Abstract
Purpose
To determine the clinical efficacy of extended targeted retinal photocoagulation (ETRP) compared to conventional panretinal photocoagulation (CPRP) in proliferative diabetic retinopathy (PDR).
Methods
In a single-masked randomized clinical trial, 270 eyes of 234 patients with naïve early or high-risk PDR were randomly assigned to receive either CPRP or ETRP (135 eyes, each treatment arm). Best-corrected visual acuity (BCVA) measurement, fundus examination, wide-field fluorescein angiography (WFFA) and optical coherence tomography were carried out before and 3 months after retinal photocoagulation. Primary outcome was early PDR regression, specified as reduction in retinal neovascularization based on WFFA at 3 months. Secondary outcomes were BCVA and central macular thickness (CMT) changes.
Results
There were significantly more high-risk PDR eyes in ETRP group compared to CPRP (109 and 94 eyes, respectively, P = 0.04). Early PDR regression occurred in 71.9 and 64.4% of eyes in the ETRP and CPRP groups, respectively (P = 0.19). The mean number of applied laser spots in the ETRP was significantly fewer than CPRP (1202 vs. 1360, respectively, P < 0.001). Mean BCVA at baseline and 3 months post-laser were 0.37 ± 0.26 and 0.47 ± 0.19 logMAR in the ETRP arm, respectively. In the CPRP arm these values were 0.40 ± 0.27 and 0.47 ± 0.24 logMAR, respectively. Although mean BCVA decreased significantly in both treatment arms (ETRP P < 0.001, CPRP P = 0.009), the difference was not significant between arms (P = 0.68). CMT increased significantly in both groups (ETRP 41.08 μm, P < 0.001, CPRP 33.31 μm, P < 0.001). Nevertheless, the difference between the groups was not significant (P = 0.26).
Conclusions
ETRP with fewer number of laser spots may be an appropriate alternative to CPRP in PDR regression at least through 3 months.
Clinical trial.gov registration number
NCT01232179.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laser photocoagulation was first used in 1969 for treatment of proliferative diabetic retinopathy (PDR), one of the principal causes of both visual impairment and blindness [1, 2]. Seminal research performed by the Diabetic Retinopathy Study (DRS) Research Group in 1976 verified the effectiveness of scatter photocoagulation in the treatment of PDR [3, 4]. They showed that panretinal photocoagulation (PRP) reduced the risk of severe visual loss in cases of PDR, especially in eyes with high-risk PDR [3, 4].
For years now, laser photocoagulation in panretinal patterns have been the mainstay of PDR treatment for inducing regression of neovascularization. Despite its benefits in reduction in severe vision loss, PRP is associated with mild visual acuity (VA) loss, macular thickening, diminished visual field, reduced color and night vision, and reduced contrast sensitivity as well as severe pain [3, 5–8]. The wide distribution pattern used in conventional panretinal photocoagulation (CPRP) was shown to have an impact on side effects and complications of laser treatment, specifically macular edema and visual loss [3, 5, 9]. It is thus suggested that reduction in the extent of PRP treatment may be advantageous [10]. This led to interest in targeted and sectorial approaches to laser treatment which have shown favorable results in PDR [11, 12]. With the aim of sparing more perfused tissue from tissue scarring compared with CPRP, targeted retinal photocoagulation (TRP) was devised to specifically treat areas of retinal capillary non-perfusion and ischemic zones. Development of more advanced imaging technologies like wide-field fluorescein angiography (WFFA) made this method feasible. Studies have shown no visual loss or worsening of central retinal thickness after TRP [13]. However, the need for additional PRP after TRP is still frequent and may be considered as a disadvantage [10, 11, 14–17].
We designed the extended TRP (ETRP) procedure to treat areas of capillary non-perfusion and intermediate ischemic zones posterior to the equator as well as the entire retina anterior to the equator. The ETRP method was expected to reduce retinal neovascularization and induce PDR regression as conventional PRP while using fewer laser spots. The comparison of these two laser techniques has been addressed in this trial.
Methods
Subjects
In this single-masked randomized clinical trial, 285 eyes from 249 diabetic patients with naive early or high-risk PDR, who attended at the retina clinic and were candidates for laser therapy, were included and divided into two arms to receive either CPRP or ETRP. The patients were considered to have early or high-risk PDR based on DRS definition [18]. Two of the authors (AR, HN) examined, graded and enrolled the patients. If there was disagreement between graders, a senior retina specialist (MS) resolved the grading. The study was carried out after receiving the approval of the research ethics committee and followed the tenets of the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (ID: NCT01232179) and performed at Labbafinejad Medical Center from October 2011 up to December 2014. All patients filled out informed consent applications and were fully apprised of the aims of the study.
