Abstract
Our aim was to compare the efficacy and safety of autologous in-situ blood coagulum versus sutures for attaching conjunctival limbal autografts (CAG) among patients undergoing primary pterygium excision over a period of 1 year. Thirty-two eyes of 32 patients with primary pterygium were randomly divided in into two groups: group I (16 eyes) underwent CAG with 10-0 monofilament nylon sutures and group II (16 eyes) underwent CAG with patient’s own in-situ blood coagulum acting as bioadhesive or fixative followed by bandaging for 48 h. Patients were followed up postoperatively on the 2nd day, 1 week, 2 weeks, 4 weeks, and 12 months. All the surgeries were done by the same surgeon. Graft success, recurrence rate, operating time, patient comfort, graft retraction or any other complication were studied. The duration of surgery was significantly less (P < 0.001) in group II (mean duration 15 ± 2 min) than group I (mean duration 67 ± 2 min). Postoperative symptoms were fewer for group II than group I. Rate of recurrence was equal in both groups (one patient in each group, 6.25 %). But complications regarding graft failure and graft retraction were more common in group II (two patients, 12.5 %) than group I (one patient, 6.25 %); however, the difference was not statistically significant (Z = 0.61). Thus, autologous in-situ blood coagulum is a useful method for graft fixation in pterygium surgery with shorter operating time and less postoperative discomfort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pterygium is a common disorder in many parts of the world, with reported prevalence rates ranging from 0.3 to 29 % [1, 2]. The main challenge of pterygium surgery is prevention of recurrence. High recurrence rates have prompted ophthalmologists to develop different adjunctive measures for recurrence prevention. Beta-radiation, excimer laser, and antineoplastic–antimetabolite drugs are some of the techniques currently used to prevent recurrence of pterygium, but these may sometimes be associated with serious complications [3–9].
Conjunctival autografting after pterygium excision is associated with lower recurrence rates (2–9 %) and relatively few sight-threatening complications [10–12]. The current method of attaching conjunctival autografts is by means of suturing. The use of suture materials requires a high degree of surgical skill and is associated with several disadvantages, including prolonged operating time, postoperative discomfort, and potential for suture-related complications such as buttonholes, suture abscesses, granuloma formation, tissue necrosis, and giant papillary conjunctivitis [13–20]. Tissue adhesives are alternative means for attaching conjunctival grafts and may shorten operating time, improve postoperative comfort, and avoid suture-related complications [19, 20]. Several studies have considered using commercial fibrin glue in ophthalmic procedures [21–26]. However, the major concern of the commercial fibrin glue is the cost and the potential risk of transmitted infection [27, 28]. Several studies on the use of autologous fibrin glue have also been reported [29–32]. This also requires laboratory backup, which may not be always available especially in developing countries. Recent cross-sectional studies also describe successful outcomes with sutureless and glue-free conjunctival autografts [33, 34]. This successfully combines all the advantages of tissue adhesive without the requirement of laboratory backup or costly procedures. The purpose of this study was to compare the efficacy and safety of sutureless glue-free autologous in situ blood coagulum with nylon sutures for attaching conjunctival autografts during pterygium surgery.
Patients and method
Thirty-two consecutive patients who underwent primary pterygium excision at our institute from April 2010 to May 2011 were prospectively enrolled. A comprehensive medical and ocular history was obtained, including patient age, gender, family, medical and ocular history. Snellen visual acuity measurement, funduscopy, applanation tonometry, slit-lamp examination, and anterior segment photography were performed preoperatively. Patients with ocular pathology other than errors of refraction, with a history of previous ocular surgery or trauma, narrow occludable angles, ocular hypertension, physiologic or glaucomatous optic disc cupping, and a family history of glaucoma, were excluded. Informed consent was obtained from all patients. The study was performed following the Declaration of Helsinki and it was approved by the ethical committee of the institute.
The pterygia were graded according to the system used by Tan et al. [15]: grade 1 (atrophic), episcleral vessels under the body of the pterygium are not obscured and clearly distinguished; grade 3 (fleshy), episcleral vessels totally obscured; and grade 2 (intermediate), all other pterygia not falling into these 2 grades.
