Abstract
Background
The aim of this study is to evaluate long-term anatomical and functional outcomes of autologous internal limiting membrane (ILM) transplantation in refractory highly myopic macular holes (HMMHs).
Methods
Retrospective interventional analysis of 13 eyes with refractory HMMH undergoing autologous ILM transplantation with gas tamponade. Best-corrected visual acuity (BCVA, Snellen), optical coherence tomography and fundus photography were scheduled at baseline and every follow-up visit (1, 3, 6, 12, 18, 24 months and the most recent). Preoperatively, we collected minimum linear diameter (MLD) and basal diameter (BD). Post-operatively, rates of external limiting membrane (ELM)/ellipsoid zone (EZ) restoration, excessive gliosis and subfoveal retinal pigmented epithelium (RPE) atrophy were evaluated.
Results
Average AXL was 31.45 ± 2.07 mm and mean follow-up was 47.2 ± 31.4 months. Anatomical success was reached in 7/13 eyes (54%), while 2 cases showed persisting HMMH, 2 cases had early recurrence and 2 cases late recurrence. BCVA went from 0.19 ± 0.18 to 0.22 ± 0.20 at final follow-up (p = 0.64), improving in 5/13 eyes (38%). One eye showed continuous ELM and EZ lines, while another eye showed an irregular ELM but no EZ. Post-operatively, 5 eyes (71%) developed progressive atrophy of the subfoveal RPE, while excessive gliosis was reported in 3 eyes (43%). Furthermore, one patient developed post-operative chronic macular edema-like changes in the perifoveal area.
Conclusion
Autologous ILM transplantation showed controversial anatomical outcomes and and poor visual results in refractory HMMH. Moreover, progressive subfoveal patchy atrophy and excessive gliosis are possible post-operative complications.
Key messages
What is known:
• Various surgical techniques have been proposed to treat refractory highly myopic macular holes (HMMHs), including autologous internal limiting membrane (ILM) flap transplantation, autologous retinal transplantation and lens capsule transplantation.
• Long-term analysis of the outcomes of autologous ILM transplantation still lacks in HMMH.
What is new:
• Primary anatomical success was reached in 54% of cases and only 38% of eyes showed improvements in visual outcomes.
• Progressive subfoveal patchy atrophy and excessive gliosis were two of the most frequent adverse effects, negatively influencing visual outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In high-income countries, degenerative myopia is a leading cause of legal blindness [1]. The spectrum of the myopic traction maculopathy (MTM), which includes inner and outer retinal schisis, foveal detachment, and lamellar or full-thickness highly myopic macular holes (HMMHs), is determined by the excessive elongation of the globe. In fact, in the posterior staphyloma, the anteroposterior traction due to the strong adhesion of the vitreous cortex combines with the tangential traction brought on by a tightened internal limiting membrane (ILM) [2,3,4]. Specifically, the formation of HMMHs, developing in around 8% of cases of MTM, relies on the breakdown of outer retinal layers and the creation of foveal cysts, caused by the coexistence on tangential and anteroposterior forces [5, 6]. Moreover, the persisting vitreous cortex traction, typical of highly myopic eyes, may lead to the development of macular hole retinal detachment (MHRD) [7,8,9].
The introduction of ILM inverted flap more than 10 years ago has significantly improved the closure rate of HMMHs, reaching around 90% even in cases complicated by MHRD [10, 11]. Nevertheless, more than 5% of myopic patients develop refractory or recurrent HMMH after first surgery [12]. In these cases, various surgical techniques have been proposed, including autologous transplantation of a free ILM flap [13], autologous neurosensory retinal flap transplantation [14], lens capsule transplantation [15] and, more recently, subretinal human amniotic membrane (hAM) patch [16, 17].
Autologous ILM was successfully positioned over regions of choroidal atrophy and over posterior retinal tears originating from the staphyloma in extremely myopic RDs [18], and a similar technique was reported for perifoveal macular holes (MHs) [19]. However, as far as we know, an analysis of surgical outcomes of autologous ILM flap transplantation in the setting of refractory HMMHs still lacks. Consequently, the aim of this research was to assess long-term functional and anatomical outcomes of pars plana vitrectomy (PPV) and free ILM transplantation in highly myopic patients with refractory MHs who already underwent ILM peel. Moreover, we focused on side effects and drawbacks of this technique.