Initial evaluation at presentation included the measurement of best-corrected visual acuity (BCVA), applanation tonometry, slit lamp biomicroscopy and fundus examination. These examinations were repeated 3 months after laser treatment. The BCVA was measured by a certified examiner using a Snellen chart and was converted into the logMAR for statistical analysis. The patients also underwent WFFA and optical coherence tomography (OCT) at baseline and 3 months after retinal photocoagulation to evaluate capillary non-perfusion areas, neovascular activity and central macular thickness (CMT). The enrolled patients did not receive any intravitreal injections (anti-VEGF or corticosteroid) during the study period.
Exclusion criteria
Patients with prior retinal laser treatment to the study eye, CMT of more than 300 microns as measured by OCT or the presence of sub- or intraretinal fluid at the center of macula, prior vitreoretinal surgery, any other intraocular surgery within the last 6 months, ongoing neovascular glaucoma, recent anti-VEGF treatment (in the last 6 months), severe cataract that could affect vision and precise laser treatment, vitreous hemorrhage severe enough to preclude peripheral retinal laser therapy, tractional retinal detachment, and not-enough dilatable pupil were excluded from the study.
Devices
The Heidelberg Retina Angiograph II (HRA2, Heidelberg engineering, Heidelberg, Germany) was used for angiography. Spectral domain OCT (SD-OCT) was performed with the Spectralis OCT (Heidelberg engineering, Heidelberg, Germany) and Topcon 3D OCT-1000 (Topcon Medical Systems, Tokyo, Japan). Quality scores for scans were expressed as the signal-to-noise ratio in decibels (dB). Quality of scans above 20 dB was considered acceptable. Macular thickness was measured in a 6-mm circle of the macular region centered on the fovea. The average of all points within the 1-mm central subfield circle was defined as the central macular thickness. Funduscopy was performed using slit lamp with 78- or 90-diopter Volk lens, and indirect ophthalmoscopy was performed using 20-diopter Volk lens. For retinal photocoagulation, we used solid-state laser devices including Ellex Integre Pro (Ellex Medical Lasers, Adelaide, Australia), LightLas 532 Green Laser Photocoagulator (LightMed, San Clemente, USA) and Nidek DC-3300 Diode Laser (Birmingham Optical Group, Birmingham, UK). Lenses employed included Volk Super Quad 160 (Volk Optical Inc, Mentor, USA) and OMRA-WF Ocular Mainster Wide Field (Ocular Instruments, Bellevue, USA).
Laser therapy intervention
In both treatment arms, laser energy was determined based on grade II DRS definition to make white to light gray burns. The nasal, inferior, superior and temporal retina was treated sequentially. The device used for photocoagulation was determined according to surgeon preference. Photocoagulation was performed in four sessions.
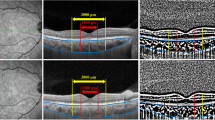
In the CPRP group, 1200–1600 laser burns with spot size of 200 µm, duration of 200 ms and spacing of 0.5 burn width were applied. Treatment started from the vascular arcade toward the periphery (Fig. 1). In the ETRP group, the entire retina anterior to the equator as well as the capillary non-perfusion areas between the vascular arcade and the equator were treated using the same spot size, spot duration and spacing between laser spots (Fig. 2). One of the authors specified the capillary non-perfusion areas on the angiograms. In order to ensure covering the entire ischemic areas as well as their margins, which usually have a high density of microaneurysms, the laser applied 1-disk diameter beyond the ischemic areas. The equator was localized in relation to the vortex ampulla.
Wide-field fluorescein angiography at baseline, showing the features of proliferative diabetic retinopathy. There are multiple hyperfluorescence areas compatible with vascular leakage from neovascularization elsewhere and neovascularization of disk. In conventional panretinal photocoagulation (CPRP) group, laser therapy was performed from the vascular arcade (shown by circle) toward the periphery
Outcome measures
The primary outcome measure was early PDR regression, defined as reduction in neovascular process based on WFFA at three months after conclusion of laser therapy compared with baseline. Secondary outcomes were changes in VA and CMT compared to the baseline at three months’ post-laser. A reduction in VA ≥2 lines was defined as VA worsening.