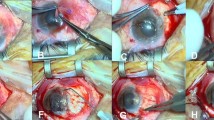
A single surgeon performed all surgeries. After instillation of topical proparacaine HCl (Alcaine; Alcon Laboratories, Fort Worth, TX, USA), the involved eye underwent standard ophthalmologic sterile preparation and draping. The pterygia were dissected from the apex using a surgical blade (No. 15) taking care to follow the surgical plane of the pterygium. Dissection was carried to the limbus. A lidocaine–epinephrine solution (Xylocaine 2 %; Astra-Zeneca, Sweden) was then injected into the pterygium head to balloon out the conjunctiva and delineate the underlying fibrovascular tissue. Blunt and sharp dissection was performed to separate the pterygium from the underlying sclera and surrounding conjunctiva. The pterygium head and surrounding atrophic conjunctival edges were then excised with Wescott scissors. The patient was then randomly assigned by coin toss to receive either nylon 10-0 sutures (group I, n = 16 eyes) or autologous fibrin in in situ blood coagulum (group II, n = 16 eyes). Only 1 eye per patient was entered in the study. For harvesting the free conjunctival autograft, we followed the technique described by Starck et al. [11]. The conjunctival donor graft site was marked on all sides with gentian violet to outline an oversized graft with an additional 1.0 mm of length and width relative to the dimensions of the graft bed. The epithelial side was marked to prevent graft inversion. The lidocaine epinephrine solution was injected into the donor conjunctiva to balloon out the area of the graft and separate it from the underlying Tenon’s capsule. By use of minimal manipulation and atraumatic conjunctival forceps and Vannas scissors, the conjunctiva was carefully dissected away from the Tenon’s capsule. Care was taken to prevent buttonholes and graft rollover. The free graft was then placed on top of the cornea and kept moist using sterile normal saline solution irrigating solution.
For group I, the graft was placed onto the bare sclera, and its 4 corners were anchored to the episclera with nylon 10-0 sutures. Care was taken to maintain the spatial orientation of the graft in relation to the limbus. The limbal side of the graft was affixed to the limbal area with horizontal mattress sutures. The sides of the graft were then attached to the surrounding conjunctiva at intervals of 1–1.5 mm with simple interrupted sutures. The sutures were removed 1 month postoperatively.
For group II, haemostasis was allowed to occur spontaneously without the use of cautery. The graft was placed on the bare sclera in such a way as to maintain the original orientation of the juxtalimbal border towards the cornea. The sclera bed was viewed through the transparent conjunctiva to ensure that residual bleeding did not lift the graft. Small central haemorrhages were tamponaded with direct compression. The free graft was held in position for 10 min by application of gentle pressure over it with a lens spatula. Care was taken to ensure that the spatial orientation was maintained and that the sides of the graft were apposed to the edges of the recipient conjunctiva. After a drying period of 10 min, the lid retractors were removed, and the patient was asked to blink several times to test graft adherence and mobility (Figs. 1, 2, 3).
Tobramycin–dexamethasone ointment (TobraDex; Alcon Laboratories) was placed in all eyes and a pressure patch applied for 48 h. Tobramycin and dexamethasone eye drops were applied 6 times daily for 1 month after the surgery.
Operating time was measured starting from placement of the lid retractors to removal at the end of surgery. The patients were followed up on the 2nd day after surgery and then on weeks 1, 2, and 4, and at 12 months. Snellen visual acuity testing and tonometry were tested during each visit. A slit-lamp examination was performed at every visit to monitor autograft integrity and development of complications such as corneal defects, symblepharon formation, giant papillary conjunctivitis, granuloma formation, and contact dermatitis graft retraction, chemosis, recurrence or any other complication.
Graft success was defined as an intact graft by the 4th week after surgery; graft failure was defined as absence of the graft by the 4th week. Recurrence was defined as any growth of conjunctiva into the cornea.
Subjective sensations of pain, foreign body sensation, tearing, and discomfort were evaluated on the first postoperative day and on weeks 1, 2, and 4 using a 5-point scale adapted from Lim-Bon-Siong and coworkers [35]: (0) none, no pain; (1) very mild, presence of pain but easily tolerated; (2) mild, pain causing some discomfort; (3) moderate, pain that partially interferes with usual activity or sleep; (4) severe, pain that completely interferes with usual activity or sleep.