Materials and methods
We conducted a retrospective mono-centric interventional analysis of patients undergoing PPV and autologous ILM transplantation to solve refractory HMMHs at the Instituto de Microcirugia Ocular, Barcelona, Spain, between January 2014 and June 2023. This research adhered the tenets of the Declaration of Helsinki and was approved by the Ethical Committee of the Instituto de Microcirugia Ocular, Barcelona. All patients signed an informed consent for data publication.
High myopia was defined as an axial length (AXL) > 26.50 mm or refractive error > 6 diopters (D). The following inclusion criteria were considered: presence of refractory HMMH with or without MHRD, surgical treatment with PPV and autologous ILM flap transplantation, a minimum 12-month follow-up time. The exclusion criteria were as follows: amblyopia, severe glaucoma, active uveitis, optic neuritis, severe cataract impairing retinal imaging, and serious systemic illness impacting ocular health. Out of 325 HMMHs undergoing surgical treatment in the study period, 13 eyes fulfilled the inclusion criteria. A flowchart of the selection process is visible in Supplemental file 1.
A complete ophthalmological anamnesis was carried out at the baseline visit, followed by a comprehensive ocular examination after pupil dilation and AXL measurement (IOLMaster 500, Carl Zeiss Meditec, Germany). At each follow-up, measurements of BCVA (Snellen equivalent), intraocular pressure (IOP), spectral domain (SD)-OCT assessment using the Cirrus 5000 high-definition OCT (Carl Zeiss, Dublin, CA) and fundus photography, using either ultra-wide Optomap (Optos GmbH, Düsseldorf, Deutschland) or Topcon TRC-NW8 fundus camera (Topcon, Tokyo, Japan), were among the examinations performed. Follow-ups were scheduled at 1, 3, 6, 12, 18, 24 months after surgery and at the most recent visit. Every patient completed a minimum of 12 months follow-up.
Surgical procedure
The same skilled vitreoretinal surgeon (C.M.) performed all surgeries using a 23-gauge system (DORC Eva Nexus, DORC, Zuidland, The Netherlands). In order to confirm earlier ILM peeling, Brilliant Blue G was initially injected within the arcades around the HMMH. Perfluorocarbon liquid (PFCL) was instilled to cover the macular area. The ILM transplant was harvested using a pinch and grasp technique from a region inside the vascular arcades, beginning from the edge of the previously removed ILM and moving in a circular fashion for about one disk diameter. Then, the ILM plug was tucked inside the HMMH under the PFCL tamponade and gently massaged to ensure correct positioning with a 23-gauge soft-tipped flute needle. If the first piece of the plug was not large enough to fill in the HMMH, a second piece of the ILM graft was harvested using a similar technique. At the end of the surgery, PFCL was removed and a fluid gas exchange using 20% sulfur hexafluoride (SF6) was carried out. The patients were asked to keep a face-down position overnight and a prone position for the first 3 post-operative days.
Scan protocol
For OCT analysis, the following scan procedures were performed for each patient: 6 × 6 mm Macular Cube, HD Cross and horizontal and vertical HD Raster 5- or 21-line. In order to guarantee the quality of each picture, the OCT scans were performed at least twice. In a fovea-centered horizontal B-scan, the caliper tool was used to measured baseline minimum linear diameter (MLD) and basal diameter (BD): the former was measured at the narrowest distance between the broken margins of the neuroepithelia with a line roughly parallel to the retinal pigment epithelium, while the latter was defined as the length of the RPE with detached photoreceptors. In cases of MHRD, the calculation of BD was not possible. In the post-operative period, anatomical closure of the HMMH was described as the disappearance of the foveal defect and no bare RPE exposed to the vitreous. Reconstitution of the external limiting membrane (ELM) or ellipsoid zone (EZ) was defined as the presence of the corresponding continuous hyperreflective line in the fovea. Moreover, rates of excessive post-operative gliosis (hyperreflective lesion with a “peak-like” aspect extending from the RPE, filling the foveal defect and ending above the retinal surface) and RPE atrophy were reported.
In the post-operative period, eyes in which the HMMH was still open at 1-month follow-up were defined as persisting HMMH, eyes in which the HMMH recurred within the first 6 months were defined as “early recurrent”, while eyes with HMMH recurring after the 6-month follow-up were defined as “late recurrent”.
