Abstract
Purpose
To introduce a novel practical technique of self-made cryopreservative fibrin glue (SMC) applied in pterygium surgery and to assess its safety and efficacy.
Methods
Forty-eight eyes of 48 patients with nasal primary pterygium were enrolled. The patients were equally assigned to 6 groups. Self-made fibrin glue was subpackaged and, respectively, cryopreserved for 3, 7, 15 days and 1, 2 and 3 months. At each time point, the asepsis of SMC was confirmed by bacterial culture and colony counting. In each group, corresponding SMC was applied to fix the autograft after the pterygium was removed (e.g., SMC 3d for group 1 and SMC 3m for group 6). All the patients were followed up postoperatively on days 1, 3, 7 and 14 and then at months 1, 3, 6. The main outcome measures included fixation success rate within two tries, postoperative discomfort, recurrence rate and complications.
Results
No colony growth was observed in all the fibrinogen and thrombin tubes sent. Five patients needed a second try with respective SMC during the autograft fixation, and there were no significant differences in SMC use times among the groups (P = 0.885). There were no significant differences in postoperative discomfort (day 1, 3, 7; P = 0.651, P = 0.269, P = 0.180, respectively) among the groups. By the end of 6-m follow-up, no infections and severe complications were observed in any group. The total recurrence rate was 3/48 (6%), and there were no significant differences in recurrences among the groups (P = 1.000).
Conclusion
SMC is safe and effective for autograft fixation in pterygium surgery. This new practical technique will benefit the patients and surgeons in developing and underdeveloped country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, fixation of limbal conjunctival autograft with fibrin glue (FG) is the most preferred choice for pterygium surgery. It causes much fewer postoperative discomforts, shortens surgery time and is with lower recurrence rates and fewer complications compared with traditional suture method [1,2,3,4,5,6,7,8,9].
Commercial Fibrin Glue Kit (CK) such as Tisseel is usually used for conjunctival autograft fixation during pterygium surgery. Although it worked efficiently, the cost of kit was not low and it might not be available in each country or region. Luckily, in most of medical institutions, there were separate fibrinogen powder and thrombin powder for injection (the main components of CK) and the total cost also was much lower than CK. We ever confirmed that a satisfactory result could be achieved for conjunctival autograft fixation when self-made fibrinogen solution and thrombin solution were mixed.
Unfortunately, in recent years, the price of blood product is skyrocketing, especially for fibrinogen powder not to mention CK. The excellent “Cut and Past” technique for pterygium is watched gradually disappears in developing country for high cost.
Here we introduced a practical packaging and cryopreservation technique for self-made FG in order to significantly reduce the average treatment cost. We proved the safety and efficacy of this novel technique in consecutive pterygium surgeries.
Methods
Self-made cryopreservation fibrin glue (SMC) preparation
Human fibrinogen 0.5 g (Boya Bio-Pharmaceutical, Jiangxi, China) was dissolved in sterile water for injection with concentration of 60 mg/ml. Lyophilizing Thrombin Powder 500 IU (Yige Pharmaceutical, Hunan, China) was dissolved in 40 mmol/l CaCl2 with concentration of 500 IU/ml. These two components then were separately subpacked into 2-ml Eppendorf tubes (1 ml each tube). Abbreviation of each component was marked on the tube cap (F stands for fibrinogen, and C stands for thrombin), and preparation date was marked on the tube wall. The tubes were put into a 72-hole box, sealed and stored at −20 °C (Fig. 1). All the processes were in sterile operation.
Bacterial culture and colony counting
Three tubes of F and three tubes of C were randomly selected every time and sent to microbiology laboratory on post-cryopreservation days 3,7,14 and months 1, 2, 3. The total bacterial colonies of each tube were counted and reported with standard flat colony counting method.
Patient enrollment and grouping
From January 1 to February 31, 2015, 48 patients with primary nasal intermediate pterygium [10] who wanted to be treated by surgery were enrolled. Among them, 20 were male and the other 28 were female. A detailed medical and ocular history was obtained, including patient age, gender and family as well as medical, systemic and ocular history. The first time the patient had found the pterygium was also recorded. Snellen visual acuity measurement, funduscopy, applanation tonometry and slit-lamp examination were performed preoperatively. Patients with ocular pathology other than early stage of cataract, mild dryness of eye, error of refraction, with a history of previous ocular surgery or trauma or with severe systemic diseases were excluded.