Results
Of the 32 patients, 18 were male (56.25 %). The mean age was 45 ± 20 years (range 23–67 years). All patients completed the 12-month follow-up period. All pterygia were nasally located. The distribution of pterygium grading was similar for both groups (Table 1).
The mean surgical duration was 67 ± 2 min for group I and 15 ± 2 min for group II. The mean operating time was significantly shorter when autologous in situ blood coagulum was used instead of nylon sutures (P < 0.001). Postoperatively, some amount of graft edema and haemorrhage was present in all eyes; it gradually subsided over time.
Subjective symptoms of pain, foreign body sensation, tearing and discomfort were fewer and disappeared more rapidly in group II than the suture group. The intensity of these symptoms was significantly lower in the group II than the suture group on all follow-up days (P < 0.001). All patients in group II were asymptomatic after 2 weeks (Figs. 4, 5, 6, 7).
In group I, total graft dehiscence or graft failure occurred in 1 eye (6.25 %) in group I in one patient following vigorous rubbing of operated eye in early postoperative period. Total graft dehiscence occurred in 1 eye (6.25 %) in group II following inadvertent early removal of bandage on early 1st postoperative day by the patient. Another patient developed graft retraction due to lack of adhesion due to accidental inclusion of Tenon‘s in the free limbal conjunctival graft. However, the difference was not found to be statistically significant (Z = 0.61) (Table 2). Recurrence was seen in one eye (6.25 %) within the follow-up period in both of the groups. None of the patients developed button hole of conjunctival graft, excessive bleeding, perforation of the globe with suture needle, injury to medial rectus, dellen, pyogenic granuloma, symblepharon formation or scleral necrosis.
Discussion
Pterygium recurrence is the most common complication of pterygium surgery and is a frequent source of frustration for patients and surgeons. The current major methods of recurrence prevention include use of mitomycin C (MMC), conjunctival autografting, and, more recently, amniotic membrane grafting. A recent meta-analysis of pterygium recurrence after surgery concluded that simple bare sclera resection alone is associated with 6 times higher odds of pterygium recurrence if a conjunctival autograft was not used and 25 times higher odds of recurrence if MMC was not used. The authors recommended that simple bare sclera excision should not be encouraged as a method of primary pterygium removal [4]. However, although intraoperative MMC is more effective than β-irradiation for prevention of pterygium recurrence, the use of MMC can be associated with sight-threatening complications such as corneoscleral melt, cataract, uveitis, secondary glaucoma, and symblepharon [3, 5, 8]. Conjunctival autografting results in lower pterygium recurrence rates compared with bare sclera excision with primary closure and use of amniotic membrane grafts [14]. Conjunctival autografting is also associated with fewer complications. Only one case of necrotizing scleritis has been reported, and this case responded to steroid treatment [17]. Although conjunctival autografting is safer and clearly more effective than bare sclera resection in preventing pterygium recurrence, a greater amount of surgical expertise and technical ability is needed to attach autografts using sutures [16, 18]. Furthermore, suture use is associated with patient discomfort and minor complications such as dellen ulcer, symblepharon, and graft dehiscence [16, 19].
Fibrin glue has been used as an alternative to sutures for securing the conjunctival grafts with a recurrence rate of 5.3 % with glue versus 13.5 % with sutures and it has been suggested that immediate adherence of the graft and lack of postoperative inflammation may inhibit fibroblast ingrowth and reduce the recurrence [20]. The main issue in using commercial fibrin glue, despite viral inactivation techniques, is transmission of infectious agents like human infection of parvovirus B19 (HPV B19) and prions [27]. Autologous fibrin, though much safer, is yet to be used widely because of the time taken to procure the fibrin and lack of laboratory facilities at all centers.
In our series, only 1 eye (6.25 %) had a recurrence. Using similar procedure as ours, Wit et al. [33] had no recurrence in 15 eyes with a mean follow up of 9.2 months. Malik et al. [34] had a recurrence rate of 2.5 % (1 out of 40) using a similar procedure.
Malik et al. [34] postulated that apposition of the lids to the bulbar conjunctiva provides a natural biological dressing and confers a unique wound-healing environment. The lids provide compression, a smooth frictionless surface, and a vascular bed with immune capability in close proximity to the injury site.