Clinical efficacy assessment
Three-months post-laser, the patients were re-examined and underwent the second WFFA. Based on clinical examination and WFFA, PDR regression was judged and considered to take place if retinopathy no longer was in the high-risk category (in the previously diagnosed high-risk PDR eyes) or neovascular activity was reduced (in the early PDR cases) [11]. One senior faculty member vitreoretinal specialist other than the authors, judged PDR regression. Re-treatment was performed based on Early Treatment Diabetic Retinopathy Study (ETDRS) guidelines. Ocular or non-ocular adverse events (AEs) were recorded at each laser session and at 3-months post-laser visits.
Sample size
In order to detect a clinically important difference of 15% in PDR regression rate between the two groups with a power of 80% and at the type I error of 0.05, 129 eyes in each arm were required. Considering the probable loss, 285 eyes were randomized in two arms (142 and 143 eyes in CPRP and ETRP groups, respectively).
Randomization and allocation sequencing
The permutated-block randomization with varying length of 4, 6, 8 and 10 was selected as the method of randomization. Random allocation sequencing was performed by a biostatistician thorough a computer-generated randomization list. Details of the series were unknown to the investigators.
Statistical analysis
All statistical analysis performed by SPSS software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). To present the data, we used mean, standard deviation, median and range. To assess the changes within groups, we used paired t test. Considering the probable correlation of bilateral samples, we used generalized estimating equation (GEE) to evaluate the difference between two groups. Effect of possible confounders such as baseline values and PDR status was considered in another GEE. Interaction analysis within GEE was used to evaluate effect modification of other variables, comparing treatment groups. To find the relation of VA changes with CMT changes, we used Spearman correlation coefficient. P value <0.05 considered statistically significant.
Results
Among 290 eligible eyes, 5 eyes were excluded since they did not sign informed consent. The remaining 285 eyes were randomly assigned in CPRP (142 eyes) and ETRP (143 eyes) groups. Intervention was discontinued in 7 eyes in CPRP and 8 eyes in ETRP groups. Reasons for withdrawal included non-compliance with laser therapy (5 and 6 eyes in CPRP and ETRP groups, respectively) and inability to attend post-laser visit (2 eyes in each group). In total, 270 eyes of 234 patients completed the study in two arms, each including 135 eyes and were included in our analyses. There were 73 (54.1%) and 68 (50.4%) males in the ETRP and CPRP arms, respectively. Mean age was 50 ± 8.3 and 49 ± 7.8 years. There was no significant difference among the treatment arms in terms of gender and age (P = 0.54 and 0.32, respectively, Table 1). Thirty-six patients underwent bilateral treatment. Sixty-seven eyes were diagnosed as early PDR and the other 203 eyes had high-risk PDR. The difference in the number of eyes with high-risk PDR was significant between the groups: 109 eyes (80.7%) in the ETRP group and 94 eyes (69.6%) in the CRPR group (P = 0.04, Table 1).
After 3 months, early PDR regression occurred in 71.9 and 64.4% of the eyes in the ETRP and CPRP groups, respectively (Table 2). Although a higher percentage of regression was noticed in the ETRP group, the difference did not reach to a significant level (P = 0.19). However, the mean number of laser spots applied in the ETRP group was significantly fewer than that of the CPRP group (1202 vs. 1360, respectively, P < 0.001, Table 1). Nonetheless, after adjustment for the number of laser spots there was no statistically significant difference between two groups in terms of RDR regression (P = 0.559, based on GEE analysis). In subgroup analysis, PDR regression occurred in 73.2 and 60.6% of eyes with early and high-risk PDR in CPRP group, respectively (P = 0.64). In ETRP arm, PDR regression occurred in 80.8 and 69.7% of early and high-risk PDR eyes, respectively (P = 0.265). PDR regression was not significantly different in early PDR eyes comparing CPRP and ETRP protocols (P = 0.649). The same result was found in eyes with high-risk PDR (P = 0.669) (Table 2).
Mean BCVA at baseline was 0.37 ± 0.26 logMAR in the ETRP group and 0.40 ± 0.27 logMAR in the CPRP group (P = 0.44). The mean BCVA 3 months post-laser was 0.47 ± 0.19 and 0.47 ± 0.24 logMAR in the ETRP and CPRP groups, respectively. Though VA decreased significantly in both groups, the difference was not significant between the groups (P = 0.68, Table 2). At 3 months, 85 (64.4%) and 82 (60.7%) eyes had stable or improved BCVA while 50 (35.6%) and 53 (39.3%) eyes had worsened VA in ETRP and CPRP groups, respectively (P = 0.54).