The main endpoint of this study was to define anatomical and visual outcomes of autologous ILM flap in refractory HMMHs. Moreover, we focused on the evolution of ILM flap during the post-operative period.
Statistical analysis
Statistical analysis was performed using IBM SPSS software, version 27.0 (SPSS Inc, Chicago, IL). The Shapiro-Wilk test was employed to assess the normality of the sample. Multiple comparisons test with Dunnet’s correction was used for continuous variables comparison between baseline and postoperative data. Correlation analysis was performed using Spearman coefficient. P < 0.05 was deemed statistically significant.
Results
Thirteen eyes of 13 patients were included in this study. Mean age at presentation was 69.0 ± 11.1 years, male/female ratio was 3/10, while 46% were right eyes. Mean number of previous HMMH surgeries was 1.2 ± 0.6 and mean subjective symptoms duration was 3.9 ± 3.5 months. Average AXL was 31.45 ± 2.07 mm. Patients were followed for 47.2 ± 31.4 months on average (range 12–120). Mean MLD at baseline was 484 ± 238 μm. Two cases (15%) initially presented with a MHRD. Patient list with demographic, clinical and OCT characteristics is visible in Table 1. None of the patients suffered intraoperative complications.
Overall, primary anatomical success was reached in 7/13 eyes (54%). Two patients (15%) with preoperative large holes (808 and 739 μm) showed persisting HMMH at 1-month follow-up but decided not to undergo further surgery. Similarly, two patients (15%) developed early recurrence at 3-months and 6-months follow-up, respectively, avoiding to undergo further operation since BCVA was stable. Finally, two patients (15%) developed late HMMH recurrence: one at the 18-months follow-up, with concomitant MHRD, and the other at the 24-months follow-up. Both of them underwent re-operation with good anatomic outcomes (Fig. 1).
B-scans of cases undergoing autologous internal limiting membrane (ILM) transplantation and experiencing surgical failure. A Persisting highly myopic macular hole (HMMH) in a patient who decided not to undergo further surgery. B Early HMMH recurrence in a patient in which anatomical success was visible at 1-month follow up, but a macular hole retinal detachment (MHRD) was highlighted at the 3-months follow up (central column, bottom image). Note the remainders of the ILM plug which were still partially attached to retinal boundaries (white arrow). C Case of late HMMH recurrence in a patient in which the ILM plug correctly sealed the hole at the 12-months follow-up. However, at month 18, the ILM plug appeared contracted toward the temporal side and a hole recurrence was identifiable
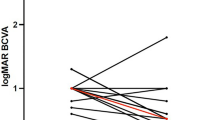
Focusing on functional outcomes, BCVA went from 0.19 ± 0.18 (Snellen equivalent) at baseline to 0.29 ± 0.24 at 12 months, 0.25 ± 0.27 at 24 months and 0.22 ± 0.20 at final follow-up. However, no significant improvement compared to baseline was reported at any follow-up (all p > 0.05) (Fig. 2). Overall, at the end of the study, BCVA improved in 5/13 eyes (38%), remained stable in 3/13 eyes (23%) and worsened in 5 eyes (38%). Two eyes (ID 03 and 10) showed at least 2-lines improvement in visual acuity.
OCT analysis and adverse events
Regarding OCT features, baseline MLD significantly correlated with the risk of refractory or early recurrent HMMH (p = 0.03), differently from baseline BD (p = 0.40). In eyes with anatomical success, foveal restoration without a visible ILM plug was reached in 3 eyes, while ILM plug was still visible in 4 eyes. However, only one eye showed continuous ELM and EZ lines, while another eye showed an irregular ELM but EZ was not visible (Supplemental file 2). The ELM/EZ complex was undetectable in any of the other eyes.
On the other hand, in patients with refractory HMMH or early HMMH recurrence (patients 07 and 09), the ILM plug was not identifiable at OCT analysis, suggesting post-operative displacement (Fig. 1A). In one patient (ID 01) who developed late HMMH recurrence, intermediate follow-up showed a visible ILM plug after 12 months. The ILM plug was no more identifiable when HMMH recurrence occurred at the 18-months follow-up (Fig. 1C). Conversely, in patient 03, no clear outer retinal restoration was visible behind the ILM plug at the 1-month follow-up. At month 3, the patient came back with a recurrent HMMH associated with MHRD, and one of the OCT scans showed how the remnants of the ILM plug were still attached to the boundaries of the detached retina (Fig. 1B).