Graft retraction, was seen in 1 eye (6.25 %) in our series which disappeared once the chemosis was controlled. It did not affect the final position of the graft. Similar occurrence of graft retraction (7.5 %) was reported by Malik et al. [34]. Tan et al. [15] advocated that risk of graft retraction could be minimised with meticulous dissection of subepithelial graft tissue. Wit et al. [33] postulated that a sutureless and glue-free graft resulted in an even tension across the whole of the graft interface and no direct tension on the free graft edges, resulting in reduced stimulus for the formation of subconjunctival scar.
Graft dehiscence is a recognized complication of using tissue glue [36]. With autologous fibrin, dehiscence occurred in 13.33 % cases and was attributed to a low concentration of thrombin and fibrinogen in the autologous glue as compared to the commercial preparation. Graft dehiscence occurred in two of our eyes of which one resulted following early bandage removal and the other was the result of the accidental inclusion of Tenon’s tissue in the free graft. The importance of a thin graft with careful dissection from the Tenon’s capsule is mandatory for a successful graft take-up.
This study compared autologous in situ blood coagulum with the use of nylon 10-0 sutures for securing conjunctival autografts. Even though nylon 10-0 is a finer material and should produce less suture-related discomfort than Vicryl 7-0, fewer postoperative symptoms were still reported when autologous in situ blood coagulum was used for attaching conjunctival autografts. It is clear from these results that grafts attached with autologous in situ blood coagulum are better tolerated than grafts attached with suture material. The advantages of using autologous in situ blood coagulum include ease of use, almost zero cost, shorter operating times, and lesser postoperative comfort. A recent study reported that the success rate of sutured conjunctival autograft can vary widely among different surgeons (range 5–82 %). This variability was attributed to significant learning curves and different surgical skill levels among different ophthalmologists [16]. Because the use of fibrin glue removes the need for the tedious suturing process, the learning curve can be shortened, and better results may be more consistently achieved despite differences in surgical expertise. Moreover, conjunctival autografting will be better accepted by the patients, because the use of autologous in situ blood coagulum produces significantly fewer symptoms.
Fibrin glue is costly, particularly for patients of developing countries who find it difficult to afford. Cost of 10-0 nylon is also substantial. In sharp contrast, in our procedure the cost of suture or bio-adhesive as fixative is virtually nil, while still having all the advantages of fibrin glue with no risk of cross-infection.
In summary, autologous in situ blood coagulum is an effective and safe method for attaching conjunctival autografts during pterygium surgery. The use of autologous in situ blood coagulum can significantly shorten operating times and produce fewer postoperative symptoms and discomfort. Long-term studies with greater numbers of cases are needed to determine whether the rate of pterygium recurrence is affected by the use of autologous in situ blood coagulum instead of suture material.
References
Moran DJ, Hollows FC (1984) Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol 68(5):343–346
Taylor HR, West S, Muñoz B (1992) The long-term effects of visible light on the eye. Arch Ophthalmol 110(1):99–104
Rubinfeld RS, Pfister RR, Stein RM (1992) Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology 99:1647–1654
Sanchez-Thorin JC, Rocha G, Yelin JB (1998) Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol 82:661–665
Hayasaka S, Noda S, Yamamoto Y, Setogawa T (1998) Postoperative instillation of low-dose mitomycin C in the treatment of primary pterygium. Am J Ophthalmol 106(715–71):8
Talu H, Tasindi E, Ciftci F, Yildiz TF (1998) Excimer laser phototherapeutic keratectomy for recurrent pterygium. J Cataract Refract Surg 24:1326–1332
Amano S, Motoyama Y, Oshika T et al (2000) Comparative study of intraoperative mitomycin C and beta irradiation in pterygium surgery. Br J Ophthalmol 84:618–621