The 48 patients were randomly assigned to 6 groups, and each contained 8. SMC 3d would be used in group 1, SMC 7d in group 2, SMC 15d in group 3, SMC 1m in group 4, SMC 2m in group 5 and SMC 3m in group 6. If there was positive result got in bacterial culture, for example, SMC 3m got positive culture but the others got negative, the group 6 would be excluded from the study, and the patients in group 6 would undergo pterygium surgery with CK.
Informed consent was obtained from all patients. This study was approved by the research ethics committees of Zhejiang Provincial People’ Hospital and adhered to the tenets of the Declaration of Helsinki.
Surgery process with SMC
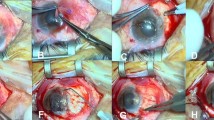
One tube of F and one tube of C would be brought into operation room with each patient. An experienced surgeon performed all surgeries. The pterygium was excised, and superotemporal autograft was harvested with conventional method. Which kind of SMC would be used (SMC 3d, 7d, 15d, 1m, 2m or 3m) to fix the autograft was blind to surgeon and patient.
One drop of F was placed on the scleral bed, and then, one drop of C was applied to active the sealant. The graft was spread over the sclera with care taken to maintain the right orientation and not to turn over the epithelial and stromal faces of the conjunctiva. The free edges of the nasal primary conjunctiva were attached to the sclera below too. Excess glue was removed, and the graft was trimmed if necessary. After a drying period of 1 min, we asked the patient to rotate eye for several times before the removal of lid speculum to ensure the graft was well stuck, otherwise SMC would be used again. If the second try was also failed, then CK would be used instead.
Tobramycin–dexamethasone ointment was applied into conjunctiva sac, and a pressure patch was applied for 24 h to restrict the eyeball from movement or blinking. From the second day on tobramycin and dexamethasone, eye drops were applied 4 times daily and tapered off over the following month.
Study and follow-up project
The cases in which CK replaced SMC were recorded in each group. All the patients were followed up postoperatively on days 1, 3, 7 and 14 and at months 1, 3, 6. In addition, during this period, they were required to see the doctor at the time any conjunctival tissue was found to have grown onto the cornea again.
Snellen visual acuity and intraocular pressure (IOP) were tested at each visit. The slit-lamp examination was performed to observe the integrity and position of graft, carefully looking for any evidence of infection, recurrence or complications. A questionnaire was designed to record the patients’ subjective symptoms during the first week follow-up. The discomfort was evaluated by a 4-point scale: (0) none; (1) somewhat bothersome; (2) quite bothersome; (3) very bothersome.
Statistics
The Kruskal–Wallis test was used to analyze the differences in postoperative symptom scores among the groups. The Chi-square test was used to evaluate the differences in SMC fixation success rate within two tries, pterygium recurrence and various complications. Value was taken to be statistically significant at P < 0.05.
Results
There were no significant differences between the groups in gender, age, area encroaching onto the cornea or the years of pterygium history. According to the result of standard bacterial culture, no colony growth was observed in all the F and C tubes sent (Fig. 2), so all of the 48 patients (6 groups) participated in the study.
There were only five patients who needed a second try with respective SMC during the autograft fixation. No patient needed CK to further ensure the fixation. There were no significant differences in SMC use times among the 6 groups (Χ 2 = 3.630, P = 0.885, Fisher’s exact test) (Table 1). There were also no significant differences in postoperative discomfort scores among the 6 groups on postoperative days 1, 3 and 7 (Χ 2 = 3.318, P = 0.651; Χ 2 = 6.406, P = 0.269; Χ 2 = 7.595, P = 0.180, respectively).
All the patients completed the 6-month follow-up. In each group, a yellowish graft edema could be observed in some eyes in the first few days postoperatively, and it resolved gradually and spontaneously. The donor site healed well in every case within 14 days (Fig. 3). By the end of the first month, all the grafts were intact and maintained in the primary position except one in group SMC 7d. It was on follow-up day 3 that we found the graft curled and the sclera was exposed. The patient recalled that he had ever rubbed his eye carelessly. Then under topical anesthesia, the graft was stuck again and it grew well from then on.