Overall, out of the 7 eyes with favorable anatomic outcome, 5 eyes (71%) developed progressive atrophy of the subfoveal RPE, identifiable as increased choroidal hypertransmission and concomitant appearance of an atrophy patch at infrared (IR) pictures and fundus photography (Fig. 3 and 4). In all cases, RPE atrophy was identifiable between the 6-month and 12-month follow-ups and was confined to the area of the pre-existing HMMH.
Case of sub-foveal patchy atrophy development during a 24-months follow-up. Although anatomical success, sub-foveal choroidal hypertransmission and retinal pigment epithelium (RPE) atrophy was identifiable starting from the 6-months follow-up (B) in infrared images (left column) and B-scans (right column). As indicated by white arrowheads, the atrophy progressively enlarged during the study period and was associated with poor visual outcomes
Long-term follow-up of a patient with refractory highly myopic macular hole (HMMH) showing successful closure but sub-foveal patchy atrophy development and late excessive gliosis. As visible with baseline fundus photography (A), infrared images at intermediate follow-ups (B-E) and final fundus photography (F), patchy atrophy progressively enlarged and involved the foveal area. B-scans (right column) showed the evolution of the ILM plug, which progressively shrunk till month 24 but then underwent progressive gliosis, clearly visible at 48-months follow-up (right column, bottom image)
Excessive gliosis was reported in 3 eyes (28%). In the first case, the lesion slowly increased over time and reached stability 12 months after surgery, not being associated with RPE atrophy. In the second case, excessive gliosis appeared 36 months after surgery and was associated with subfoveal patchy atrophy (Fig. 4). Finally, in the third case (ID 02), the lesion increased in the first 12 months but concomitant subfoveal RPE atrophy developed. During the successive follow-ups, the gliosis showed progressive reduction and regression 54 months after surgery, while the RPE atrophy had progressively enlarged (Fig. 5).
Evolution of excessive gliosis and sub-foveal patchy atrophy through a 54-months follow-up in infrared images (left column) and B-scans. Cystic degeneration of the ILM plug was visible at the 3-months follow-up (B, right column), and left space to increasing excessive gliosis and enlarging RPE atrophy (white arrowheads) through 6- and 12-months follow-ups (C and D). Finally, at 54-months follow-up, subfoveal atrophy had further enlarged, but excessive gliosis regressed, even if no restoration of outer retinal layers was identifiable
Furthermore, patient 11 developed post-operative chronic macular edema (ME)-like changes in the perifoveal area. The ME was highlighted starting from the 6-month follow-up and assumed a microcystic-like aspect, being localized in the inner nuclear layer (INL) (Fig. 6). Finally, patient 12 developed an extra-foveal myopic choroidal neovascularization (mCNV), which required 3 anti-vascular endothelial growth factor (anti-VEGF) injections.
Case of persisting macular edema-like changes in the perifoveal area of a refractory highly myopic macular hole (HMMH) treated with autologous internal limiting membrane (ILM) transplantation. Microcystic changes of the inner nuclear layer (red arrowheads) appeared at the 3 months follow-up (B, right column) and were still visible 75 months after surgery (E, right column). Moreover, the patient gradually developed an enlarging sub-foveal patchy atrophy associated with poor visual outcomes
Discussion
Various surgical techniques based on the ‘scaffold theory’ have been proposed to treat primary and refractory MHs. These techniques include the inverted ILM flap, lens capsular flap transplantation and autologous ILM transplantation, all relying on the same healing process, which involves a membrane-like tissue that acts as a scaffold for the multiplication and movement of Müller cells. Previous research showed that the ILM flap is encircled by activated Müller cells, which also generate the same factors, and displays neurotrophic and growth factors [20]. Moreover, when Müller cells are cocultured with the ILM alone, Müller cell migration and proliferation are stimulated by ILM components of collagen IV, laminin, and fibronectin. These results corroborated the idea that adding an ILM flap considerably increases gliosis, which improves the prognosis for patients with refractory MHs [21].