Dadeya S, Kamlesh Khurana C, Fatima S (2002) Intraoperative daunorubicin versus conjunctival autograft in primary pterygium surgery. Cornea 21:766–769
Akarsu C, Taner P, Ergin A (2003) 5-Fluorouracil as chemoadjuvant for primary pterygium surgery: preliminary report. Cornea 22(522–52):6
Kenyon KR, Wagoner MD, Hettinger ME (1985) Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 92:1461–1470
Starck T, Kenyon KR, Serrano F (1991) Conjunctival autograft for primary and recurrent pterygia: surgical technique and problem management. Cornea 10:196–202
Allan BD, Short P, Crawford CJ (1993) Pterygium excision with conjunctival autografting: an effective and safe technique. Br J Ophthalmol 77:698–701
Chen PP, Ariyasu RG, Kaza V (1995) A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol 120:151–160
Prabhasawat P, Barton K, Burkett G, Tseng SC (1997) Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 104:974–985
Tan DT, Chee SP, Dear KB, Lim AS (1997) Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol 115:1235–1240
Ti SE, Chee SP, Dear KB, Tan DT (2000) Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol 84:385–389
Sridhar MS, Bansal AK, Rao GN (2002) Surgically induced necrotizing scleritis after pterygium excision and conjunctival autograft. Cornea 21:305–307
Cohen RA, McDonald MB (1993) Fixation of conjunctival autografts with an organic tissue adhesive [letter]. Arch Ophthalmol 111(1167–116):8
Koranyi G, Seregard S, Kopp ED (2004) Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol 88:911–914
Zauberman H, Hemo I (1988) Use of fibrin glue in ocular surgery. Ophthalmic Surg 19(2):132–133
Lagoutte FM, Gauthier L, Comte PR (1989) A fibrin sealant for perforated and preperforated corneal ulcers. Br J Ophthalmol 73(9):757–761
Mandel MA (1990) Closure of blepharoplasty incisions with autologous fibrin glue. Arch Ophthalmol 108(6):842–844
Bartley GB, McCaffrey TV (1990) Cryoprecipitated fibrinogen (fibrin glue) in orbital surgery. Am J Ophthalmol 109(2):227–228
Kajiwara K (1990) Repair of a leaking bleb with fibrin glue. Am J Ophthalmol 109(5):599–601
Kaufman HE, Insler MS, Ibrahim-Elzembely HA, Kaufman SC (2003) Human fibrin tissue adhesive for sutureless lamellar keratoplasty and scleral patch adhesion: a pilot study. Ophthalmology 110(11):2168–2172
Ratnalingam V, Eu AL, Ng GL, Taharin R, John E (2010) Fibrin adhesive is better than sutures in pterygium surgery. Cornea 29(5):485–489
Hino M, Ishiko O, Honda KI (2000) Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol 108(1):194–195
Kawamura M, Sawafuji M, Watanabe M (2002) Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg 73(4):1098–1100
Ozcan AA (2008) Autologous human fibrin glue in multilayered amniotic membrane transplantation. Ann Ophthalmol (Skokie) 40(2):107–109
Gammon RR, Prum BE Jr, Avery N, Mintz PD (1998) Rapid preparation of small-volume autologous fibrinogen concentrate and its same day use in bleb leaks after glaucoma filtration surgery. Ophthalmic Surg Lasers 29(12):1010–1012
Asrani SG, Wilensky JT (1996) Management of bleb leaks after glaucoma filtering surgery. Use of autologous fibrin tissue glue as an alternative. Ophthalmology 103(2):294–298
Mandel MA (1992) Minimal suture blepharoplasty: closure of incisions with autologous fibrin glue. Aesthetic Plast Surg 16(3):269–272
Wit D, Athanasiadis I, Sharma A, Moore J (2010) Sutureless and glue free conjunctival autograft in pterygium surgery: a case series. Eye 24:1474–1477
Malik KPS, Goel R, Gupta A, Gupta SK, Kamal S, Malik VK, Singh S (2012) Efficacy of sutureless and glue free limbal conjunctival autograft for primary pterygium surgery. Nepal J Ophthalmol 4(8):230–235
Uy HS, Reyes JM, Flores JD, Lim-Bon-Siong R (2005) Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology 112:667–671
Srinivasan S, Slomovic AR (2007) Eye rubbing causing conjunctival graft dehiscence following pterygium surgery with fibrin glue. Eye 21:865–867
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choudhury, S., Dutta, J., Mukhopadhyay, S. et al. Comparison of autologous in situ blood coagulum versus sutures for conjunctival autografting after pterygium excision. Int Ophthalmol 34, 41–48 (2014). https://doi.org/10.1007/s10792-013-9790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-013-9790-y