As shown in Table 1, the difference of baseline CMT was insignificant between the groups: 245 ± 26 vs. 248 ± 28 µm in the ETRP and CPRP groups, respectively (P = 0.36). Three months following laser, mean CMT increased to 285 ± 40 and 281 ± 44 µm in the ETRP and CPRP groups, respectively (Table 2). Although these changes were significant in both arms (41.08 µm in the ETRP group, 95% CI 33.52–48.64, P < 0.001 and 33.31 µm in the CPRP group, 95% CI 24.90–41.72, P < 0.001), the difference between the groups was not significant (P = 0.26). Furthermore, the changes of VA (logMAR) and CMT were found to be correlated positively among patients (r = 0.165, P = 008). However, eyes with worsened BCVA had greater increase in CMT (mean increase difference 23 µm, 95% CI 12–36, P < 0.001) compared to the eyes with stable or improved BCVA.
None of the eyes developed tractional retinal detachment during the study. Additionally, no ocular or non-ocular AEs related to the study intervention were detected by the investigators or reported by patients.
Discussion
In this trial, we prospectively investigated the clinical efficacy of a modified targeted retinal photocoagulation defined as ETRP compared to the conventional PRP described by DRS Research Group. It was shown that at 3 months following laser therapy, the ETRP technique was successfully effective in early PDR regression (71.9%) compared to the CPRP technique (64.4%). Although both techniques resulted in BCVA worsening and CMT increase in some cases, they were comparable in this regard.
Some earlier studies compared the efficacy of other PRP techniques with the conventional one. For instance, in a prospective randomized clinical trial by Blankenship, mid-peripheral PRP combined with either peripheral or central retinal photocoagulation in only 50 PDR eyes were evaluated [10]. The purpose was to spare the posterior fundus from laser treatment. After 6 months, the rate of disk neovascularization regression was similar, 67 and 60% in peripheral and central retinal photocoagulation groups, respectively. In another study, Plumb et al. [14] compared panretinal versus peripheral photocoagulation on regression of disk neovascularization in PDR eyes, using xenon arc and argon laser. At 3 months following laser therapy, 9 among 22 patients needed additional laser therapy (41%) in the peripheral photocoagulation group. They concluded that treatment of the peripheral retina only might be inadequate in many cases of PDR. Anti-VEGF therapy is another treatment modality in treating PDR [19]. In a paper published recently by diabetic retinopathy clinical research (DRCR) network, they concluded that treatment of PDR cases with ranibizumab resulted in visual acuity and neovascularization regression rate that was non-inferior to PRP treatment [20]. Nonetheless, the cost and probable ocular and systemic adverse effects of anti-VEGF treatment should be kept in mind.
Regarding targeted PRP technique, one study investigated the clinical effects of targeted pattern scan laser photocoagulation (200 µm, 20-ms pulse) in the treatment of naive PDR cases [11]. They used PASCAL (PAttern SCAnning Laser) method of photocoagulation applied to zones of retinal capillary non-perfusion and intermediate retinal ischemia in 28 eyes, guided by wide-field fluorescein angiography. At 12-week visit, 10 eyes (37%) needed and underwent repeat TRP treatment. Despite small sample size, this case series showed that the treatment of only non-perfusion areas might not be adequate to achieve PDR regression. In our study therefore, we applied a modified TRP named as ETRP and treated more retinal areas in addition to the mid-peripheral non-perfused areas. We selected to treat the peripheral retina anterior to the equator plus the non-perfused areas of the mid-periphery in order to diminish the laser spots. In addition to this assumed advantage, we succeeded to a comparable regression rate in retinal neovascularization with fewer number of laser spots (1202 in the ETRP vs. 1360 in the CPRP groups) which was indicative of less retinal destruction. To the best of our knowledge, this method had not been performed and investigated before. Furthermore, the method of laser photocoagulation of non-perfused areas in PDR is underway and its results have not been published yet.
In the present study, the need for additional laser treatment was almost similar between the ETRP and CPRP groups (28.1 vs. 35.6%, respectively). The higher number of high-risk PDR cases in the ETRP group might indicate the greater efficacy of ETRP. However, post hoc power analysis showed that our study had 54% power to detect the observed difference (7.5%). A power level of 80, 90 and 95% would require 245, 327 and 404 eyes in each study arm, respectively, necessitating a large multicenter trial.