In our research, we evaluated the role of autologous ILM flap transplantation in the treatment of recurrent HMMH. Overall, primary anatomical success was reached in 54% of cases and only 38% of eyes showed improvements in visual outcomes. In two cases, we reported anatomical failure at the first post-operative follow-up. Both patients had a preoperative large HMMH (808 and 739 μm), suggesting that this category of patients have higher risk of early displacement of the ILM plug and may require different intraocular tamponades. Rizzo et al. suggested that other tamponading agents, including “heavy” silicone oils, have been effective in closing chronic MHs [22]. However, consistent with the study of Giansanti et al., we chose a gas tamponade in our series since we looked for a high tamponading force during the initial postoperative period [23], and possible incorrect postoperative positioning may have resulted in the two cases of displacement we reported. On the other side, the use of longer acting gases (e.g. C3F8) or the use of viscoelastics substances as surgical adjuvants to hold the flap in place could have been an option to optimize post-operative anatomical outcomes [24], but was not assessed in our research.
Moreover, 4 patients developed early (in the first 6 post-operative months) or late recurrence of the HMMH, which occurred even 2 years after surgery. When compared to reports on hemmetropic refractory MHs, in which free ILM transplantation showed success rates of 91–100%, (21 − 16 di giansanti, giansanti) anatomic results in HMMH are sensibly lower. However, high myopia is a well-known risk factor for MH recurrence and our case series had a mean AXL of 31.45 mm, which may have detrimentally impacted success rates of the technique.
In patients who reached anatomical success, only one eye showed visible restoration of the ELM/EZ complex, while another eye showed the presence of an irregular ELM. None of the other eyes (71%) showed restoration of outer retinal layers, thus leading to poor visual outcomes. Giansanti et al., in their case series, observed good recovery of outer retinal layers and good visual improvement [23]. On the other side, several studies hypothesized that the closure of a recurrent MH undergoing ILM transplantation was associated with the prolonged proliferation of glial tissues in the fovea with fibrotic and depigmentation phenomena, which often hampered the restoration of outer retinal layers, consistent to the findings of our research [25, 26].
Moreover, starting at the 6- and 12-months follow-up, 71% of eyes developed RPE subfoveal atrophy, which was detectable in infrared OCT scans and in fundus photography, as a white roundish lesion with well-defined borders in the foveal area slowly enlarging during the study period. Previous reports in literature highlighted the occurrence of RPE atrophy after MH surgery but addressed this complication to the use of vital dyes, such as trypan blue and indocyanine green [27, 28], and, more recently, Brilliant blue [29]. Nevertheless, all these case showed a diffuse RPE atrophy involving larger areas or the entire posterior pole. In our research, the RPE atrophy had a nummular aspect, similar to patchy atrophy (PA), and progressively developed in the area of the pre-existing hole, suggesting that the ILM plug, even in cases of anatomical success, often fails to integrate with the surrounding retina and alters the homeostasis in the foveal area, eventually leading to RPE sufferance. Notably, a clinical evidence of myopic atrophic maculopathy with PA was already visible preoperatively in all of these eyes, thus suggesting a pre-existing choroidal circulation imbalance, which, combined with scleral fragility of myopic eyes and the choriocapillaris disappearance, enhances the vulnerability of the RPE, as demonstrated in previous research [30]. We hypothesize that the presence of the ILM plug, since being a scaffold of non-vascularized tissue, may be an additive element hastening the development of PA in the foveal area.
Moreover, we reported excessive gliosis in 3 eyes (43%). As previously reported for the inverted flap technique performed in HMMH, the ILM has a critical function in encouraging the hyperproliferation of Müller cells. Ye et al. recently reported a 42% rate of excessive gliosis in HMMH undergoing ILM inverted flap, showing that the risk is enhanced when AXL is more than 29.985 mm [31]. In our research, all eyes experiencing excessive gliosis had an AXL > 31 mm, consistent with the idea that AXL may be an independent factor for the development of this side effect. Previous studies claimed that highly myopic eyes have an altered ILM microstructure and Müller cells symmetry [32], and that the inverted ILM flap may induce anomalous activation of Müller cells which move along the pathologically altered ILM “scaffold” and ultimately result in excessive gliosis [33, 34].
