoperative largest basal diameter was 1146.7 ± 413.7 µm (range, 653–1768 µm), mean preoperative widest inner-opening diameter was 1287.1 ± 271.5 µm (range, 980–1696 µm), and mean preoperative narrowest inner-opening diameter was 788.9 ± 148.8 µm (range, 644–1100 µm). The boundary between the graft and the MH edge could be clearly observed 1 month after ANRFFT as a hyporeflective or hyperreflective line (Figs. 1 and 3). At the final follow-up, these hyporeflective or hyperreflective lines between the graft and the original edge of the MH beginning to disappear approximately 3 months after surgery were not obvious or even disappeared in all eyes on OCT images, resulting in fusion with the surrounding retinal tissue (Figs. 1, 2, 3). Complete anatomic closure of MH on OCT was achieved in all eyes at the final follow-up (Figs. 1, 2, 3). While total restoration of ELM was observed in one eye, partial restoration of ELM was noted in others (Figs. 1, 2, 3). The average preoperative ELM defect was 1450.3 ± 306.5 µm (range, 1044–1908 mm), which decreased to 533.1 ± 399.2 µm (range, 0–1156 µm) at the final follow-up (t = 5.799, P = 0.001).
Cystic changes seen in the inner layers of the graft on OCT occurred in one eye 3 months after silicone oil removal (Fig. 1), which caused a visual acuity decrease from 20/80 to 20/125. Fluorescein angiography was performed and did not reveal any dye leakage. Optical coherence tomography angiography (OCTA) demonstrated limited vascularization of the graft at the level of superficial capillary plexus (Fig. 1). These cystic changes well documented on OCT “en face” imaging, which may be due to degeneration, did not respond to intravitreal anti-vascular endothelial growth factor and steroid treatments (Fig. 1). Although these cystic changes were persistent at the final follow-up, BCVA was still better than the preoperative BCVA in this case.
Complications
The most common intra-operative complication was bleeding from the harvest site during the separation of the graft (57.1%), which was easily controlled by raising the infusion pressure and diathermizing the offending bleeder vessels. Postoperative complications were RD (n = 1), cystoid macular edema (CME)-like cystic changes (n = 1) and significant reactive hyperplastic pigmentary changes (n = 1) (Figs. 1 and 2). While reactive pigment epithelial hyperplasia developed three months after surgery, the other complications occurred after silicon oil removal. Overall, a total of 3/7 eyes (42%) having postoperative complications related to the ANRFFT technique were noted.
Discussion
The treatment of an idiopathic macular hole could achieve a 90% closure rate or more with modern vitrectomy [15]. However, treatment of eyes with refractory MH after failed ILM peeling surgery became a challenging issue for anatomic and functional success [4]. In recent years, a variety of modified surgical techniques including extended ILM peeling [16], autologous free ILM flap transplantation [9] and lens capsular flap transplantation [5] have been described in the treatment of refractory macular holes. All these methods facilitate the closure of most MHs. Most of these techniques involve the same healing mechanism, in which membrane-like tissue provides a basement membrane enabling better proliferation of glial cells. Nevertheless, few patients still have persistent MH even after multiple surgeries. Furthermore, the tissue to be used as a flap is extremely important in terms of functional recovery. Therefore, ANRFFT becomes a reasonable approach for the repair of large refractory MH. In this study, ANRFFT was performed for refractory large IMHs in eyes that underwent prior failed multiple surgeries.
Surgical options for refractory MH after multiple surgeries are limited. Although impressive outcomes have been reported with autologous ILM flaps [9, 17, 18], harvesting suitable ILM free flap can be problematic in eyes that underwent previous extensive ILM peeling. Similar to ILM flap, autologous lens capsular flaps have been used with favorable outcomes [19]. However, harvesting the anterior lens capsule is practical only in patients undergoing combined cataract and MH surgery and is also applicable in pseudophakic eyes with an intact posterior capsule. Despite the optimism of the results of autologous free ILM flap and lens capsule techniques for refractory MHs, these approaches have their own several disadvantages and challenges. Firstly, both ILM and capsule free flaps tend to roll over and float into the vitreous cavity, and easily dislodge during operation or postoperative days. Secondly, a rolled segment of the peeled ILM or capsule other than single-layered transplanted flap is usually placed inside the MH, which often has to be mechanically positioned with the potential for iatrogenic trauma. Finally, they both act as a scaffold for proliferation of glial cells. Thereby, very limited restoration of ellipsoid zone (EZ) and ELM defects was observed in cases of refractory MHs after these surgical approaches [20].