There were 1 conjunctival cyst and 4 mild conjunctival shrinkages and 5 grafts overlying the limbus during the follow-up, but none needed further treatment. No cases of infection, anaphylactic reaction, symblepharon, scleral thinning, necrosis or severe visual acuity threatening complications were observed. At 6 month, there were 3 recurrences (total, 6%). One was in SMC 3d group found in month 4, one was in SMC 7d group found in month 3, and another was in SMC 2m group found in month 6. There were no significant differences in recurrences among the groups (Χ 2 = 4.047, P = 1.000, Fisher’s exact test) (Table 2).
Discussion
Since 2004 Dr. Koranyi G introduced the “cut and paste” technique in pterygium surgery, this excellent technique was soon accepted worldwide.
As the first one, our team carried out this new technique in China in 2005 and proved the Chinese CK could also do well in pterygium surgery. After we did the report at national annual meeting, the technique became popular in China.
The recent meta-analysis for FG versus sutures in pterygium surgery also proved the superiority of FG. It showed that using FG could result in less recurrence, shorten the operation time and lead to less postoperative discomforts compared with sutures [11].
At that time, the price of a CK was about 300 RMB Yuan, but now in 2015 this figure became over 1200. Surgeons had to turn back to the traditional method of autograft suture fixation or MMC after pterygium excision, although ”cut and paste” had obvious advantage in operation time, comfort and complications. To solve this problem, we designed a series of study. In previous research, we had proved mixing the separate fibrinogen and thrombin product could fix the autograft.
According to the results of this study, if we kept strict sterile operation during preparation, subpackage and cryopreservation, then SMC stored for 3 months would still be safe, which was also proved by subsequent clinical observation. SMC stored for different times (from 3d to 3m) got similar effect on autograft fixation and no case needed CK instead. Several cases needed two tries of SMC, and the reason might be that during the first try, the glue did not evenly distributed on the sclera surface which caused weak adhesion in some area. The F and C components were both prepared for 1 ml each tube, and every time only 1–2 drops (0.05–0.1 ml) each would be used, so a set of SMC could supply at least 10 tries considering the fact of natural loss.
During the 6-m follow-up, no severe complications including infection occurred. Common complications such as conjunctival cyst, mild conjunctival shrinkages and grafts overlying the limbus were observed in only a few cases. Previous published studies had confirmed the predominant efficacy of CK in pterygium surgery with the recurrence of 0–9.8% [1, 12,13,14]. We expected the total midterm recurrence rate for the SMC to be similar with that of CK reported, and it turned out to be 3/48 (6%). There was no significant difference in recurrences among the different SMC groups, so SMC 3m was as effective as SMC 3d. This meant we did not need to finish all the pterygium surgeries in one day, and with the SMC technique, the patient could be operated on at any time. The midterm follow-up and absence of control group may be the limitations of this study, and a prospective randomized and controlled study with a long term follow-up is now in progress (SMC 2m VS CK).
Another interesting technique of autologous blood instead of CK appeared in recent years. The autologous blood can eliminate the risk of transmitting infectious as well as any autoimmune or allergic reactions and avoid the cost of CK, the burden of its preparation and suspicion of its sterilization status [15]. But this new method might be more effective in small-size pterygium than larger one. Meanwhile, it was less stable, had a higher recurrence rate and took more surgery time compared with CK [16,17,18].
Additionally, the safety of SMC is of considerable importance. Human infection of parvovirus B19 (HPV B19) has been reported after the use of CK from different manufacturers. The allergen was believed to be the bovine protein aprotinin, which was included in the CK as an antifibrinolytic to inhibit clot breakdown [19, 20]. But the component of SMC contains no aprotinin. In our study, none of the patients experienced infection or anaphylactic reactions. Adherence to rigid aseptic techniques can help preclude the transmission of pathogens. The price of human fibrinogen 0.5 g is ~ 1000 RMB Yuan, which is approximately four times as much as one nylon 10-0 suture. The human fibrinogen can be dissolved in 8–10 ml of sterile water, and during the surgery only one drop of the fibrinogen solution is needed to attach the conjunctival autograft. If we subpacked the solution into 1-ml Eppendorf tube (each containing 0.5 ml, 10 drops), one bottle of human fibrinogen 0.5 can be used for up to 20 patients. Then, the average cost of the SMC becomes affordable and may be cheaper than a suture.