In a Western blot analysis of highly myopic eyes with foveoschisis, the ILM showed increased levels of the acidic glial fibrillary protein (GFAP), a marker of reactive gliosis and stress for Müller cells [20]. Stressed Müller cells have a higher tendency to multiply quickly when damage connected to surgery takes place [35]. In refractory HMMH, already damaged Müller cells undergo further surgical stress due to intraoperative handling of the ILM transplant in the foveal area. Furthermore, we suggest that the presence of the ILM plug enhances the re-activation of Müller cells which leads to anomalous gliosis developing in the first post-operative months. As already demonstrated, excessive glial growth may have an impact on the rebuilding of the photoreceptor layer and, consequently, on visual outcomes [31, 34]. This hypothesis is consistent with our results, in which the patients developing this lesion did not show any restoration of ELM/EZ, and BCVA at the end of the follow-up period was equal to baseline.
Our study has several limitations, primarily due to the intrinsic nature of this retrospective research, which lacked a control group. Second, the sample size was relatively small and the follow-up periods were heterogeneous, even if all the patients completed at least 12 months of follow-up and in some cases we were able to study OCT changes happening 6 or 7 years after surgery. In addition, other functional outcomes, such as post-operative metamorphopsia, significantly impact quality of life and could have been investigated.
After a long term experience with HMMHs and after highlighting the shortfalls of autologous ILM flap transplantation, our group progressively abandoned this option in favor of newly proposed techniques, such as the use of hAM patches [36] and macular hydrodissection [37], which demonstrated better anatomical and functional outcomes, even if reports with long-term follow-ups are not yet available in literature. A similar process occurred with the use of macular buckling, which had been our mainstay for the management of MTM [38], but was then supplanted in our practice by less invasive techniques with lower rates of early and late complications.
In conclusion, autologous ILM transplantation with gas tamponade showed controversial results in recurrent HMMH, either in terms of anatomical and functional outcomes, with reconstitution of outer retinal layers being visible only in rare cases and a high risk of hole recurrence. Moreover, we highlighted the development of several post-operative complications, such as progressive subfoveal patchy atrophy and excessive gliosis, further limiting visual results.
Data availability
The data that support the findings of this study are available from the corresponding author, MMC, upon reasonable request.
References
Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, Yasuzumi K, Nagaoka N, Saka N, Yoshida T, Tokoro T, Mochizuki M (2010) Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 117:1595–1611. https://doi.org/10.1016/j.ophtha.2009.11.003
Lin CW, Ho TC, Yang CM (2015) The development and evolution of full thickness macular hole in highly myopic eyes. Eye (Lond) 29:388–396. https://doi.org/10.1038/eye.2014.312
Gaucher D, Haouchine B, Tadayoni R, Massin P, Erginay A, Benhamou N, Gaudric A (2007) Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol 143:455–462. https://doi.org/10.1016/j.ajo.2006.10.053
Johnson MW (2012) Myopic traction maculopathy: pathogenic mechanisms and surgical treatment. Retina 32 Suppl 2S205–210. https://doi.org/10.1097/IAE.0b013e31825bc0de
Kakinoki M, Araki T, Iwasaki M, Ueda T, Sano H, Hirano Y, Moriya Y, Sawada O, Takamura Y, Sakamoto T, Kanda T, Ohji M, Japan Clinical Retinal Study T (2019) Surgical outcomes of Vitrectomy for Macular Hole Retinal detachment in highly myopic eyes: a Multicenter Study. Ophthalmol Retina 3:874–878. https://doi.org/10.1016/j.oret.2019.04.026
Gu X, Hu Z, Qian H, Fransisca S, Mugisha A, Wang J, Zhang Z, Zhang W, Ji J, Liu Q, Xie P (2021) Perfluorocarbon Liquid-assisted inverted internal limiting membrane flap technique Versus Internal limiting membrane peeling for highly myopic Macular Hole Retinal detachment. Retina 41:317–323. https://doi.org/10.1097/IAE.0000000000002853