In contrast to a lens capsule flap or an ILM flap, neurosensory retinal flap is a thicker, robust tissue, which can be positioned on the surface of the MH with a negligible iatrogenic trauma [13]. Using similar ANRFFT technique described by Grewal et al. [13] in the present study, anatomic closure and significant visual improvement were achieved in all seven eyes. OCT showed significant improvement in macular integrity with gradual recovery of the ELM defects in all eyes, with full restoration in one eye. It was also observed that the graft tissue remained in situ and tightly adhered to surrounding retinal tissue in all eyes at the final follow-up. Recently, Multicenter International Collaborative Study Group reported that the ANRFFT achieved a high degree of anatomical success with a closure rate of nearly 90% in eyes with refractory MH, and functional improvement with a rate of 53% in eyes with anatomic closure [14]. Although findings in the present study are consistent with those reported by the International Collaborative Study Group, a higher rate of functional improvement was observed in the present study. The limited improvement in vision observed in their study could be related to existence of high myopia and comorbid conditions. During follow-up, they also observed a prominent microstructural regeneration of the outer retina with improved restoration of EZ and ELM in the majority of eyes. Recently, the study by Rizzo et al. [12] demonstrated that a recurrent MH could also be repaired by human amniotic membrane tissue with the restoration of the foveal structure. This technique achieved an anatomic closure of MHs in all eyes with an improvement in vision.
Our findings are also consistent with previous study by Chang et al. [21] which assessed the effectiveness of ANRFFT for refractory large MH that already underwent at least two ILM peeling surgeries. They reported a 90% closure rate which most of them had partial restoration of EZ after ANRFFT. Thus, this surgical technique not only acts as a scaffold but also promotes some functional improvement with a partial restoration of the outer retinal layer.
Although the underlying mechanism remains unclear, the mechanism for an autologous retinal transplant seems to be different than just a scaffold. The autologous retinal flap integrating the original retina tissue with a significant restoration of outer layers as seen on OCT images indicates the rebuilding of connection between the graft and the surrounding retinal tissue. The possible mechanisms participating functional and mechanical integration of the graft with the surrounding retina can be derived from previous experimental and animal work. Cells expressing the markers of neuroepithelial stem/progenitor cells (NSCs) giving rise to all distinct cell types of the neuroretina during development of the human retina were demonstrated in Müller glia in the peripheral adult human retina [22]. Although these cells seem to be quiescent in the uninjured adult human retina, retinal injuries can activate NSCs with Müller glial characteristics in the peripheral retina [22]. Recently, it has been shown that by optimizing the culture conditions, Müller glia isolated from the peripheral retina during vitrectomies can be an efficient source for producing cells with properties of rod photoreceptors [23]. Additionally, Johnsen et al. [24] demonstrated that glial-like Müller cells could develop reactive gliosis and respond to changes as a result of injury. Although Müller glia show neurogenic properties in the early postnatal retina of rodents and monkeys [25, 26], no evidence for their in vivo neurogenesis in response to injury has yet been observed in humans. It is hard to know whether de novo synaptic reconstruction between photoreceptors in the graft retina and bipolar cells in the recipient retina occurs in human eyes. However, experimental studies demonstrated the synaptic formation between the host bipolar cells and graft photoreceptors confirmed by immunohistochemical and multi-electrode array (MEA) recordings analysis [27, 28]. In correspond to this finding, another experimental study on monkeys reported by Tu et al. [29] demonstrated that human induced pluripotent stem cells-derived retina used for transplantation therapy in retinal degeneration resulted in visual function recovery at the grafted area. Thus, the author suspected that both the reactive gliosis and neurogenic properties of Müller glia cells in the graft obtained from peripheral retina might participate functional and mechanical integration of the transplanted retina with the surrounding host retina. Nevertheless, the exact functional and mechanical integration mechanisms still need to be clarified.
Although the partial or total dislocated graft rates after ANRFFT have been reported as the most common complications with an incidence between 5 to 33% [14, 30], the graft tissue remained in situ in all eyes in the present study.
In the present study, reactive RPE hyperplasia located in the macular region with a diffuse pattern developed in one case, which may be due to surgical trauma, light or dye toxicity to the RPE. In spite of this complication, the final visual acuity significantly improved.
The author observed CME-like cystic changes restricted to the inner layers of the graft in one eye, which showed very poor blood flow signal on OCTA. Therefore, it may be hypothesized that the transplanted retina takes its blood supply from the choroid and the cystic changes observed in the inner layer of the graft may related to inadequate nourishment of the inner layers. Similarly, Multicenter International Collaborative Study Group reported CME-like changes after ANRFFT [14]. However, in contrast to their reports, in the present study, the existence of CME-like changes worsened the visual outcome and did not resolve even after intravitreal treatments although final BCVA was still better than the preoperative BCVA.