In conclusion, SMC is safe and effective for autograft fixation in pterygium surgery. After subpackage, the cost of material on each patient is very low. This new practical technique will benefit the patients and surgeons in developing and underdeveloped country.
References
Koranyi G, Seregard S, Kopp ED (2004) Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol 88:911–914
Uy HS, Reyes JM, Flores JD et al (2005) Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygiumexcision. Ophthalmology 112:667–671
Bahar I, Weinberger D, Dan G et al (2006) Pterygium surgery: fibrin glue versus Vicryl sutures for conjunctival closure. Cornea 25:1168–1172
Jiang J, Yang Y, Zhang M et al (2008) Comparison of fibrin sealant and sutures for conjunctival autograft fixation in pterygium surgery: one-year follow-up. Ophthalmologica 222:105–111
Hall RC, Logan AJ, Wells AP (2009) Comparison of fibrin glue with sutures for pterygium excision surgery with conjunctival autografts. Clin Exp Ophthalmol 37:584–589
Cha DM, Kim KH, Choi HJ et al (2012) A comparative study of the effect of fibrin glue versus sutures on clinical outcome in patient undergoing pterygium excision and conjunctival autografts. Korean J Ophthalmol 26:407–413
Koranyi G, Artzén D, Wijk T (2013) Learning curve in the cut and paste method for surgery of primary pterygium. Acta Ophthalmol 91:463–468
Cagatay HH, Gokce G, Ekinci M et al (2014) Long-term comparison of fibrin tissue glue and vicryl suture in conjunctival autografting for pterygium surgery. Postgrad Med 126:97–103
Jiang J, Gong J, Li W et al (2015) Comparison of intra-operative 0.02% mitomycin C and sutureless limbal conjunctival autograft fixation in pterygium surgery: five-year follow-up. Acta Ophthalmol 93:e568–e572
Tan DT, Chee SP, Dear KB, Lim AS (1997) Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol 115(10):1235–1240
Romano V, Cruciani M, Conti L, Fontana L (2016) Fibrin glue versus sutures for conjunctival autografting in primary pterygium surgery. Cochrane Database Syst Rev 12:CD011308
Koranyi G, Seregard S, Kopp ED (2005) The cut and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand 83:298–301
Marticorena J, Rodríguez-Ares MT, Touriño R et al (2006) Pterygium surgery: conjunctival autograft using a fibrin adhesive. Cornea 25:34–36
Pfister RR, Sommers CI (2005) Fibrin sealant in corneal stem cell transplantation. Cornea 24:593–598
Kurian A, Reghunadhan I, Nair KG (2015) Autologous blood versus fibrin glue for conjunctival autograft adherence in sutureless pterygium surgery: a randomised controlled trial. Br J Ophthalmol 99(4):464–470
Nadarajah G, Ratnalingam VH, Mohd Isa H (2017) Autologous blood versus fibrin glue in pterygium excision with conjunctival autograft surgery. Cornea 36(4):452–456
Boucher S, Conlon R, Teja S et al (2015) Fibrin glue versus autologous blood for conjunctival autograft fixation in pterygium surgery. Can J Ophthalmol 50(4):269–272
Choudhury S, Dutta J, Mukhopadhyay S et al (2014) Comparison of autologous in situ blood coagulum versus sutures for conjunctival autografting after pterygium excision. Int Ophthalmol 34(1):41–48
Morita Y, Nishii O, Kido M et al (2000) Parvovirus infection after laparoscopic hysterectomy using fibrin glue hemostasis. Obstet Gynecol 95(6 Pt 2):1026
Hino M, Ishiko O, Honda KI et al (2000) Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol 108(1):194–195
Acknowledgements
This study was supported by major scientific and technological project of Science Technology Department of Zhejiang Province (No. 2013C03048-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is nothing to declare about financial support or relationships that may pose conflict of interest.
Rights and permissions
About this article
Cite this article
Gong, JW., Chen, JH., Shen, T. et al. Self-made cryopreservative fibrin glue applied in pterygium surgery: a novel practical technique. Int Ophthalmol 38, 1295–1300 (2018). https://doi.org/10.1007/s10792-017-0629-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0629-9