Garcia-Arumi J, Martinez V, Puig J, Corcostegui B (2001) The role of vitreoretinal surgery in the management of myopic macular hole without retinal detachment. Retina 21:332–338. https://doi.org/10.1097/00006982-200108000-00006
Kadonosono K, Yazama F, Itoh N, Uchio E, Nakamura S, Akura J, Sawada H, Ohno S (2001) Treatment of retinal detachment resulting from myopic macular hole with internal limiting membrane removal. Am J Ophthalmol 131:203–207. https://doi.org/10.1016/s0002-9394(00)00728-5
Parolini B, Palmieri M, Finzi A, Besozzi G, Lucente A, Nava U, Pinackatt S, Adelman R, Frisina R (2021) The new myopic traction Maculopathy Staging System. Eur J Ophthalmol 31:1299–1312. https://doi.org/10.1177/1120672120930590
Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J (2014) Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina 34:664–669. https://doi.org/10.1097/IAE.0000000000000042
Mete M, Alfano A, Guerriero M, Prigione G, Sartore M, Polito A, Pertile G, (2017) Inverted Internal limiting membrane flap technique versus complete internal limiting membrane removal in myopic macular hole surgery A comparative study. Retina 37:1923–1930. https://doi.org/10.1097/IAE.0000000000001446
Hattori K, Kataoka K, Takeuchi J, Ito Y, Terasaki H (2018) Predictive factors of Surgical outcomes in Vitrectomy for myopic traction Maculopathy. Retina 38(Suppl 1):S23–S30. https://doi.org/10.1097/IAE.0000000000001927
Chen S-N, Hsieh Y-T, Yang C-M (2018) Multiple free internal limiting membrane flap insertion in the treatment of macular hole-associated retinal detachment in high myopia. Ophthalmologica 240:143–149
Grewal DS, Mahmoud TH (2016) Autologous neurosensory Retinal Free Flap for Closure of Refractory Myopic Macular Holes. JAMA Ophthalmol 134:229–230. https://doi.org/10.1001/jamaophthalmol.2015.5237
Chen S-N, Yang C-M (2016) Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina 36:163–170
Caporossi T, Pacini B, De Angelis L, Barca F, Peiretti E, Rizzo S (2020) Human amniotic membrane to close recurrent, high myopic macular holes in pathologic myopia with axial length of ≥ 30 mm. Retina 40:1946–1954
Caporossi T, Governatori L, Gambini G, Baldascino A, De Vico U, Ripa M, Scampoli A, Carlà MM, Rizzo C, Kilian R (2022) Treatment of recurrent high myopic macular hole associated with retinal detachment using a human amniotic membrane. Jpn J Ophthalmol 66:518–526
Rizzo S, Tartaro R, Barca F, Bacherini D, Franco F, Caporossi T (2018) Autologous Internal limiting membrane fragment transplantation for Rhegmatogenous Retinal Detachment due to Paravascular or Juxtapapillary Retinal breaks over patchy Chorioretinal Atrophy in Pathologic Myopia. Retina 38:198–202. https://doi.org/10.1097/IAE.0000000000001636
Kumase F, Morizane Y, Kimura S, Toshima S, Shiraga F (2017) Autologous Internal limiting membrane transplants successfully close a large parafoveal retinal hole. Acta Med Okayama 71:255–257. https://doi.org/10.18926/AMO/55209
Shiode Y, Morizane Y, Matoba R, Hirano M, Doi S, Toshima S, Takahashi K, Araki R, Kanzaki Y, Hosogi M, Yonezawa T, Yoshida A, Shiraga F (2017) The role of Inverted Internal limiting membrane flap in Macular Hole Closure. Invest Ophthalmol Vis Sci 58:4847–4855. https://doi.org/10.1167/iovs.17-21756
Zhang L, Li X, Lin X, Wu M (2019) Nerve growth factor promotes the proliferation of Muller cells co-cultured with internal limiting membrane by regulating cell cycle via Trk-A/PI3K/Akt pathway. BMC Ophthalmol 19:130. https://doi.org/10.1186/s12886-019-1142-x
Rizzo S, Belting C, Genovesi-Ebert F, Cresti F, Vento A, Martini R (2006) Successful treatment of persistent macular holes using heavy silicone oil as intraocular tamponade. Retina 26:905–908. https://doi.org/10.1097/01.iae.0000250006.76155.3d
Giansanti F, Tartaro R, Caporossi T, Bacherini D, Savastano A, Barca F, Rizzo S (2019) An internal limiting membrane plug and gas endotamponade for recurrent or persistent macular hole. Journal of ophthalmology 2019