The strengths of this study are uniform cases with no other comorbidity, the use of same techniques by a single surgeon, the use of same long acting intraocular tamponade (SO) and adequate follow-up. Nevertheless, this study has some limitations, including a retrospective design, lack of standardized imaging, a small sample size, no comparison group and randomization. Therefore, further prospective clinical studies that include larger sample sizes with comparison group are needed to investigate the efficacy and safety of ANRFFT.
In conclusion, ANRFF transplantation seems to be an effective surgical option in cases with refractory large idiopathic MHs. This technique can promote recovery of the outer retinal structure resulting in functional improvement. A number of complications including CME-like changes in the graft, retinal detachment and reactive pigment epithelial hyperplasia were observed after ANRFFT. Further studies are warranted to clarify whether the plausible mechanism leading to improved functional outcome might be the neural integration of transplanted retina to surrounding host retina.
Code Availability
Not applicable.
References
McCannel CA, Ensminger JL, Diehl NN, Hodge DN (2009) Population based incidence of macular holes. Ophthalmology 116:1366–3669. https://doi.org/10.1016/j.ophtha.2009.01.052
Rao P, Yonekawa Y, Abbey AM, Shah AA, Wolfe JD, Faia LJ (2017) Prevalence and surgical outcomes of macular hole in eyes with age-related macular degeneration. Ophthalmol Retina 1(2):158–164. https://doi.org/10.1016/j.oret.2016.09.014
Liu L, Enkh-Amgalan I, Wang NK et al (2018) Results of macular hole surgery: evaluation based on the international vitreomacular traction study classification. Retina 38:900–906. https://doi.org/10.1097/IAE.0000000000001647
Valldeperas X, Wong D (2008) Is it worth reoperating on macular holes? Ophthalmology 115:158–163. https://doi.org/10.1016/j.ophtha.2007.01.039
Chen SN, Yang CM (2016) Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina 36:163–170. https://doi.org/10.1097/IAE.0000000000000674
Charles S, Randolph JC, Neekhra A, Salisbury CD, Littlejohn N, Calzada JI (2013) Arcuate retinotomy for the repair of large macular holes. Ophthalmic Surg Lasers Imaging Retina 44:69–72. https://doi.org/10.3928/23258160-20121221-15
Felfeli T, Mandelcorn ED (2019) Macular hole hydrodissection: surgical technique for the treatment of persistent, chronic, and large macular holes. Retina 39:743–752. https://doi.org/10.1097/IAE.0000000000002013
Wong R, Howard C, Orobona GD (2018) Retina expansion technique for macular hole apposition report 2: efficacy, closure rate, and risks of a macular detachment technique to close large full-thickness macular holes. Retina 38:660–663. https://doi.org/10.1097/IAE.0000000000001705
Morizane Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, Kawata T, Hosogi M, Shirakata Y, Okanouchi T (2014) Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol 157(861–869):e1. https://doi.org/10.1016/j.ajo.2013.12.028
Michalewska Z, Michalewski J, Adelman RA, Nawrocki J (2010) Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 117:2018–2025. https://doi.org/10.1016/j.ophtha.2010.02.011
Lai CC, Hwang YS, Liu L, Chen KJ, Wu WC, Chuang LH, Kuo JZ, Chen TL (2009) Blood-assisted internal limiting membrane peeling for macular hole repair. Ophthalmology 116:1525–1530. https://doi.org/10.1016/j.ophtha.2009.02.025
Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, Giansanti F (2019) A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina 39(Suppl 1):S95–S103. https://doi.org/10.1097/IAE.0000000000002320
Grewal DS, Mahmoud TH (2016) Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol 134:229–230. https://doi.org/10.1001/jamaophthalmol.2015.5237
Grewal DS, Charles S, Parolini B, Kadonosono K, Mahmoud TH (2019) Autologous retinal transplant for refractory macular holes: multicenter international collaborative study group. Ophthalmology 126(10):1399–1408. https://doi.org/10.1016/j.ophtha.2019.01.027
Rahimy E, McCannel CA (2016) Impact of internal limiting membrane peeling on macular hole reopening: a systematic review and meta-analysis. Retina 36(4):679–687. https://doi.org/10.1097/IAE.0000000000000782
Al Sabti K, Kumar N, Azad RV (2009) Extended internal limiting membrane peeling in the management of unusually large macular holes. Ophthalmic Surg Lasers Imaging 40:185–187. https://doi.org/10.3928/15428877-20090301-03