In our study, 50 (35.5%) and 53 (39%) eyes lost ≥2 lines of vision in the ETRP and CPRP groups, respectively. This was comparable to other published reports, showing a range of 25–43% of eyes with VA worsening after laser [12–24]. In the diabetic retinopathy study, severe visual loss occurred in more than 10% of eyes after panretinal photocoagulation [4]. In Blankenship report, there was 8 and 24% of eyes with ≥2 lines VA worsening in the peripheral and central retinal photocoagulation groups, respectively [10]. However, Muqit et al. observed no change in VA at 4 and 12 weeks in 28 cases after pattern laser photocoagulation [11].
We found a positive linear correlation between VA and CMT changes. Therefore, CMT increase was the possible reason of BCVA worsening in our cases. McDonald and Schatz also reviewed 175 eyes and similar to our results reported that macular edema was the main cause of VA deterioration following PRP [25]. Other reasons for post-PRP diminishing VA in diabetic retinopathy include associated systemic diseases like hypertension, hyperlipidemia, nephropathy and also poor glycemic control [26–28]. The effects of these factors could be influential in longer period of times and therefore were not addressed in our study.
Present study demonstrated that mean CMT increased in both ETRP and CPRP groups (41 and 33 µm, respectively) which was almost similar. Central macular thickening after laser therapy has been shown in numerous other studies [29–31]. McDonald and Schatz [25] reported increased macular edema in 43% of their cases, occurred 6–10 weeks after PRP. Soman et al. [30] reported 15% increase in CMT after PRP in eyes with PDR without clinically significant macular edema. We found 18 and 15% CMT rise in the ETRP and CPRP groups, respectively. Additionally, type of macular edema based on OCT findings has been shown to be more relevant and better correlated with visual outcome than the quantitative estimation of foveal thickness [30].
The main limitation of our study was short-term follow-up. Although, due to new tendency to inject anti-VEGF in PDR patients and developing macular edema in some of our cases, it was not possible to continue the study longer than 3 months post-laser, without anti-VEGF injection [19, 20]. The other limitation was not matching the associated systemic diseases and the glycemic control between the two groups. However, singh and co-workers evaluated the effect of systemic factors like serum glucose, HbA1c level and blood pressure on BCVA achieved with ranibizumab for treatment of DME in the RISE and RIDE studies and found no associations between systemic factors (baseline values or change from baseline) and mean change of BCVA at month 24 [32]. Also, we did not consider the effects of this new treatment approach on other aspects of visual function other than central visual acuity, such as visual field. In addition, we did not perform an early post-treatment evaluation by fluorescein angiography to ensure covering all ischemic areas by laser spots. However, our study was powered by prospective design and randomized allocation of patients, high sample size, limited exclusion criteria and determining CMT changes as well.
In conclusion, the present study disclosed that ETRP was a sensible substitution for CPRP to induce PDR regression. Considering the fewer number of laser spots and applying the laser to non-perfused mid-peripheral retina based on WFFA, one would conclude that ETRP induces less posterior retinal destructive effect compared to the conventional method while preserves the same therapeutic effect. Future studies with larger sample size, longer follow-up and testing VF may further confirm our results.