Chou HD, Chong YJ, Teh WM, Chen KJ, Liu L, Chen YP, Yeung L, Hwang YS, Wu WC, Lai CC (2021) Nasal or temporal internal limiting membrane flap assisted by Sub-perfluorocarbon Viscoelastic Injection for Macular Hole Repair. Am J Ophthalmol 223:296–305. https://doi.org/10.1016/j.ajo.2020.09.023
Pires J, Nadal J, Gomes NL (2017) Internal limiting membrane translocation for refractory macular holes. Br J Ophthalmol 101:377–382. https://doi.org/10.1136/bjophthalmol-2015-308299
Ra H, Lee WK (2018) Contralateral Autologous Internal Limiting Membrane Transplantation for Closure of a refractory Macular Hole: Surgical technique. Ophthalmic Surg Lasers Imaging Retina 49:e75–e77. https://doi.org/10.3928/23258160-20180907-10
Imai H, Azumi A (2014) The expansion of RPE Atrophy after the inverted ILM flap technique for a chronic large Macular Hole. Case Rep Ophthalmol 5:83–86. https://doi.org/10.1159/000360693
Jain S, Kishore K, Sharma YR (2013) Progressive atrophy of retinal pigment epithelium after trypan-blue-assisted ILM peeling for macular hole surgery. Indian J Ophthalmol 61:235–237. https://doi.org/10.4103/0301-4738.111180
Federico O, Anniken BJ, Carlos M (2021) Diffuse retinal pigment epithelium atrophy following pars plana vitrectomy for high myopic macular hole assisted by Brilliant Blue G: a case report. Am J Ophthalmol Case Rep 23:101148. https://doi.org/10.1016/j.ajoc.2021.101148
Xie S, Fang Y, Du R, Yokoi T, Takahashi H, Uramoto K, Yoshida T, Ohno-Matsui K (2020) Abruptly emerging vessels in eyes with myopic patchy chorioretinal atrophy. Retina 40:1215–1223
Ye X, Wang J, Qiu W, Chen Y, Shen L (2023) Excessive Gliosis after Vitrectomy for the highly myopic Macular hole: a spectral domain Optical Coherence Tomography Study. Retina 43:200–208. https://doi.org/10.1097/IAE.0000000000003657
Vogt D, Stefanov S, Guenther SR, Hagenau F, Wolf A, Priglinger SG, Schumann RG (2020) Comparison of Vitreomacular Interface Changes in Myopic Foveoschisis and Idiopathic Epiretinal membrane Foveoschisis. Am J Ophthalmol 217:152–161. https://doi.org/10.1016/j.ajo.2020.04.023
Chen L, Wei Y, Zhou X, Zhang Z, Chi W, Gong L, Jiang X, Zhang S (2018) Morphologic, biomechanical, and compositional features of the Internal limiting membrane in Pathologic Myopic Foveoschisis. Invest Ophthalmol Vis Sci 59:5569–5578. https://doi.org/10.1167/iovs.18-24676
Chen Y, Wang J, Ye X, Yu J, Tao J, Lin L, Wu S, Qu J, Shen L (2021) The role of internal limiting membrane flap for highly myopic macular hole retinal detachment: improving the closure rate but leading to excessive gliosis. Front Med 8:812693
Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, Charteris DG (2005) Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 46:329–342. https://doi.org/10.1167/iovs.03-0518
Caporossi T, Governatori L, Gambini G, Baldascino A, De Vico U, Ripa M, Scampoli A, Carla MM, Rizzo C, Kilian R, Rizzo S (2022) Treatment of recurrent high myopic macular hole associated with retinal detachment using a human amniotic membrane. Jpn J Ophthalmol 66:518–526. https://doi.org/10.1007/s10384-022-00953-w
Felfeli T, Mandelcorn ED (2019) MACULAR HOLE HYDRODISSECTION: Surgical technique for the treatment of Persistent, Chronic, and large Macular Holes. Retina 39:743–752. https://doi.org/10.1097/IAE.0000000000002013
Alkabes M, Mateo C (2018) Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefe’s Archive Clin Experimental Ophthalmol 256:863–877
Acknowledgements
Not applicable.
Funding
Not applicable.
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Approved by Instituto de Microcirugia Ocular, Barcelona, Spain.
Consent to publication
The authors affirm that human research participants provided informed consent for publication of the images.
Informed consent
Written informed consent was obtained from all participants.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carlà, M.M., Mateo, C. Shortfalls of free autologous internal limiting membrane transplantation for highly myopic refractory macular holes in a long term follow-up. Graefes Arch Clin Exp Ophthalmol (2024). https://doi.org/10.1007/s00417-024-06533-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00417-024-06533-7