Dai Y, Dong F, Zhang X, Yang Z (2016) Internal limiting membrane transplantation for unclosed and large macular holes. Graefes Arch Clin Exp Ophthalmol 254(11):2095–2099. https://doi.org/10.1007/s00417-016-3461-4
Pires J, Nadal J, Gomes NL (2017) Internal limiting membrane translocation for refractory macular holes. Br J Ophthalmol 101(3):377–382. https://doi.org/10.1136/bjophthalmol-2015-308299
Peng J, Chen C, Jin H, Zhang H, Zhao P (2018) Autologous lens capsular flap transplantation combined with autologous blood application in the management of refractory macular hole. Retina 38(11):2177–2183. https://doi.org/10.1097/IAE.0000000000001830
Lee SM, Kwon HJ, Park SW, Lee JE, Byon IS (2018) Microstructural changes in the fovea following autologous internal limiting membrane transplantation surgery for large macular holes. Acta Ophthalmol 96:e406–e408. https://doi.org/10.1111/aos.13504
Chang YC, Liu PK, Kao TE, Chen KJ, Chen YH, Chiu WJ, Wu KY, Wu WC (2019) Management of refractory large macular hole with autologous neurosensory retinal free flap transplantation. Retina. https://doi.org/10.1097/IAE.0000000000002734
Johnsen EO, Frøen RC, Albert R, Omdal BK, Sarang Z, Berta A, Nicolaissen B, Petrovski G, Moe MC (2012) Activation of neural progenitor cells in human eyes with proliferative vitreoretinopathy. Exp Eye Res 98:28–36. https://doi.org/10.1016/j.exer.2012.03.008
Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V (2011) Adult human Muller glia cells are a highly efficient source of rod photoreceptors. Stem Cells 29:344–356. https://doi.org/10.1002/stem.579
Johnsen EO, Froen RC, Olstad OK, Nicolaissen B, Petrovski G, Moe MC, Noer A (2018) Proliferative cells isolated from the adult human peripheral retina only transiently upregulate key retinal markers upon induced differentiation. Curr Eye Res 43:340–349. https://doi.org/10.1080/02713683.2017.1403630
Fischer AJ, Hendrickson A, Reh TA (2001) Immunocytochemical characterization of cysts in the peripheral retina and pars plana of the adult primate. Invest Ophthalmol Vis Sci 42(13):3256–3263
Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA (2008) Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA 9 105(49):19508–19513. https://doi.org/10.1073/pnas.0807453105
Iraha S, Tu HY, Yamasaki S et al (2018) Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation. Stem Cell Rep 10(3):1059–1074. https://doi.org/10.1016/j.stemcr.2018.01.032
Mandai M, Fujii M, Hashiguchi T, Sunagawa GA, Ito SI, Sun J, Kaneko J, Sho J, Yamada C, Takahashi M (2017) iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Rep 8(1):69–83. https://doi.org/10.1016/j.stemcr.2016.12.008
Tu HY, Watanabe T, Shirai H et al (2019) Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine 39:562–574. https://doi.org/10.1016/j.ebiom.2018.11.028
Wu AL, Chuang LH, Wang NK, Chen KJ, Liu L, Yeung L, Chen TL, Hwang YS, Wu WC, Lai CC (2018) Refractory macular hole repaired by autologous retinal graft and blood clot. BMC Ophthalmol 18(1):213. https://doi.org/10.1186/s12886-018-0898-8
Funding
The author has no proprietary or commercial interest in any materials discussed in this article.
Author information
Authors and Affiliations
Contributions
Design of the study, conduct of the study, analysis and interpretation and literature search were done by the author (KS).
Corresponding author
Ethics declarations
Conflict of interest
The author reports no conflict of interest.
Availability of data and material
All data will be available upon request.
Consent to participate
All participants gave written informed consent for participation in the study.
Consent to publication
All participants gave written informed consent for their data to be published.
Ethical approval
The study adhered to the tenets of Declaration of Helsinki and was approved by the Institutional Review Board/Ethics Committee of Ankara Training and Research Hospital, Ankara, Turkey.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (MP4 42813 kb)
Rights and permissions
About this article
Cite this article
Sonmez, K. Autologous neurosensory retinal transplantation for large refractory idiopathic macular hole. Int Ophthalmol 41, 1415–1425 (2021). https://doi.org/10.1007/s10792-021-01716-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01716-1