References
Beetham WP, Aiello LM, Balodimos MC, Koncz L (1969) Ruby-laser photocoagulation of early diabetic neovascular retinopathy: preliminary report of a long-term controlled study. Trans Am Ophthalmol Soc 67:39–67
Pascolini D, Mariotti SP (2010) Global estimates of visual impairments: 2010. Br J Ophthalmol 96:614–618
Diabetic Retinopathy Study Research Group (1976) Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 81:383–396
Diabetic Retinopathy Study Research Group (1981) Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 88:583–600
Early Treatment Diabetic Retinopathy Study Research Group (1991) Early photocoagulation for diabetic retinopathy, ETDRS report number 9. Ophthalmology 98(5 Suppl):766–785
Fong DS, Girach A, Boney A (2007) Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina 27:816–824
Rand LI, Prud’homme GJ, Ederer F, Canner PL (1985) Factors influencing the development of visual loss in advanced diabetic retinopathy, Diabetic Retinopathy Study (DRS) report no. 10. Invest Ophthalmol Vis Sci 26:983–991
Watanachai N, Choovuthayakorn J, Patikulsila D, Ittipunkul N (2015) Changes in central macular thickness following single session multispot panretinal photocoagulation. J Ophthalmol. doi:10.1155/2015/529529
Kaiser RS, Maguire MG, Grunwald JELD et al (2000) One-year outcomes of panretinal photocoagulation in proliferative diabetic retinopathy. Am J Ophthalmol 129:178–185
Blankenship GW (1988) A clinical comparison of central and peripheral argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 95:170–177
Muqit MM, Marcellino GR, Henson DB et al (2013) Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol 91:251–258
Japanese Society of Ophthalmic Diabetology, Subcommittee on the Study of Diabetic Retinopathy Treatment (2012) Multicenter randomized clinical trial of retinal photocoagulation for preproliferative diabetic retinopathy. Jpn J Ophthalmol 56:52–59
Muqit MM, Young LB, McKenzie R et al (2013) Pilot randomised clinical trial of Pascal TargETEd Retinal versus variable fluence PANretinal 20 ms laser in diabetic retinopathy: PETER PAN study. Br J Ophthalmol 97:220–227
Plumb AP, Swan AV, Chignell AH, Shilling JS (1982) A comparative trial of xenon arc and argon laser photocoagulation in the treatment of proliferative diabetic retinopathy. Br J Ophthalmol 66:213–218
Reddy S, Hu A, Schwartz SD (2009) Ultra-wide field fluorescein angiography guided targeted retinal photocoagulation (TRP). Semin Ophthalmol 24:9–14
Vine AK (1985) The efficacy of additional argon laser photocoagulation for persistent, severe proliferative diabetic retinopathy. Ophthalmology 92:1532–1537
Okun E (1968) The effectiveness of photocoagulation in the therapy of proliferative diabetic retinopathy (PDR). (A controlled study in 50 patients). Trans Am Acad Ophthalmol Otolaryngol 72:246–252
Diabetic Retinopathy Study Research Group (1978) Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 85:82–106
Osaadon P, Fagan XJ, Lifshitz T, Levy J (2014) A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 28:510–520
Writing Committee for the Diabetic Retinopathy Clinical Research Network (2015) Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 314:2137–2146
Sl Fine, Patz A (1987) Ten years after the diabetic retinopathy study. Ophthalmology 94:739–740
Asher R, Hunt S, Hamilton AM et al (1981) Photocoagulation for optic disc new vessels in diabetes mellitus. Int Ophthalmol 3:79–85
Lim AS, Khoo CY, Ang BC, Chiang C (1985) Argon laser photocoagulation in diabetic retinopathy: five-year review of 697 treated eyes. Ann Acad Med Singapore 14:252–260
Bailey CC, Sparrow JM, Grey RH, Cheng H (1999) The national diabetic retinopathy laser treatment audit. III. Clinical outcomes. Eye 13:151–159
McDonald HR, Schatz H (1985) Macular edema following panretinal photocoagulation. Retina 5:5–10
Rodriguez-Fontal M, Kerrison JB, Alfaro DV, Jablon EP (2009) Metabolic control and diabetic retinopathy. Curr Diabetes Rev 5:3–7
Rema M, Sujatha P, Pradeepa R (2005) Visual outcomes of pan-retinal photocoagulation in diabetic retinopathy at one-year follow-up and associated risk factors. Indian J Ophthalmol 53:93–99
Davis MD, Fisher MR, Gangnon RE et al (1998) Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: early treatment diabetic retinopathy study report #18. Invest Ophthalmol Vis Sci 39:233–252
Shimura M, Yasuda K, Nakazawa T et al (2009) Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol 247:1617–1624
Soman M, Ganekal S, Nair U, Nair KGR (2012) Effect of panretinal photocoagulation on macular morphology and thickness in eyes with proliferative diabetic retinopathy without clinically significant macular edema. Clin Ophthalmol 6:2013–2017
Brucker AJ, Qin H, Antoszyk AN et al (2009) Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol 127:132–140
Singh RP, Habbu K, Ehlers JP et al (2016) The impact of systemic factors on clinical response to ranibizumab for diabetic maculae edema. Ophthalmology 123:1581–1587
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
The study was carried out after receiving the approval of the research ethics committee of Shahid Beheshti University of Medical Sciences and followed the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nikkhah, H., Ghazi, H., Razzaghi, M.R. et al. Extended targeted retinal photocoagulation versus conventional pan-retinal photocoagulation for proliferative diabetic retinopathy in a randomized clinical trial. Int Ophthalmol 38, 313–321 (2018). https://doi.org/10.1007/s10792-017-0469-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0469-7